Carl Zimmer's Blog, page 23
August 14, 2013
The Renewed Hope for Virus-Repaired Genes: My New Story for Wired
Since the mid-1900s, medical researchers have dreamed of fixing genetic disorders by supplying people with working versions of genes. By the late 1990s, that dream–known as gene therapy–seemed very, very close. Scientists were developing engineered viruses that would infect patients with DNA that would allow their bodies to make the proteins they needed to survive.
But then, in 1999, a young man who had volunteered for a trial died. The whole field of gene therapy went into a tailspin. Only in recent years has it recovered.
I’ve written a story for Wired about that turnaround, focusing on the career of the scientist who oversaw that fateful 1999 trial, James Wilson. For the past fourteen years Wilson been hunting for better viruses for gene therapy, and his viruses are now involved in some of the most promising research for treating diseases ranging from hemophilia to blindness. To find out more about Wilson and gene therapy, check out “The Fall and Rise of Gene Therapy.”
August 8, 2013
A Most Interesting Bone
Let me just say at the outset that this post is about the baculum. Some of you may not want to read about the bone found in the penises of many mammal species. I want to give you a chance to head off for tamer blogs. But you might want to stick around. There’s some real science below–and some evolution in action.
***
Last week in the New York Times I wrote about the evolution of monogamy (here and here). The occasion for the articles were two new studies in which scientists analyzed hundreds or thousands of species of mammals, tracing the evolution of monogamy and other social arrangements. This big-picture approach to evolution can yield some important insights, but the finer details are hard to make out.
If you look at us humans (monogamy, polygamy, and assorted other stuff) versus chimpanzees (monogamy is for losers!), you’re only looking at the tips of two deep branches. Chimpanzees may be our closest living relatives, but our common ancestor with them lived about seven million years ago. After the two lineages split from that ancestor, they’ve been evolving in different directions ever since. We can make some inferences about what that evolution was like based on ourselves, chimpanzees, and other living mammal species. But this kind of research doesn’t give us a visceral sense of how the sexual habits of mammals evolve from generation to generation.
That’s why it was so interesting to come across a new study from Leigh W. Simmons and Renée Firman at the University of Western Australia. They’ve been able to observe sexual evolution of mammals unfold in their laboratory. Last week’s studies were like a satellite view of the continents. Here, we’re down on the ground.
How males and females live with one another depends on the conditions in which their species lives. If a single male can mate with lots of females, for example, he will end up with a lot of offspring. But if the females are spread out too far, he may not be able to guard them all from other males who want to mate with them too. In such cases, natural selection may favor males that prefer to stick with just one female.
A seed beetle phallus. Photo by Johanna Ronn
In species where males compete with each other a lot for females, evolution may produce new pieces of anatomy. Some males evolve extravagant horns to fight off rivals. Even their genital anatomy can change. This is likely to happen when females mate with many males. The males fight against each other even during sex. Some male insects, for example, using spiny genitals to scrub out their competitors’ sperm.
Evolutionary biologists hypothesized that these extravagant sexual organs were the result of an evolutionary race between males. They found support for this idea when they compared individual males to each other. It turned out that the males that had the most offspring tended to have the spiniest penises.
More recently, researchers have started to watch these organs evolve. In 2011, for example, Swiss scientists reported a study they carried out on seed beetles, which have spines on their genitals for scrubbing away rival sperm.
The Swiss scientists isolated each male with just one female. In that arrangement, the males had no competition for mates. The researchers then let the beetles mate for 21 generations. The spines on the male’s genitals got measurably smaller. That’s just what scientists had predicted based on evolutionary theory. Without any competition from other males, there was no advantage to spiny penises.
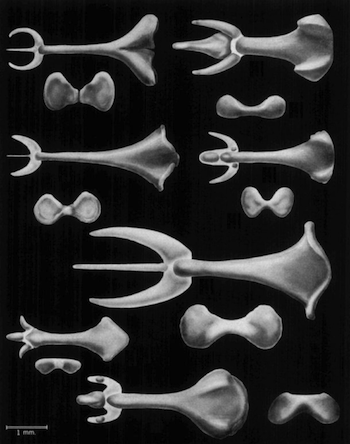
The bacula of rice rats and voles. From Stockley 2012 http://dx.doi.org/10.1016/j.cub.2012....
Simmons has documented a lot of the evidence for this evolution of male genitals in insects, and now he and Firman have turned their attention to mammals. To be more precise, they’ve turned their attention to the baculum–a bone that they call “one of the most puzzling enigmas of mammal morphology.”
The baculum is long in some species and stubby in others; it can be straight or hooked, barbed or shaped like Neptune’s trident. In a few species, like our own, it’s just missing altogether.
Scientists have developed several possible explanations for its existence. Some have suggested that by making the penis rigid, the baculum lets a male deliver more sperm into a female. Those extra sperm may outnumber those of rival males. Others have suggested that the baculum helps the sperm travel further towards an egg. Still others have proposed that it stimulates the female, triggering ovulation.
All three hypotheses have something in common: the baculum evolves thank to its ability to translate mating into fathering. In June, some British researchers published a study that supported that idea. They studied house mice, a species in which females mate with many males each time they’re ovulating. The scientists found that male mice with a wider baculum had more mouse pups than other males.
Simmons and Firman took this research to the next logical step. They reasoned that this difference between male mice should drive the evolution of the baculum. To find out, they ran an experiment similar to the one run by the Swiss scientists. They created two groups of mice: promiscuous maters and monogamous maters.
The promiscuous females got to mate with three males in each cycle. The monogamous ones only got to mate with one. They bred the mice for 27 generations and then took a look at their bacula. As with the seed beetles, the baculum evolved. It became thicker in the promiscuous group and thinner in the monogamous one. For the first time, scientists had observed the baculum evolving.
The experiment still doesn’t solve the mystery of what the baculum for, but Simmons and Firman do have an idea about that–at least for mice. They think that the baculum helps male mice stimulate the female reproductive tract. That stimulation may make it more likely that the male fertilizes the female’s eggs, or raises the odds that a fertilized egg successfully implants itself in her uterus.
If the baculum is indeed driven by sexual selection in mammals, the question naturally arises: where’s ours? “Why human males lack a baculum remains enigmatic,” Paula Stackley, a biologist at the University of Liverpool, wrote last December in Current Biology.
Among primates, monogamous species tend to have much smaller bacula than species where males compete for mating. So it wouldn’t be crazy to assume that the shift towards monogamy in our ancestors made the human baculum disappear altogether (except for a very, very few scary cases).
Things aren’s so simple as all that, however. Chimpanzees, our closest relatives, are far from monogamous. You’d think they had a huge baculum, but it’s only about the size of a grain of rice–about five times smaller than a baboon’s baculum. In fact, all the great apes have tiny bacula. For some mysterious reason, this mysterious bone has been vanishing in our ancestors for some ten million years or more. While the bacula can evolve in a matter of weeks in a scientific experiment, its evolution can stretch out across deep time, as well.
August 7, 2013
Henrietta Lacks, Genomes, And The Collaborations That Make Science Work
In the New York Times, I report on a pretty remarkable pair of events that have just taken place in the world of genome science–and both having to do with Henrietta Lacks. Cells taken from Lacks’s body in 1951–now known as HeLa cells–have revolutionized cell biology. But neither she nor her family had any say about their use. This woeful situation came to a head recently when it turned out that scientists had sequenced the genome of HeLa cells without any contact with the family. Now the NIH and the Lacks family have worked out an arrangement for controlling access to the genetic data, and today scientists unveiled a high-quality sequence of the genome full of interesting insights into cell biology. But, as I explain in my article, the ethics of genome sequencing are far from settled. Check it out.
August 6, 2013
De-Extinctions and Straw Men
In my feature on de-extinction in the April issue of National Geographic, I tried to capture the debate in the scientific community about whether we should try to bring vanished species back to Earth. It’s been gratifying to see a spirited, sustained conversation going on ever since. The prospect of de-extinction raises important issues that have to be grappled with. Is it better to spend money trying to revive a mammoth or to secure a vast swath of rain forest? Are objections to de-extinction driven by a flawed notion of what’s natural? Would it make more sense to use the emerging tools of biotechnology to prevent endangered species from disappearing, rather than attempting to bring back the extinct ones?
But I’m frustrated by a column by George Monbiot that just appeared in the Guardian, entitled, “Resurrecting woolly mammoths is exciting but it’s a fantasy.” Monbiot singles out National Geographic for scoffing, declaring,
the double-page painting published by National Geographic in April, depicting tourists in safari vehicles photographing a herd of Siberian woolly mammoths roaming the Siberian steppes, is pure fantasy: the animals it shows are mumbo-jumbos.
(We Yanks use mumbo-jumbo to refer to gibberish, but after reading Monbiot’s piece, I did some dictionary-ing and discovered that the Brits use it to refer to a meaningless idol.)
It’s not Monbiot’s position that bothers me. In my article, I wrote about harsh critics of de-extinction as well as advocates. It’s the way he frames his argument at the outset:
There is an obvious, fatal but widely overlooked problem with de-extinction.
Wow! Both obvious and fatal. Not just obvious and fatal, but also widely overlooked! What could this problem be, a problem that conservation biologists and molecular biologists who are exploring de-extinction have somehow failed to notice, a problem that Monbiot is here–at last–to unveil?
This:
The scarcely credible task of resurrection has to be conducted not once but hundreds of times, in each case using material from a different, implausibly well-preserved specimen of the extinct beast. Otherwise the resulting population will not be genetically viable.
Really? That’s it?
I felt a distinct lack of surprise at Monbiot’s big reveal. That’s because I had addressed this very issue in my own article four months ago, noting that reviving a single animal is not the same as bringing back an entire species.
But I didn’t go so far as saying that this was a “fatal” problem, because I discussed the issue with the scientists I interviewed. You’d think from reading Monbiot’s column that these scientists hadn’t the faintest clue of this problem. I picture them sitting in front of their screens, reading Monbiot’s revelations, and smacking their foreheads all at once, roaring, “Of course! How stupid of us!”
You’d have to be a truly stupid scientist to not be aware that the long-term viability of a species depends on a genetically viable population. If a small population is only made up of nearly genetically identical individuals, they run the risk of inbreeding, which can make them unhealthy, vulnerable to diseases, and even infertile.
The scientists exploring de-extinction are aware of this challenge, and they have actually given this matter some thought. They have ideas about how to deal with it. There’s a good debate to be had over whether those ideas could really work in practice, but Monbiot shows no signs of being familiar with them.
Monbiot is arguing that de-extinction cannot work, period, because it would require discovering an intact cell for every individual animal or plant scientists wanted to produce. There are several reasons why this is wrong. For one thing, scientists already have the technology required to engineer diversity into a species.
Museums have hundreds of preserved passenger pigeons, for example, and those birds are not clones of one another. By sequencing the DNA from a number of passenger pigeons, scientists could learn about the genetic diversity of the species. Based on the experiments scientists are already doing on animal cells, it’s conceivable that researchers could synthesize gene variants and plug them into the genome of an extinct animal. By engineering the genomes of the pigeons, scientists would create a flock containing some of the genetic viability that existed before the species became extinct.
If scientists can produce a few dozen genetically diverse passenger pigeons–or gastric brooding frogs, or thylacines, for that matter–it’s an open question whether those creatures could seed a sustainable population. Monbiot seems to be down on the whole idea of restoring small populations. He points to European bison, which have gone from 54 animals to 3,000, but which still have trouble with inbreeding.
But there are more heartening stories, too. Northern elephant seals were hunted down to the same population level, and today their numbers are up to 160,000.
Now we’ve drifted off the original course, though. We are no longer talking about de-extinction, but about the broader question of captive breeding. There’s another good debate to be had about whether to save the black-footed ferret and the California condor. But I guess it’s not as fun as shouting mumbo-jumbo!
August 4, 2013
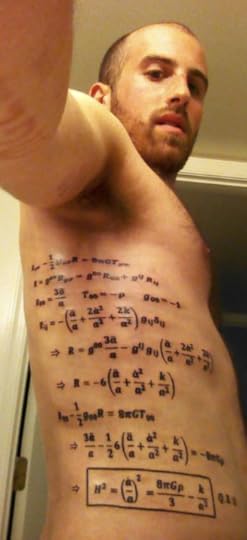
A Big Universe Deserves A Big Equation (Science Ink Sunday)
Adam Platz writes,
“The equation is called the Friedmann equation and, simply put, governs the expansion of space in a homogenous universe such as our own. In the 1920s Alexander Friedmann, a Russian astrophysicist, sought to unite Einstein’s recently conceived theory of general relativity with a general model for our universe’s behavior. The Freidmann equation resulted. From its basic form, one can derive the density of the universe at a given time, the pressure, the mass, the age of the universe, and finally the rate of expansion of the universe (found in a term known as the Hubble constant). In 2008 during my senior year at Dartmouth, my senior seminar in astrophysics focused in part on this equation. I always found the equation to be elegant and beautiful. My own little god equation. Explaining where we came from and what we are made of. That year I thought up the idea of the tattoo and decided that if in 5 years I was still interested in the ink, I would get it. And so I did in 2013.”
The Wikipedia page for the Friedmann equation is suitably intense. The American Institute of Physics has a more accessible history of Friedmann and other early twentieth-century cosmologists.
You can see the rest of the Science Tattoo Emporium here or in my book, Science Ink: Tattoos of the Science Obsessed.
August 2, 2013
Monogamy On the Brain: My New “Matter” Column for the New York Times
On Monday I reported in the New York Times on new research into the evolution of monogamy in mammals. For this week’s “Matter” column, I follow up with some thoughts on our somewhat monogamous species, and how a shift in mating patterns may have driven the evolution of our giant brains. Check it out.
Meet the Animats
Here is the story of how simple video-game creatures evolved a memory.
These simple creatures were devised by a group of scientists to study life’s complexity. There are lots of ways to define complexity, but the one that they were interested in exploring has to do with how organisms behave.
Every creature from a microbe to a mountain lion can respond to its surroundings. E. coli has sensors on its surface to detect certain molecules, and it processes those signals to make very simple decisions about how it will move. It travels in a straight line by spinning long twisted tails counterclockwise. If it switches to clockwise, the tails unravel and the microbe tumbles.
A worm with a few hundred neurons can take in a lot more information from its senses, and can respond with more behaviors. And we, with a hundred billion or so neurons in our brains have a wider range of responses to our world.
A group of scientists from Caltech, the University of Wisconsin, Michigan State University, and the Allen Brain Institute wanted to better understand how this complexity changes as life evolves. Does life get more complex as it adapts to its environment? Is more complex always better? Or–judging from the abundance of E. coli and its fellow microbes on the planet today–is complexity overrated?
There are two massive problems with trying to answer these questions. One is that it’s hard to run an experiment on living things to watch them evolve different levels complexity. The other is that it’s difficult to measure that complexity in a precise way. Simply counting the number of neurons in a brain isn’t good enough, for example. If a hundred billion neurons are joined together randomly, they won’t generate any useful behavior. How those neurons work together matters, too.
There are more precise ways to think about this complexity. William Bialek of Princeton has proposed that complexity is a measurement how much of the future an organism can predict from the past. Giulio Tononi of the University of Wisconsin has proposed the complexity is a measure of how many parts of a brain can separately process information, and how well they combine that information into a seamless whole. (I wrote more about Tononi’s Integrated Information Theory in the New York Times.)
Both Bialek and Tononi have laid out their theories in mathematical terms, so that you can use them to measure complexity in terms of bits. You can say the complexity of a system is precisely 10 bits. You don’t have to just throw up your hands and say, “It’s complicated.”
Unfortunately, there’s still a catch. As powerful as these theories may be, they only allow scientists to calculate the complexity of a brain (or any other information-processing system) if they can measure all the information in it. There are so many bits of information flooding through our brains, and in such an inaccessible way, that it’s pretty much impossible to actually calculate their complexity.
Chris Adami of Michigan State and his colleagues decided to overcome these hurdles–of observing evolution and measuring information precisely–by programming a swarm of artificial creatures which they dubbed animats.
To create animats, the scientists first had to create the world in which they would struggle to survive, reproduce, and evolve. The scientists put them in a maze made of a series of walls. To move forward, the animats had to crawl along each wall to find a doorway. If they passed through the doorway, they could move forward to the next wall, where they could search for a new door. The animats that traveled through the most walls were then able to reproduce.
Here’s a diagram of the animat’s anatomy:

Edlund et al 2011. doi:10.1371/journal.pcbi.1002236.g002
The red triangles, marked 0 through 2, are simple eyes. All they do is sense whether they are next to an obstacle or not. The pink triangle marked 3 is a sensor that senses whether it’s in a doorway or not. Sensors 4 and 5 are collision detectors that sense whether the animat has crashed into the upper or lower borders of the maze. The information that these senses register is as simple as can be: they’re either on or off.
That information–on or off–flows from the senses to the animat’s brain–the circles marked 6, 7, 8, and 9. Each sense may be linked to one circle, or two, or all of them. The links may be strong or weak. If an eye has a strong connection to one of the circles, it may flip every time the eye senses an obstacle. A weak connection may mean that it only flips a quarter of the time the eye sees something. The parts of the brain can be linked to each other, too, helping to switch each other on and off.
Finally, the animat has legs, the green trapezoids marked 10 and 11. The brain can send signals to the legs, as can the sensors. The legs can respond in one of four ways–move left, move right, move forward, or do nothing.
To launch their experiment, the scientists created 300 animats with randomly generated instructions for how each part of their body worked. They then dropped each animat into an identical maze and run their programs for 300 steps.
In those 300 steps, a lot of the animats just meandered up and down their first walls, making no progress. At the end of the run, the scientists grabbed the 30 animats that had gone the furthest and let them reproduce.
Each animat got to produce ten new offspring. Their offspring inherited the same code as their parent, but each position in the code had a small chance of mutating. These mutations could strengthen or weaken a link between an eye and a part of the brain, or could add an entirely new link. The parts of the brain could change the signals they sent to each other, or to the legs. Again, the scientists didn’t program in changes that would make the animats faster. The mutations dropped into the animat genomes at random.
The scientists then set the animats into their mazes again and let 300 steps pass by. Once again, they picked the 30 that managed to travel the furthers to reproduce. This process is natural selection in its essence. Some organisms have more offspring thanks to inherited variations in their genes, and new variation can arise through mutations. Over many generations, this process can spontaneously change how organisms work.
One of the luxuries of digital evolution is that you can let it run practically ad infinitum. The scientists let the animats reproduce for 60,000 generations. And in that time, the animats evolved into much better maze-travelers.
Here, in glorious Pong-era video, is an animat from the 12th generation. The top panel shows it moving through the full maze, while the lower left panel zooms in on the animat. The lower right panel shows the activity in the animat’s brain. Note how it takes its own sweet time meandering up and down the walls:
And here is an animat from the 60,000th generation. It moves with assurance and swiftness. The researchers were able to calculate the perfect strategy for an animat, and this evolved specimen had reached 93% of the ideal performance. (The early animat from the 12th generation only performed at 6%.)
You might be wondering what those red arrows are in the doorways. They’re clues. Each arrow tells which direction to go to find the next doorway. The doorway sensor can respond to those signals, but at the start of the experiment, the animats have no way to use the information.
But after thousands of generations, some of the animats evolved the ability to pick up the clues. Their brain evolved a wiring allow it to store the information they picked up in each doorway and use it guide their movements till they got to the next doorway–whereupon they kicked out the old information and recorded the information in the new doorway. Once the animats evolved this simple memory, their performance skyrocketed.
It’s startling to open the virtual skull of the animats and looking at what their evolved brains look like. Here are two different animats after 49,000 generations.

Edlund et al 2011. doi:10.1371/journal.pcbi.1002236.g006
Both animats don’t even use three of the four parts of their brain–that’s why only the circle marked nine is shown in the diagrams. Each one has evolved different patterns of inputs and outputs. It’s hard to break apart the systems into individual circuits and say that they do anything in particular. The behavior of the animat emerges from the whole network.
Thanks to the design of the experiment, the scientists could measure the complexity of the animats as they evolved–with a collossal amount of computing time. This graph shows the complexity of animats along the Y axis, using a measurement of Tononi’s integrated information. The color of the dots represents which generation each animat came from-blue is from the early generations, and red from the latest ones. And, finally, the X axis shows how fast the animats can travel, as a percentage of the highest possible speed an animate could possibly go.

Joshi et al., 2013 doi:10.1371/journal.pcbi.1003111.g005
There are two lessons from this graph, which can seem contradictory.
As the animats get better at getting through the maze, they get more complex. No 50% animat is less complex than a 20% animat.
To see whether selection really was essential to the rise of complexity, the scientists ran a test on some highly evolved, highly complex animats. At the end of each maze run, they didn’t pick out the top 10 percent of the animats as the parents of the next generation. They just picked 30 animats at random from all 300. After 1,000 generations without selection, the animats were pretty much hopeless, hardly able to find a single doorway. And their complexity crashed.
This research shows that an increase in complexity comes with adaptation–at least for animats. But look again at the graph. Look at the animats at any given level of fitness. Some of them are more complex than others. In other words, some animats can race along at the same speed as other animats that are twice as complex. All that extra complexity seems like a waste.
In the world of animats, evolving a better brain requires a minimal increase in complexity, so as to take in more information and make better use of it. Extra complexity doesn’t necessary make an animat better at traveling the maze, although it may provide the raw material for further evolutionary advances.
It would be interesting to see what would happen if some of the rules for animats were changed. In this experiment there was no cost to extra complexity–something that may not be true in the real world. The human brain makes huge demands of energy–twenty times more the same weight of muscle would. There’s lots of evidence that efficiency has a strong influence on the anatomy of our brains. Perhaps we might have more complex brains if we did. And if the animats had to pay a cost for extra complexity, they would evolve only the bare minimum. That’s an experiment I’d like to see. And don’t forget that arcade music….
Sources:
Edlund JA, Chaumont N, Hintze A, Koch C, Tononi G, et al. (2011) Integrated Information Increases with Fitness in the Evolution of Animats. PLoS Comput Biol 7(10): e1002236. doi:10.1371/journal.pcbi.1002236
Joshi NJ, Tononi G, Koch C (2013) The Minimal Complexity of Adapting Agents Increases with Fitness. PLoS Comput Biol 9(7): e1003111. doi:10.1371/journal.pcbi.1003111
July 31, 2013
Archaeopteryx’s Evolutionary Humiliation Continues
Few extinct species have emerged from the Earth with more fanfare than Archaeopteryx. In 1861, workers in a limestone quarry in Germany discovered the impression of a single 145-million-year-old feather. Hermann von Meyer, the paleontologist who first studied, almost thought it was a forgery, until he compared the impressions of the feather on the upper and lower layers of limestone in which it was discovered. “No draughtsman could produce anything so real,” he declared.
Soon von Meyer was working on another fossil: the entire body of the animal that grew such feathers. It had some hallmarks of birds, such as feathered wings, but it also had more reptilian traits seen on no bird today, such as teeth and a long, bony tail. Von Meyer dubbed both fossils Archaeopteryx, meaning “ancient wing.” Darwin had just published the Origin of Species two years earlier, and he couldn’t ask for a better piece of evidence for evolution. “It is a grand case for me,” he confided to a friend.
For over a century Archaeopteryx stood as the crucial fossil for scientists wanting to understand how reptiles evolved into feathered flyers. But starting in the late 1900s, new fossils began to emerge, and, as I wrote in National Geographic in 2011, they revealed a gradual transformation of ground-running dinosaurs into birds.
Even with all the new company of feathered dinosaurs, Archaeopteryx still held an exceptional position in study of the origin of birds. It seemed to be the closest relative to living birds with an anatomy suited to flying, with traits such as long arms. In a very real sense, it was still the first bird.
Now Archaeopteryx is sinking back into the crowd of primitive birds and feathered dinosaurs. As Ed Yong has ably explained, a fresh wave of fossils are coming to light. They reinforce the argument that paleontologists have agreed on for a couple decades now: birds evolved from a lineage of dinosaurs called theropods. But it’s less clear now how exactly Archaeopteryx fits into that evolution. It might still be closely related to the ancestors of living birds, or there might be non-flying theropods that were more closely related. Combine this with the recent discoveries of heavily feathered dinosaurs–feathered down to their feet, in fact–and the possibility emerges that dinosaurs evolved into flyers more than once. We look up in the sky today and see the results of only one of those transitions.
Today comes a new study that gives Archaeopteryx a further push back into the crowd. A team of researchers at the American Museum of Natural History and the University of Texas have taken a look at Archaeopteryx‘s brain, and it’s pretty unexceptional.
Scientists have long known that the brains of living birds are quite exceptional. Compared to reptiles, birds have brains that are huge in proportion to their body. “Hyperinflated” is the word that scientists like to use to describe them.
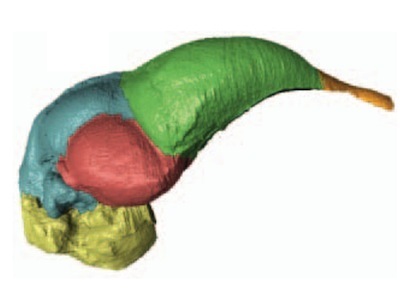
Archaeopteryx brain reconstructed from a fossil. Colors mark brain regions: olfactory bulbs (orange), cerebrum (green), optic lobes (pink), cerebellum (blue) and brain stem (yellow). From Balanoff et al., Nature 2013
When it first became clear that birds evolved from theropods, scientists took a look at dinosaur brains to trace this hyperinflation. They found that Archaeopteryx’s brain fell in between that of distantly related theropods, such as Tyrannosaurus rex, and living birds. What’s more, its structure resembled that of birds–at least compared to other dinosaurs. The visual centers were expanded, and the regions of the brain it used to process sound were big. Its brain was, it seemed, ready for flight.
With so many new bird-like dinosaur fossils to peruse, the American Museum team decided to make a more detailed comparison of brain cases from 28 species in total.
In this new study, Archaeopteryx no longer pops out. The researchers found, in fact, that several species of feathered dinosaurs, such as a troodontid called Zanabazar and an oviraptor called Conchoraptor, have brains that are bigger, relative to their body size, than Archaeopteryx.
The scientists also drilled down to look at the sizes of different regions of the brain and found that the only bird-like brain region in Archaeopteryx is the olfactory bulb, which it uses for smelling. But some other dinosaurs have bulbs that are just as big.

A 3D rendering from CT scans of the troodontid dinosaur Zanabazar junior. In this image the endocast (brain) is rendered opaque and the skull transparent.
Amy Balanoff, AMNH
It’s possible that the common ancestor of Archaeopteryx and other close relatives of birds had already evolved a more bird-like brain than other dinosaurs. It’s also possible that the different linages of dinosaurs that were closely related to birds evolved even bigger brains in parallel. If a bird-like brain was essential for the mental challenge of flying through the air, then these other dinosaurs had what it took for flight. It will be up to future paleontologists and ornithologists to figure out how flight shapes the brain, and how well other feathered dinosaurs could fly. But Archeopteryx will only be one among many species that they consider when they tackle those questions.
[Update 7/31 2 pm: I fixed the etymology of Archaeopteryx, thanks to a commenter.]
July 30, 2013
The Mystery of the Monogamous Mammals
The golden lion tamarin is a monogamous primate. A male and a female will bond for life, mating only with each other, and cooperating to rear their family. Over 200 other mammal species are also monogamous, which is puzzling. Why do males stick around? In the news section of today’s New York Times, I have an article on two new studies on this mystery. It’s an example of science as a work in progress: the studies end up with very different conclusions. Check it out.
July 28, 2013
A Cellular Homage to Mom (Science Ink Sunday)
 Sara Faust writes, “The Science Ink collection has been a favorite among my friends and me for a long time, and I am excited to finally be able to submit for your consideration a (hopefully) unique nerdy tattoo of my own. After explaining to the tattoo artist and various family friends that, no, it is not a raisin or a boot tread or a pill, it will be a relief to share it with people who perhaps will appreciate the humor of an endosymbiotic twist on the classic “Mom” ink. The moment I first learned about mitochondrial DNA, my immediate reaction was, ‘That would make a great Mom tattoo…’ Years later, it’s finally a reality!”
Sara Faust writes, “The Science Ink collection has been a favorite among my friends and me for a long time, and I am excited to finally be able to submit for your consideration a (hopefully) unique nerdy tattoo of my own. After explaining to the tattoo artist and various family friends that, no, it is not a raisin or a boot tread or a pill, it will be a relief to share it with people who perhaps will appreciate the humor of an endosymbiotic twist on the classic “Mom” ink. The moment I first learned about mitochondrial DNA, my immediate reaction was, ‘That would make a great Mom tattoo…’ Years later, it’s finally a reality!”
For more on this gift from our mothers, all the way back to Mitochondrial Eve and beyond, read this.
You can see the rest of the Science Tattoo Emporium here or in my book, Science Ink: Tattoos of the Science Obsessed.