Richard Conniff's Blog, page 74
May 1, 2013
Antibiotic Resistance Spills Over to Wildlife

Banded mongoose at Botswana’s Chobe National Park
For the next few months, many posts from Strange Behaviors will also be appearing at TakePart, the web site for Participant Pictures (“An Inconvenient Truth,” “Charlie Wilson’s War,” “Lincoln,” and many more). I’ll start those posts here and then give you a link to jump to the body of the story at Take Part. Here’s the first such item:
Banded mongooses don’t get the love like their celebrated cousins the meerkats. Their eyes aren’t quite as soulful, and they don’t spend as much time standing around on their hind legs looking human. Even so, these small, highly social creatures are a favorite with visitors to sub-Saharan Africa, nosing around the camp in small groups, searching for beetles, millipedes, and other choice foods. But now, improbably, banded mongooses have turned up in the middle of a global health crisis.
It is, on the surface, a familiar story about the greatest miracle drugs in modern medicine: Massive overuse of antibiotics has rapidly caused bacteria to develop resistance, meaning that many human illnesses, from a common urinary tract infection to tuberculosis, are becoming difficult or impossible to treat. Antibiotic resistance is so widespread that, according to new research, it occurs even in wildlife living in a national park in southern Africa.
The new study, published last week in the scientific journal EcoHealth, raises questions about overuse of antibiotics, the hidden costs of ecotourism, and the need for more careful management of protected wildlife areas. Diseases spilling over from animals to humans have “the potential to spark a global pandemic,” according to the authors, and the new data add the alarming prospect that some emerging pathogen—the next SARS or swine flu—may be resistant to antibiotic treatment from Day One.
To read the rest of the article, click here.


April 24, 2013
How to Destroy a Mainland Madagascar
This is a review I wrote for the Wall Street Journal:
Gold Rush in the Jungle
By Dan Drollette Jr.
(Crown, 310 pages, $25)
If you delight, as I do, in strange, colorful animals, and like to see a lot of them at the same time, the usual strategy is to visit islands, where isolation has a way of breeding eccentricity. But over the past few decades, Vietnam has revealed itself, improbably, as a sort of mainland Madagascar. It is a mother lode of newly described species, many of them upland refugees in a region cut off from mainland Asia during the ice age.
![[image]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381172172i/4443193.jpg) Recent discoveries there include a tube-nosed bat named M. beelzebub for its devilish good looks, a walking catfish, a striped rabbit, and, just this year, a flying frog and a turquoise-headed crocodile lizard. The saola, a large forest-dwelling antelope first described in 1992, is so odd that it required the invention of a new genus, Pseudoryx. Since no one has been able to keep a saola alive in captivity, the species remains an enigma—and an enigma that is probably en route to extinction: Vietnam resembles Madagascar not just in the diversity of its wildlife but in its catastrophic rush to destroy it.
Recent discoveries there include a tube-nosed bat named M. beelzebub for its devilish good looks, a walking catfish, a striped rabbit, and, just this year, a flying frog and a turquoise-headed crocodile lizard. The saola, a large forest-dwelling antelope first described in 1992, is so odd that it required the invention of a new genus, Pseudoryx. Since no one has been able to keep a saola alive in captivity, the species remains an enigma—and an enigma that is probably en route to extinction: Vietnam resembles Madagascar not just in the diversity of its wildlife but in its catastrophic rush to destroy it.You might think that outsiders had already taken care of that. During what is known there as “the American War,” U.S. forces doused Vietnam with about 20 million gallons of herbicides and defoliants, destroying 7,700 square miles of forest. Earlier, in the French colonial era, a single big-game hunter in the Annamite Mountains gunned down 600 deer, 50 tigers and panthers, and 40 elephants (about as many as now survive in the entire nation).
But the era of mass extinctions has really taken off as Vietnam has developed into an economic powerhouse, with average annual GDP growth of 6.3% over the past dozen years. The rising middle class has so far developed an appetite for the natural world only in the most literal sense: The craving for exotic meats and traditional medicines often leads to what naturalists call “empty forest syndrome.” In 1992, for instance, Vietnam designated Cát Tiên National Park, north of Ho Chi Minh City, as a reserve for mainland Asia’s last population of Javan rhinos. But the government never provided adequate protection, particularly during a world-wide rhino-poaching crisis said to be largely driven by Vietnam itself. At Cát Tiên in 2010, poachers butchered the nation’s last surviving rhino for the imaginary medicinal value of its horn.
In “Gold Rush in the Jungle,” science writer Dan Drollette Jr. attempts to tell this story of discovery amid pell-mell destruction. He focuses primarily on the work of Tilo Nadler, an East German immigrant whose Endangered Primate Rescue Center south of Hanoi has become a final refuge for many species, including some rescued from Vietnam’s rampant illegal traffic in wildlife and some new species discovered by Mr. Nadler’s team.
Having started in 1993 with a few acres and a budget of just $20,000 a year, Mr. Nadler now maintains about 15 species in captivity, mainly highly endangered monkeys. To critics who argue for protecting whole habitats rather than plucking out a few charismatic species for captive breeding, Mr. Nadler replies: “The biggest problem in Vietnam is that there is no time for education on environmental issues. It takes twenty years to see the effects of an education program, and these species don’t have even ten years. . . . You can’t put animals back in the wild if they are extinct.”
It is a timely story, and with plenty of potential take-home. Mr. Drollette points out, for instance, that the U.S. is by far the biggest customer for wood furniture from Vietnam. That bedroom set with the attractive price tag? It often comes at an unspoken cost: We are supplying the cash to cut down the forests where Mr. Nadler’s monkeys used to live.
Unfortunately, “Gold Rush in the Jungle” is a dismally inept book, starting with the title, which risks reinforcing the widespread suspicion in emerging countries that naturalists are somehow cashing in on their discoveries. Mr. Drollette even quotes an unintentionally hilarious line from Michael Crichton’s novel “Congo” about a biologist driven to discover new species by “his insensate lust for fame.” (Look for your favorite taxonomist edging Kim Kardashian off the cover of next week’s People magazine.)
The book’s flat-footed language often sounds like the narration in a Wes Anderson film, minus the irony: “His take on the situation was that the horror and the beauty associated with the country coexisted simultaneously, much like what is found in Mother Nature.” It’s also riddled with minor errors. It wasn’t Ernst Mayr who coined the term “island biogeography”; it was Robert MacArthur and E.O. Wilson. It wasn’t other biologists who said “God created, Linnaeus arranged”; it was Linnaeus himself. And on a personal note, it wasn’t “some scientists” who proposed creating a “Wall of the Dead” to commemorate the naturalists who have lost their lives in the search for new species. That was me, and I’m a writer, not a scientist.
But the real problem with this book is that Mr. Drollette almost never takes the reader out into the forest to show us through his own eyes just what we are losing. He seems to consider a 25-minute walk from a paved road a voyage into Vietnam’s “lost world.” He gets unnerved being alone in a room with, of all things, a captive civet, a small, mongoose-like mammal with a fondness for fruit, coffee beans and fermented palm sap. His big wildlife encounter involves the moths gathered on a bedsheet under a porch light in Mr. Nadler’s yard.
It is a pity, because there is still time for the outside world to help Vietnam protect the natural wonders that are its best real shot at a sustainable future. Or maybe we should just despair. When Vietnamese VIPs tour his captive breeding facility, Mr. Nadler tells Mr. Drollette that they often conclude by saying: “We’ve seen your animals. Where’s your restaurant? Now we want to eat exotic animals.”


April 23, 2013
Her Last Chance at a Baby (Body Eclectic–Part 1)
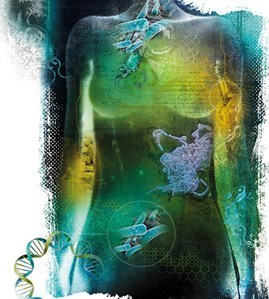
(Illustration: Stephanie Dalton Cowan)
This is a piece I wrote for the May issue of Smithsonian Magazine, about life on the human body.
Of all the cases Barbara Warner has faced as a pediatrician specializing in newborns, the one that sticks hardest in her mind involved a couple who had been trying for years to have children. Finally, in 1997, the woman was pregnant. She was in her mid-40s. “This was her last chance,” says Warner. Then, too soon, she gave birth to twins. The first child died at two weeks of respiratory failure, at the time the most common killer of preterm babies.
A week later—it happened to be Thanksgiving Day—Warner folded down the blanket on the surviving twin, and even now she draws in her breath at the memory. The baby’s belly was reddened, shining and so swollen “you could have bounced a nickel off it.”
It was necrotizing enterocolitis, or NEC, little known outside neonatal intensive care units, but dreaded there as a sudden, fast-moving bacterial inflammation of the gut. On the operating table, a surgeon opened the baby boy’s abdomen and immediately closed it again. The intestinal tract from stomach to rectum was already dead. Warner, in tears, returned the child to die in the arms of his shattered parents.
“It’s 15 years later, and there’s nothing new,” Warner says bleakly as she moves among her tiny patients, each one covered in tubes and bathed in soft violet light, in a clear plastic incubator. NEC is still one of the leading killers of preterm babies. But that may soon change, thanks to a startling new way of looking at who we are and how we live.
Over the past few years, advances in genetic technology have opened a window into the amazingly populous and powerful world of microbial life in and around the human body—the normal community of bacteria, fungi and viruses that makes up what scientists call the microbiome. It’s Big Science, involving vast international research partnerships, leading edge DNA sequencing technology and datasets on a scale to make supercomputers cringe. It also promises the biggest turnaround in medical thinking in 150 years, replacing the single-minded focus on microbes as the enemy with a broader view that they are also our essential allies.

A baby recovers from surgery for NEC (Photo: Mark Katzman)
The subject matter is both humble and intimate. In Warner’s neonatal care unit at St. Louis Children’s Hospital, researchers studying NEC have analyzed every diaper of almost every very low-weight baby delivered there over the past three years. They don’t expect to find a single pathogen, some killer virus or bacteria, the way medical discovery typically happened in the past. Instead, says Phillip Tarr, a Washington University pediatric gastroenterologist who collaborates with Warner, they want to understand the back-and-forth among hundreds of microbial types in the newborn’s gut—to recognize when things go out of balance. Their goal is to identify the precise changes that put a baby on track to developing NEC and, for the first time, give neonatal care units crucial advance warning.
A separate research group demonstrated early this year that secretions from certain beneficial microbes seem to relieve the deadly inflammation characteristic of NEC. So doctors may soon see into life-or-death processes that until now have been hidden, and take action to address them.
The new insights into NEC suggest why the microbiome suddenly seems so important to almost everything in the medical and biological worlds, even our understanding of what it means to be human. We tend to think that we are exclusively a product of our own cells, upwards of ten trillion of them. But the microbes we harbor add another 100 trillion cells into the mix. The creature we admire in the mirror every morning is thus about 10 percent human by cell count. By weight, the picture looks prettier (for once): Altogether an average adult’s commensal microbes weigh about three pounds, roughly as much as the human brain. And while our 21,000 or so human genes help make us who we are, our resident microbes possess another eight million or so genes, many of which collaborate behind the scenes handling food, tinkering with the immune system, turning human genes on and off, and otherwise helping us function. John Donne said “no man is an island,” and Jefferson Airplane said “He’s a peninsula,” but it now looks like he’s actually a metropolis.
Read Body Eclectic part 2


Opening the Black Box of Human Health (Body Eclectic Part 2)

Relman (Photo: Lea Suzuki / San Francisco Chronicle / Corbis)
The modern microbiome era started in the late 1990s, when David Relman, an infectious disease physician at Stanford University, decided to get a sample of the microbes in his own mouth. It’s a simple process: A dentist scrapes a sort of elongated Q-tip across the outer surface of a tooth, or the gums, or the inside of a cheek. These samples typically look like nothing at all. (“You have to have a lot of faith in the invisible,” one dentistry professor advises.)
Back then, such samples normally went to a laboratory to be grown in a petri dish for analysis, a good way to study those microbes that happen to be at home in a petri dish. Relman had the bold idea of adding DNA sequencing as a way of seeing every living thing. In the years since, the cost of sequencing has plunged and taking swab samples from various neighborhoods of the body for DNA analysis has become the standard practice of microbiome research.
In the laboratory, each Q-tip sample ends up in one of 96 little wells on a plastic collection plate smaller than a paperback book. A technologist then puts the plate on a sort of paint shaker, with a pebble and some detergent in each well to break open the cell walls, the first step in extracting the DNA. The resulting liquid gets drawn up by a pipetter—imagine a device with eight tiny turkey basters in a row—and transferred to wells in a series of eight more collection plates, each step taking the sample closer to pure DNA. The finished product then goes to the sequencer, a countertop device that looks about as impressive as an automated teller machine married to a bar refrigerator. But what it tells us about our own bodies is astonishing.
It’s not just that there are more than 1,000 possible microbial species in your mouth. The census, as it currently stands, also counts 150 behind your ear, 440 on the insides of your forearm and any of several thousand in your intestines. In fact, microbes inhabit almost every corner of the body, from belly button to birth canal, all told more than 10,000 species. Looked at in terms of the microbes they host, your mouth and your gut are more different than a hot spring and an ice cap, according to Rob Knight, a microbial ecologist at the University of Colorado. Even your left and right hands may have only 17 percent of their bacterial species in common, according to a 2010 study.
But the real news is that the microbial community makes a significant difference in how we live and even how we think and feel. Recent studies have linked changes in the microbiome to some of the most pressing medical problems of our time, including obesity, allergies, diabetes, bowel disorders and even psychiatric problems like autism, schizophrenia and depression. Just within the past year, for instance, researchers have found that:
•Infants exposed to antibiotics in the first six months of life are 22 percent more likely to be overweight as toddlers than unexposed infants, perhaps because antibiotics knock down essential microbes.
•A lack of normal gut microbes early in life disturbs the central nervous system in rodents, and may permanently alter serotonin levels in the adult brain. Scientists suspect that the same could hold for humans.
•Just giving enough food to starving children may not permanently fix their malnutrition unless they also have the “right” digestive micro-organisms, according to a study of kids in Malawi.
Researchers generally can’t say for sure if changes in the microbiome cause certain conditions, or merely occur as a consequence of those conditions. Even so, the intriguing correlations have stirred up intense scientific interest, particularly with the publication last June of the first results from the Human Microbiome Project, a $173 million effort by the National Institutes of Health. The aim of that project was to establish a normal profile of microbial life in 300 healthy individuals. For the medical community, it was like discovering a new organ within the human body—or more than that, a whole new operating system. Suddenly doctors had “another lever,” as an article in the American Journal of Epidemiology put it this January, “to pry open the proverbial black box” of human health and sickness.
Read the Body Eclectic Part 3


Foodie Gift Giving: A Stool Sample? (Body Eclectic Part 3)
How Our Microbes Keep Us HealthyThe public has also embraced the microbiome, beginning a few years ago when researchers at Washington University noticed a curious fact about obesity: Fat mice have more of a bacterial group called Firmicutes in their guts and thin mice have more Bacteroidetes. Feed the mice the same diet, and the ones with more Firmicutes extract more calories and lay on more fat. When the same differences showed up in humans, it seemed to explain the common complaint of many overweight people that they get fat just smelling food their skinny friends gorge on with impunity.
Such studies have stirred up remarkable enthusiasm in a subject matter most people would once have dismissed as yucky, gross or worse. It’s as if people suddenly loved Gulliver’s Travels for the passage where Jonathan Swift depicts a scientifically inclined student trying to return human excrement to the foods from which it originated.
This past winter, two rival efforts invited microbiome enthusiasts to submit their own fecal, oral, genital or skin samples for microbial analysis, and each raised more than $300,000 from crowd-funded donations typically under $100 apiece. The first effort, managed by Rob Knight’s Colorado lab and called American Gut, emphasized participation by top scientists in the field. Prevention magazine ranked the project’s $99 “map of your very own gut bacteria ecosystem” among its top 10 foodie gifts for the holidays. (For romantics, the $189 “Microbes for Two” package included analysis of a stool sample for both you and your partner. Or your dog.)
Microbiome excitement has spread to venture capitalists, who have so far invested in at least four start-ups with the aim of developing new microbiome-focused drugs and diagnostic tools. At Second Genome outside of San Francisco (motto: “The most important genome in your body may not be your own”), chief executive Peter DiLaura has nearly $10 million in seed money and a plan to get to clinical testing within three years for drugs targeted at common conditions like ulcerative colitis, where the microbiome probably plays a causative role.
That timetable may seem optimistic, especially given that research on the first genome—that is, the human genome—has barely begun to produce the abundance of new therapies originally predicted. But at least in theory it ought to be easier to manipulate individual microbes. According to researchers in the field, several major drug companies working on diabetes and obesity now have research units dedicated to the microbiome. The big toothpaste and mouthwash companies are also investigating microbial methods to prevent tooth decay.
Even before such products come to market, merely being able to characterize a person’s microbiome may yield direct medical benefits. Research suggests that each of us has a distinct microbial fingerprint, with individual variation based on diet, family, medical history, ethnic or regional background, and a host of other factors. These differences seem to matter in ways both large and small. For instance, a person may have certain gut bacteria that alter the effect of a drug—even blocking a remedy as common as acetaminophen, the pain-relieving ingredient in Tylenol. At present, doctors sometimes fumble from one prescription to the next before finally hitting on the drug that helps a given patient. The ability to consult that patient’s microbiome profile could make it easier to get there on the first try.
Even so, some researchers worry that the microbiome movement may be promising too much too soon.
Read the Body Eclectic Part 4


Overselling the Microbiome (Body Eclectic Part 4)
When a scientific team recently suggested that changes in gut bacteria could protect against stroke, Jonathan Eisen of the University of California at Davis lambasted them for “absurd, dangerous, self-serving claims that completely confuse the issue of correlation versus causation.” Eisen, a specialist in microbial genomics, now regularly presents “overselling the microbiome” awards on his blog. He says he doesn’t doubt the ultimate importance of the microbiome: “I believe the community of microbes that live in and on us is going to be shown to have major influences.” But believing that “is different from actually showing it, and showing it doesn’t mean that we have any idea what to do to treat it. There is danger here.”
For instance, probiotics, dietary supplements containing live bacteria, are generally harmless. Most contain the same microbes people have been consuming more or less forever. But exaggerated reports about beneficial microbes may lead people to regard the supplements as a cure-all, warns Richard Sharp, a bioethicist at the Cleveland Clinic. Manufacturers are careful not to claim specific health benefits because that would force them to undertake the kind of safety and effectiveness tests required for drugs. “But if somebody says they have a cure for everything,” says Rob Knight, “it’s probably a cure for nothing.” Still, U.S. probiotic sales were up 22 percent last year.
Researchers say they are only beginning to realize how subtle the interactions among our microbial species can be. They hope ultimately to develop probiotics that are correspondingly precise. But in the meantime, if the microbiome is like a symphony, then adding in current probiotics may be the equivalent of performing the piano solo with your elbows.
In certain rare circumstances, hitting the wrong notes may prove deadly. Administering probiotics before treatment seemed to make sense to the physicians in one study of severe acute pancreatitis, a bacterial inflammation of the pancreas. The theory, says the lead author, a Dutch gastroenterologist named Marc Besselink, was that a dose of beneficial microbes might crowd out dangerous microbes. That kind of “competitive exclusion” has worked well in some other conditions. But the pancreatitis patients receiving probiotics died more than twice as often as those who did not. The deaths occurred only in the most severe cases, where organ failure was already underway, and there was nothing to raise concern about the way most people use probiotics. But it was a wake-up call: The microbiome is a complicated system and we are only beginning to understand what happens when we tinker with it.


Killing Off Our Microbial Support System (Body Eclectic Part 5)
Blindly tinkering with the microbiome is, however, exactly what some researchers say we have been doing, willy-nilly, for more than 70 years, since the dawn of the antibiotic era. For Martin Blaser, a physician at New York University’s School of Medicine, one trend stands out: The typical child in the developed world now receives 10 to 20 courses of antibiotic treatment by the age of 18, often for conditions where these drugs do little or no good. “For two or three generations we’ve been under the illusion that there is no long-term cost to using antibiotics,” says Blaser, eyebrows rising over the tops of his wire-rimmed eyeglasses. It certainly hasn’t seemed like a cost for the child being treated, and only remotely for society at large (because excess use can lead to antibiotic resistance). But “you can’t have something this powerful,” says Blaser, “and change something as fundamental as our microbiome, at a critical time in development, and not have an effect.”
Though they have always known that antibiotics kill “good” bacteria as well as “bad,” doctors generally assumed the body’s microbial community was resilient enough to bounce back. But new studies show that the microbiome struggles to recover from repeated assaults, and may lose species permanently. Blaser suspects that diversity loss is cumulative, worsening from one generation to the next. He calls it “the disappearing microbiota hypothesis.” It’s like somebody played the piano solo with a two-by-four.
Along with the antibiotics, Blaser blames our obsession with cleanliness and antibacterial soaps and lotions. In addition, about 30 percent of American children are now born by Caesarean section. They start life without the microbiome they would normally have picked up passing through the mother’s birth canal, and some research suggests that this puts them at a disadvantage. Studies show that a diverse microbial community is essential to jump-start a baby’s immune system, establish a healthy digestive tract and even help shape the growing brain. Blaser doesn’t think it’s a coincidence that children now face an epidemic of medical disorders in all these areas, and that the surge in incidence tracks with an increase in Caesarean births and the introduction of powerful new antibiotics in the 1970s and ’80s.
“Here’s the point,” he says. “You have 10 or 12 diseases that are all going up dramatically, more or less in parallel—diabetes, obesity, asthma, food allergies, hay fever, eczema, celiac disease. They’re not going up 2 or 3 percent, they’re doubling and quadrupling. Each one may have a different cause. Or there could be one cause that’s providing the fuel, and my hypothesis is that it’s the disappearing microbiota.”
For Blaser, the decline of one “bad” bacterial species represents what’s happening to the entire microbiome. Helicobacter pylori, which lives in the human stomach, became notorious in the 1980s after scientists demonstrated that it is the essential precondition for almost all peptic ulcers and stomach cancers. The microbe was already on the decline from sanitary improvements and routine antibiotic use, but doctors then began directly targeting H. pylori in adults, incidentally meaning parents were less likely to pass the microbe on to their children. Today, while up to 100 percent of children in developing countries have Helicobacter, only about 6 percent of American kids do—and the latter is ostensibly a good thing.
“It’s good and it’s bad,” says Blaser. A study last year traced the human association with H. pylori back at least 116,000 years into our evolutionary history. “The idea that an organism that has been with us that long is disappearing in a century is striking,” says Blaser. “The good news is that it means less ulcers and less gastric cancer. The bad news is that it means more childhood-onset asthma and more esophageal reflux.” In certain circumstances, at certain times, Blaser argues, H. pylori may have protective effects not yet fully recognized.
The medical community has thus far resisted the rehabilitation of H. pylori. When Blaser first proposed that doctors would eventually find themselves reintroducing the species into American children, David Y. Graham, a gastroenterologist at the Baylor College of Medicine, replied in print, “The only good Helicobacter pylori is a dead Helicobacter pylori.” Of Blaser, he says, “He’s good at selling things.” Graham thinks Blaser is wrong to ascribe beneficial effects to H. pylori, and he worries Blaser’s message will dissuade people from seeking needed treatments.
Douglas Morgan, a Vanderbilt University gastroenterologist and epidemiologist, credits Blaser with pointing out the dual character of H. pylori. But the species may just look like the key player protecting against immune disorders because a simple medical test makes it the easiest to measure. Other microbes that rise and fall along with it could really drive the process, Morgan says.
Still, the attack on antibiotics doesn’t come casually. Blaser is a past president of the Infectious Diseases Society of America. Physicians who share his medical specialty depend utterly on antibiotics to treat patients suffering from pneumonia, heart valve infections and a deadly host of other disorders. But infectious disease specialists also see the cost being paid for their reliance on antibiotics, says Relman, a fellow microbiome researcher, physician and current president of the Infectious Diseases Society. These doctors have become dismayingly accustomed to saving patients’ lives, he says, only to see them go home and develop a crippling and sometimes fatal case of Clostridium difficile. “C. diff.,” as it’s known, is an intestinal infection with chronic diarrhea, and incidence in the United States has more than doubled since 2000. The problem almost always results from antibiotic use that has destroyed the normal population of microbes, clearing the way for just one, C. difficile, to dominate. So far, the only conventional remedy is another antibiotic.


“There Is No Yuck Factor for People Who Are This Sick” (Body Eclectic Conclusion)
In a procedure room at Rhode Island Hospital in Providence, a gastroenterologist named Colleen Kelly sprays a little air freshener, says “Breathe through your mouth” and then opens a plastic container of donor material, delivered fresh this morning by a relative of today’s patient. Kelly mixes it into a half liter of saline solution, then shakes it up like a bartender mixing a mai tai. She draws the liquid off into a half-dozen syringes the size of handheld bicycle pumps, and then it’s time to wheel in the patient.
The idea of fecal transplants is not new. Veterinarians have long used them to treat livestock with digestive troubles. Human cases in the United States, though rare, date back at least to the 1950s. But the procedure has become more common recently because it seems to cure C. diff. infection. Janet O’Leary, a medical imaging technologist in Massachusetts, went to Kelly for the procedure last October. “I told my boyfriend what I was going to do,” she recalls, “and he said, ‘I absolutely don’t believe it. You’re making this up.’”
Her personal physician felt almost as horrified. “It’s considered fringe, and this is how medicine in America works,” O’Leary says. “It’s not a drug. Nobody’s making money off it. Yet. It’s not being pushed by a dozen companies. It’s just a natural way to get the normal flora back in your gut. My response is that there is no ‘yuck factor’ for people who are this sick.”
O’Leary had come down with C. diff. after a vacation trip on which she used a powerful antibiotic for turista. Back home, her doctor prescribed another round of the same antibiotic, and the problem just got worse. A different antibiotic followed, and then repeated courses of a third antibiotic. It got so bad O’Leary couldn’t go to work at her hospital. She became a patient instead. “This wasn’t getting better. It was pretty scary, and the doctors were saying they might try another round of antibiotics, or I might lose part of my colon.”
Instead, O’Leary contacted Kelly, one of a few dozen gastroenterologists around the country now performing fecal transplants. The donor is usually a family member, says Kelly, and must be screened beforehand to ensure against introducing known pathogens. The procedure itself is a basic colonoscopy. But on the way back out, Kelly screws those bicycle-pump syringes into the instrument panel of her colonoscope and injects the contents at various points in the colon. The phrase is to “seed them through,” planting a healthy microbiome like a landscaper installing a new garden.
Of 94 C. diff. patients she has treated, Kelly says, all but three have overcome the infection. She’s now participating in a National Institutes of Health study to test the effectiveness of the procedure against a placebo in a double-blind clinical trial. She also foresees a time when a carefully designed probiotic manufactured in a laboratory will obviate the need for a human donor. One researcher has already begun testing an experimental version. It’s named RePOOPulate.
For the rest of us, the idea of fecal transplants, or of ulcer-causing bacteria as our sometime-friends, or of babies being anointed into humanity at birth by their mother’s microbiome, will no doubt continue to sound a little gross for a while to come. But here’s a way to put that in perspective: Vaccination also sounded gross when Edward Jenner figured out in the 1790s that inoculating people with pus from a cow could protect them from smallpox. And it was gross in 1928 when Alexander Fleming began the process of turning a moldy growth into penicillin. But vaccines and antibiotics would go on, in time, to become the most important discoveries in the history of medicine, and they now routinely protect billions of people from disease.
Coming to understand our microbes not as enemies, but as intimate partners could change our lives at least as dramatically, with time and proper testing. Asked recently about the prospects for microbiome research, one scientist not directly involved put it this way: “To make an analogy, we’re roughly a year after Fleming found penicillin.”


April 17, 2013
Into the Heart of the Outbreak (Guardians Against a Global Pandemic Part 1)

May 2, 2013: Flight 801 Riyadh, Saudi Arabia, to New York’s JFK.
Aboard: 368 souls and a virus that, over the next 10 years, will kill 20 million people. Are you prepared?
This is a story I wrote for the May issue of Men’s Health magazine:
Last September, a 49-year-old Qatari man who’d recently traveled to Saudi Arabia was hospitalized in Doha with a nasty respiratory illness. He deteriorated rapidly, and doctors promptly airlifted him to a London hospital, where he wound up on life support with kidney and lung failure. From respiratory tract samples, investigators soon teased out an unknown coronavirus. It turned out to be the same virus that had just killed an otherwise healthy 60-year-old in Saudi Arabia.
For one tense moment, epidemiologists thought they might be witnessing a replay of the devastating 2003 SARS epidemic, also brought on by a coronavirus. But the threat this time looked worse: Three million people were about to descend on Saudi Arabia for the hajj, a Muslim pilgrimage to Mecca already well known for the overnight global redistribution of illnesses via passenger jet.
Disease detectives of all specialties caught the next available flights into the heart of the potential outbreak. Epidemiologists tracked down anyone who had been even remotely associated with the victims. Veterinarians wearing protective gear went to a farm that one of the victims had visited and took samples from hundreds of domestic and wild animals to identify the species from which the virus had jumped to humans. This effort, unseen by the public but involving hundreds of experts around the world, soon established that the disease did not, in fact, spread easily from one person to another. The hajj wasn’t a hot zone after all.
It was a lucky break. As of early March, the new virus had sickened only 14 people and killed eight. But the episode was also a reminder that the supply of emerging diseases in the modern world is almost eye-bleedingly endless, and that they can turn up anywhere. One such pathogen, West Nile virus, killed 243 people in the United States last year. And a Denver hospital last summer experienced an alarming outbreak of a notorious New Delhi “superbug,” a bacteria with broad resistance to almost all antibiotics. Health officials will tell you that the Big One, a disease outbreak on the order of the influenza pandemic of 1918, could happen any day—and that sooner or later it almost certainly will.
They’ll also tell you that men in particular need to pay attention to the potential hazards: We travel more than women, particularly for business. Our trips tend to take us to more-remote destinations. So maybe it shouldn’t come as a surprise that we also have a much higher incidence of malaria, dengue fever, hepatitis, and Legionnaires’ disease (which last year killed 13 people in Quebec City, and three at a downtown Chicago Marriott hotel)—and perhaps other diseases yet unknown.


The Thing That Makes a Virus Hunter Nervous (Guardians: Part 2)

The CDC’s Marty Cetron
The good news? Science has become remarkably adept at identifying and containing potential outbreaks right at the start, even in the most remote locations, and often when only a handful of people—rather than hundreds—have become sick. In other words, they generally halt the outbreak before it can turn up on a 747 bound for New York City.
Some of the credit goes to rapidly advancing technologies, from Internet data mining to DNA fingerprinting. In the early 1980s, for instance, it took 3 devastating years to identify the virus that causes AIDS. With modern gene sequencing, says Columbia University virus hunter W. Ian Lipkin, M.D., it would take just 48 hours today. And part of the credit belongs to governments, which have learned painful lessons about the consequences of allowing a new disease to get out of hand: Since 1981, AIDS has killed more than 30 million people worldwide, with no end in sight.
But if we are lucky enough to see another year pass without some pandemic lurching up out of nowhere to kill vast swaths of humanity, it’s mainly because of the people who now constantly watch for early signs of trouble—as well as the ones who parachute in when things go wrong to save lives and stop epidemics. They tend to be unusual characters, people who can chat casually about “flavors” of Ebola and about the addictive thrill of their work on the front lines of possible outbreaks. But they also know firsthand what it takes to keep the world safe—and how to stay healthy themselves, even as people all around them die.
At CDC headquarters in Atlanta one day recently, as the coronavirus investigation was wrapping up, a daily map of trouble spots included an Ebola outbreak in the Democratic Republic of the Congo, Marburg fever in Uganda, cholera in Haiti, polio in Pakistan, and dengue fever in Portugal. Hantavirus, which is transmitted through urine, droppings, or saliva mainly from deer mice (and which also disproportionately affects men), had recently killed three vacationers at Yosemite National Park, and a case of Crimean-Congo hemorrhagic fever had just turned up in, of all places, Glasgow, Scotland.
It is a dangerous world out there, especially because of the kinds of travel we now consider normal. In his office in the division of global migration and quarantine at the CDC, director Martin Cetron, M.D., plays a computerized display tracking a single day’s passenger flights, streams of yellow lights gently flowing in from the farthest corners of the earth, coalescing in bright megalopolitan splotches of light, then radiating outward again. “This is what makes me nervous,” he says.
Nearly a billion people a year cross international borders, some of them inevitably carrying infections. Each international flight landing on U.S. runways also carries, on average, 1.6 live mosquitoes. In 1999, one theory holds, some of these jet-setting mosquitoes may have delivered West Nile encephalitis to New York. West Nile has since spread to 48 states and killed about 1,500 in the United States. As bad as that outbreak was, afflictions that are far more widespread may yet come if what Dr. Cetron calls the “invisible infrastructure” of disease prevention ever falters.
Simon Richardson, now 29, spent much of the past 6 years backpacking his way from Australia, through Southeast Asia and India, and around Africa, never experiencing anything worse than “the odd tummy bug.” He was a rafting guide in New Zealand, a trekking guide in Thailand, and a scuba instructor in Mozambique. Finally, he returned home to England and joined the British Army, ranking in the top 2 percent on the fitness test. Then the pain hit, in the left side of his chest.
“I thought I pulled a muscle, so I stopped lifting weights for a few weeks. Then I thought it was flu. But it just kept getting worse and worse.” He went from being able to run a mile in under 5 minutes to a point where he couldn’t run a mile at all. In the hospital, doctors took a sample of lung tissue with an endoscopic tube and gave him a diagnosis of tuberculosis that “was like getting punched in the stomach.” His friends just gave Richardson a blank stare when he told them. Most remembered tuberculosis only from old movies where pale victims coughed up blood and then died.





