Carl Zimmer's Blog, page 19
November 22, 2013
November 21, 2013
Old Age In the Embryo: My New Matter Column for the New York Times
One of the hallmarks of aging is a process called senescence. Cells stop dividing and release a distinctive blend of chemicals that cause inflammation and other effects. It’s thus a big surprise that scientists have now found senescent cells in embryos. For my new column for the New York Times, I take a look at this remarkable similarity between old and new–and how it changes our understanding of how we developed from an egg. Check it out.
November 15, 2013
A Long Way Left Up Darwin’s Mountain
One of the things I like about a long-running blog is that I can revisit long-running stories whenever I feel like it. And one of the longest of those stories has been unfolding in a lab at Michigan State University since 1988. That year, a biologist named Richard Lenski began rearing Escherichia coli from a single microbe. The bacteria, which he raised in a dozen separate flasks, all faced the same challenge: endure a starvation diet that their lab-pampered ancestors had not suffered.

The twelve flasks that contain the bacteria in Lenski’s long-term experiment. Photo by Michael Wiser
Every few hours, the bacteria reproduced. Each morning, the scientists took a few drops from each flask and moved these colonists to a fresh flask. Mutations arose, which the descendants inherited. Some helped the bacteria grow faster than their cousins, and natural selection spread them across the population.
It’s been 25 years–and 50,000 generations–since Lenski started the experiment, and it just won’t quit. Here are a few of the pieces I’ve written about it over the years:
–In 2007, I wrote about Lenski and the field of experimental evolution for the New York Times.
–In 2008, I dedicated a chapter to Lenski’s work in my book, Microcosm, which is a biography of E. coli. If Google will cooperate, I’m going to embed the chapter here. The blog post continues below it…
–In 2009, I wrote a magazine feature for BBC Knowledge
–One of Lenski’s lines of bacteria even went off in an unexpected direction, evolving the ability to feed on citrate in the presence of oxygen. This might represent the birth of a new species. I blogged about that research here and here.
Yet there is still more to learn from these bugs. In this week’s Science, Lenski and two members of his lab–Michael J. Wiser and Noah Ribeck–took a close look at the evolution of the bacteria over the course of the entire experiment.
One of the great strengths of this experiment is that Lenski is an obsessive hoarder. He and his team freeze bacteria every 500 generations, filling freezer after freezer with them. They can then thaw out a few of the bacteria and put them in a dish with the latest generation and see how fast they each grow under identical conditions. Think of it as microbial Hunger Games.
These contests allow the scientists to precisely gauge what’s known as relative fitness–a measurement of natural selection. In the early years of the experiment, the fitness of the bacteria rose very fast. Within 5,000 generations, all twelve lines were growing 50% faster than the original microbe Lenski started the experiment with. Then they slowed down. By 20,000 generations, they were 75 percent faster.
These results lead to an obvious question: were the bacteria coming to the end of their increase in fitness? You can think of evolution in these cases like a mountain, with the elevation of any spot on the mountain as the average fitness of a population. Perhaps the mountain the bacteria in Lenski’s lab were climbing had steep slopes at low elevations. But now they were getting to the gentle climb just before reaching the mountain’s peak.
So the scientists thawed out some bacteria and pitted them against each other. Even at 50,000 generations, it turns out, the bacteria are still getting faster. Between generations 40,000 and 50,000, their fitness increased by 3 percent.
The scientists then competed the newest bacteria against ancestors from 41 generations across the past 25 years. They plotted the data on a graph and then looked for the curve that fit the dots best. The data do not indicate the mountain is going to flatten out. Instead, the best curve is generated by a mathematical relationship called a power law. In this model, the bacteria will improve by smaller steps in the future, but they will never stop improving. The power law is so powerful that the scientists can plug data into it only up to generation 20,000 and then accurately predict the next 30,000 generations.
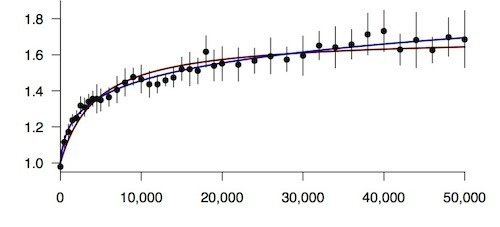
Fitness of E. coli over 50,000 generations. (Ancestor=1) Blue curve from a power law; red curve asymptotic. Wiser et al Science 2013
To explain this result, the scientists turned to some of the previous experiments that researchers have carried out on Lenski’s bugs. Over the years, they’ve looked closely at the DNA of some of the bacteria, in order to pinpoint new mutations and track them over the generations. By 20,000 generations, the bacteria had already acquired several dozen beneficial mutations. If the scientists engineered the ancestral bacteria with each of those mutations, they grew faster.
These adaptations arose thanks to the fact that mutations spontaneously occur at random in the bacteria. Most of the mutations are harmful, either killing the bacteria outright or slowing down their grow so that their descendants eventually disappear from their flask. But a few are beneficial.
Of all the possible beneficial mutations, most of them provide a small benefit, while a few provide big ones. After a few thousand generations, most of the big mutations probably occurred, leaving only small ones to continue improving the bacteria. And each time a new mutation arises, it has to interact with all the mutations that came before it. Some of those interactions may actually be harmful to the bacteria, reducing the overall benefit. The more time passes, the more mutations there are that may impose that cost.
Yet the experiment also shows that the bacteria still have the capacity to grow even faster. There’s a creativity in the genome that is inexhaustible–despite the fact that just about every spot in the microbe’s DNA has mutated by now. Again, the interaction between mutations gets the credit. In some cases, mutations can’t be beneficial until a series of other beneficial mutations have first evolved.
The creativity of evolution means that this experiment will keep yielding results for a long time. It’s possible that eventually the bacteria will hit some ceiling imposed by physics. There may be an upper limit to how fast DNA can be copied, for example, or how thick cell walls can get.
But no one knows when the bacteria will hit that wall. Lenski and his colleagues estimate that if they could run the experiment for another million years, the bacteria would keep speeding up. Today it takes one microbe 55 minutes to become two. In a million years (or 2.5 billion generations) it would take 23 minutes.
Lenski’s getting towards retirement, so he won’t be running a million-year experiment. Perhaps someone can create a robotic, solar-powered time capsule to take over. As long as the experiment can run, these bacteria will be ready to surprise.
PS: After 25 years, Lenski has evolved too–he has started to blog. Here’s his own post on the new paper.
November 14, 2013
Where Dogs Are From: My New Column for the New York Times
I suspect I will be writing about the science of dogs as long as I’m writing about science at all. These creatures, despite being so familiar, have so much left to reveal. For my new “Matter” column at The New York Times, I look at the latest research on the origin of dogs, based on new analysis of DNA from ancient dog (or wolf?) fossils. For now, there’s a battle over where dogs first called home. Check it out.
November 13, 2013
How Our Minds Went Viral
Did viruses help make us human? As weird as it sounds, the question is actually a reasonable one to ask. And now scientists have offered some evidence that the answer may be yes.
If you’re sick right now with the flu or a cold, the viruses infecting you are just passing through. They invade your cells and make new copies of themselves, which burst forth and infect other cells. Eventually your immune system will wipe them out, but there’s a fair chance some of them may escape and infect someone else.
But sometimes viruses can merge into our genomes. Some viruses, for example, hijack our cells by inserting its genes into our own DNA. If they happen to slip into the genome of an egg, they can potentially get a new lease on life. If the egg is fertilized and grows into an embryo, the new cells will also contain the virus’s DNA. And when that embryo becomes an adult, the virus has a chance to move into the next generation.
These so-called endogenous retroviruses are sometimes quite dangerous. Koalas, for example, are suffering from a devastating epidemic of them. The viruses are spreading both on their own from koala to koala and from parents to offspring. As the viruses invade new koala cells, they sometimes wreak havoc on their host’s DNA. If a virus inserts itself in the wrong place in a koala cell, it may disrupt its host’s genes. The infected cell may start to grow madly, and give rise to cancer.
If the koalas manage to survive this outbreak, chances are that the virus will become harmless. Their immune systems will stop their spread from one host to another, leaving only the viruses in their own genomes. Over the generations, mutations will erode their DNA. They will lose the ability to break out of their host cell. They will still make copies of their genes, but those copies will only get reinserted back into their host’s genome. But eventually they will lose even this feeble ability to replicate.
We know this is the likely future of the koala retroviruses, because we can see it in ourselves. Viruses invaded the genomes of our ancestors several times over the past 50 million years or so, and their viral signature is still visible in our DNA. In fact, we share many of the same stretches of virus DNA with apes and monkeys. Today we carry half a million of these viral fossils, which make up eight percent of the human genome. (Here are some posts I’ve written about endogenous retroviruses.)
Most of this viral DNA is just baggage that we hand down to the next generation. But sometimes mutations can transform viral DNA into something useful. Tens of millions of years ago, for example, our ancestors started using a virus protein to build the placenta.
But proteins aren’t the only potentially useful parts that we can harvest from our viruses.
Many human genes are accompanied by tiny stretches of DNA called enhancers. When certain proteins latch onto the enhancer for a gene, they start speeding up the productions of proteins from it. Viruses that infect us have enhancers, too. But instead of causing our cells to make more of our own proteins, these virus enhancers cause our cells to make more viruses.
But what happens when a virus’s enhancer becomes a permanent part of the human genome? Recently a team of scientists carried out a study to find out. They scanned the human genome for enhancers from the youngest endogenous retroviruses in our DNA. These viruses, called human-specific endogenous retroviruses, infected our ancestors at some point after they split off from chimpanzees some seven million years ago. We know this because these viruses are in the DNA of all living people, but missing from other primates.
Once the scientists had cataloged these virus enhancers, they wondered if any of them were now enhancing human genes, instead of the genes of viruses. If that were the case, these harnessed enhancers would need to be close to a human gene. The scientists found six such enhancers.
Of these six enhancers, however, only one showed signs of actually boosting the production of the nearby gene. Known as PRODH, it encodes an enzyme that’s involved in making signaling molecules in the brain. And if the enzyme isn’t working properly, the brain can go awry.
In 1999, scientists shut down the PRODH gene in mice and found a striking change in their behavior. They ran an experiment in which they played a loud noise to the mice at random times. Then they started playing a soft tone just before the noise. Normal mice learn to connect the two sounds, and they become less startled by the loud noise. But mice without PRODH remained as startled as ever.
Other researchers have also found evidence for the importance of PRODH in the human brain. In some studies, mutations to the gene have been linked to schizophrenia, for example. (One study has failed to find that link, though.) A mutation that deletes the PRODH gene and its surrounding DNA has been linked to a rare psychiatric disorder, called DiGeorge syndrome.
Once the scientists had found the virus enhancer near PRODH, they took a closer look at how they work in human cells. As they report in the Proceedings of the National Academy of Sciences this week, they searched for the activity of PRODH in tissue from human autopsies. PRODH is most active in the brain–and most active in a few brain regions in particular, such as the hippocampus, which organizes our memories.
The new research suggests that the virus enhancer is partly responsible for PRODH becoming active where it does. Most virus enhancers in our genome are muzzled with molecular caps on our DNA. That’s probably a defense to keep our cells from making proteins willy-nilly. But the hippocampus and other regions of the brain where PRODH levels are highest, the enhancer is uncapped. It may be left free to boost the PRODH gene in just a few places in the brain.
The scientists also found one protein that latches onto the virus enhancer, driving the production of PRODH proteins. And in a striking coincidence, that protein, called SOX2, is also produced at high levels in the hippocampus.
What makes all this research all the more provocative is that this situation appears to be unique to our own species. Chimpanzees have the PRODH gene, but they lack the virus enhancer. They produce PRODH at low levels in the brain, without the intense production in the hippocampus.
Based on this research, the scientists propose a scenario. Our ancestors millions of years ago were infected with a virus. Eventually it became lodged in our genome. At some point, a mutation moved the virus enhancer next to the PRODH gene. Further mutations allowed it to helped boost the gene’s activity in certain areas of the brain, such as the hippocampus.
The scientists can’t say how this change altered the human brain, but given what we know about brain disorders linked to the PRODH gene, it could have been important.
It’s always important approach studies on our inner viruses with some skepticism. Making a compelling case that a short stretch of DNA has an important function takes not just one experiment, but a whole series of them. And even if this enhancer does prove to have been one important step in the evolution of the human brain, our brains are also the result of many other mutations of a far more conventional sort.
Still, the intriguing possibility remains. Perhaps our minds are partly the way they are today thanks to an infection our ancestors got a few millions of years ago.
[For more on the mighty influence of these tiny life forms, see my book A Planet of Viruses.]
November 7, 2013
The Microbiome and the Future of Medicine
On Wednesday I was in New York to participate in the Wired Health Conference. I interviewed Martin Blaser of NYU on the microbiome and what it means for the future of medicine. We managed to cover a lot of ground, which you can watch in the video, which I’ve embedded below.
You and Your Microbiome from WIRED on FORA.tv
November 4, 2013
Our Speckled Brains
It’s not exactly true to say that each of us has our own genome. We have genomes. Some of us, known as chimeras, have genomes from more than one person. The cells of children linger behind in their mothers; in the womb, cells from twins can intermingle. The rest of us non-chimeras can trace our genomes to one origin–the fertilized egg from which we developed. But as the cells in our bodies divided, they sometimes mutated, creating a panoply of genetic variation known as mosaicism.
I wrote about chimeras and mosaics in September in the New York Times. My article was a status report of sorts. Scientists have known about our many genomes for decades. But with the advent of single-cell genome sequencing, they’re now learning some surprising things about our genetic multitudes. As a status report, my story was far from the final word. And now, just a couple months later, a new study has come out that sheds more light on a place where our mosaic nature can have huge consequences: our brains.
For a long time, scientists who study mosaicism have focused their attention on its dark side. In the 1960s, for example, scientists recognized that cancer cells were the result of our mosaic nature. Mutations arose in a line of cells, and eventually those mutations drove the cells to grow quickly and develop into tumors. And since then mosaicism research has continued to revolve around diseases. A number of rare diseases such hemimegalencephaly–in which one side of the brain is bigger than the other–have been traced to mutations that arise in developing cells.
This is important research, but it risks providing a lopsided view of our mosaic nature. We are left to wonder how many genomes a healthy person can have. Scientists have started to shift their attention from disease to health, and they’re finding that we can have a surprisingly large amount of variation with no apparent ill effect. In the latest issue of Science, Fred Gage of Salk Institute for Biological Studies and his colleagues provide a deep look into the mosaic nature of healthy brains.
First, they watched the brain’s mosaic emerge. They grew three colonies of human stem cells, rearing each of them in a broth of nutrients. Mixed into that broth where chemicals that coaxed the stem cells to develop into neurons. The scientists then plucked out 40 of these neurons and analyzed their genomes. Thirteen of the 40 cells had changed markedly from their ancestors. Some had accidentally gained an extra copy of a chromosome, while others had copies of smaller chunks of DNA. In other neurons, chunks of DNA had been chopped out. The changes were never the same, which meant that they had originated separately.
The scientists then turned their attention to real brains. They took tissue samples from three healthy people who had died in their twenties in accidents. From those samples, the scientists isolated 110 neurons and surveyed their genomes. In those neurons, they found a similar pattern to the one they saw in their dishes of stem cells. Forty-five out of the 110 neurons had either extra copies of DNA or missing segments. Again, none of the neurons shared the same mutations. That finding means it’s unlikely that the neurons share mutations that arose in a single neuron early in development. Instead, new mutations kept emerging as the brains matured and neurons divided.
Far from being a rare, dangerous fluke, in other words, mosaic neurons turn out to be abundant in our brains. The figure at the bottom of this post shows how this new study expands our understanding of how we become mental mosaics.
With so much mutating going on in our brains, it may be hard to believe that our brains can work at all. In a commentary accompanying the paper, Evan Macosko and Steven McCarroll of Harvard sketch out some defenses our brains may have against this genomic messiness. For one thing, mutations tend to emerge in the parts of the genome that a cell uses least. So many of the mutations that Gage and his colleagues have discovered may affect genes that don’t matter in the brain anyway.
Even if a mosaic neuron does turn out to be defective, the brain may have ways to prevent it from causing much trouble. When the brain develops, it starts by producing an abundance of connections between its neurons. Only later does it then prune many of those connections back. The brain may take its pruning shears to defective mosaic neurons with particular vigor, cutting them off from conversations with other cells.
It’s even possible that those misfit neurons can let our brains perform in new ways, Macosko and McCarroll suggest. The brain may not just tolerate diversity. It may depend on it.
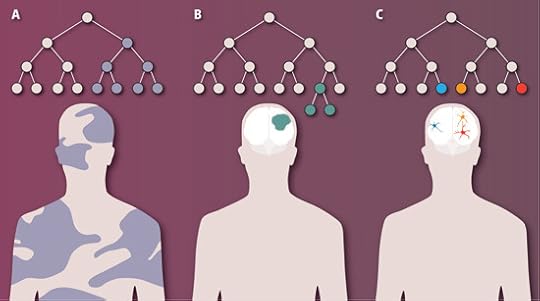
A: If a cell mutates very early in development, its descendants will be found across much of the body. B: A mutation that arises later in the brain and causes cells to proliferate may be easily detected. C: A subtler mosaic forms when neurons experience unique, late-developing mutations. From Macosko & McCarroll, Science 2013
October 31, 2013
Happy Halloween from Nature’s Zombies
In case snakes weren’t enough of a fright-fest for you this Halloween, here’s a video that I narrated for National Geographic about one of nature’s spookier creatures. (One of many, as I explain in Parasite Rex.)
Snakes on the Brain: My New Column for the New York Times
Snakes inhabit our fears and stories. Why do they have such a hold on us? For my New York Times column this week, I take a look at a provocative theory that snakes have shaped our evolution since our primate ancestors first clambered through the trees. This week, a new study added a neurological twist to this idea, as scientists offered evidence that some of our neurons may be exquisitely sensitive to snakes. Check it out.
(And just to stress that our relationship with snakes isn’t all about fear, here’s one of my favorite images from my book Science Ink)
October 28, 2013
The Code of Life: My New Feature for Nautilus Magazine
One of the strangest episodes in the history of biology occurred in 1953. A physicist named George Gamow, who is best known for his work on the Big Bang, sat down and read a new paper by two biologists named James Watson and Francis Crick. They reported that DNA is arranged as a double helix. Gamow then wondered how DNA encodes proteins. DNA used four “letters” in its genes, while proteins are built from a chemical alphabet of twenty amino acids. He realized that the question turned life into a problem in cryptography. Gamow came up with an explanation for life’s code that turned out to be wildly wrong. But it prompted other researchers to crack the code.
In the new issue of Nautilus (a new magazine about science and philosophy), I’ve written a feature about the history and future of the genetic code. There’s still a lot we don’t understand very well about the genetic code–for example, why all living things use the same one. But at the same time, scientists are learning how to alter the code, to generate forms of life fundamentally different from anything that came before. Check it out.




