Carl Zimmer's Blog, page 22
September 11, 2013
“Therefore His Shipmates Called Him Mad”: The Science of Moby Dick
One of the best things about a blog is that it can function as a public sketch pad, where I can try out ideas that aren’t quite right for a full-blown magazine feature, book, or newspaper article. Sometimes those verbal sketches can mature into something more.
In November, for example, I was inspired by an online reading of Moby Dick to praise Melville as a science writer. Soon afterwards, I was contacted by the Los Angeles Public Library, which was planning a month-long celebration of the book that has just kicked off. They asked if I would write an essay about the science behind the novel, which they could include in the program for the final event on October 5.
It was a great pleasure to dig deeper into Melville’s life and times, and reflect on how his scatter-shot education in pre-Darwinian biology shaped his book.
Here’s how the piece starts:
“To have one’s hands among the unspeakable foundations, ribs, and very pelvis of the world; this is a fearful thing. What am I that I should essay to hook the nose of this leviathan!”
Ishmael asks himself this question at the beginning of “Cetology,” the thirty-second chapter of Moby Dick. Up till this point, the narrative of Moby Dick, as Ishmael recounts his experiences joining the crew of the Pequod, feels fairly straightforward. Readers who bought the novel when it first came out in 1851 probably found it similar to Melville’s previous novels of the sea, like Mardi and White-Jacket. But then Ishmael abruptly turns into a peculiar sort a naturalist. He dedicates an entire chapter to whale taxonomy in absurdly exhaustive detail. Later in the novel, he writes chapters dedicated to the anatomy of whales, their fossils, and their ecology.
Those chapters put off many readers and critics in Melville’s day. All that science felt like a massive distraction from the central story of Ahab’s mad pursuit of the White Whale. And even today, I’d wager that a lot of readers page quickly through the long passages about whale flukes and whale brains. But the science of Moby Dick is as superfluous to the novel as lungs are to the body. Melville used science to elevate the hunt for a single sperm whale into a metaphysical tragicomedy.
The entire essay is available at the event’s web site. Check it out.
September 9, 2013
The Second Draft of the History of Science
I’ve been hearing good things for a while now about Retro Report, a journalism project that produces 10-to-20-minute-long videos about what happened to big headline stories from decades ago. I’m now gobbling up my Monday morning watching their backlist. It’s excellent stuff, and I’ve been trying to figure out why I like it. I think it’s because the programs get beyond the simple “Where Are They Now?” format. The journalists who make the pieces really report the stories–they go back and find people who were in the midst of the news to interview them, and then they discover the surprising course of the story after it fell away from the world’s attention.
What really surprised me about Retro Report is that most of their categories are related in one way or another science. I write about new scientific research, and so my job requires me to keep my eyes locked on the future, trying to figure out what some discovery or invention will mean to generations to come. And the longer you spend in this job, the more you start asking yourself, “Hey, what happened to…?” In many cases, things quietly take an unexpected–but revealing–turn. Retro Report shows that going back to a story about science can reveal important lessons about what’s going on today, but ones you may not have predicted.
Here, for example, is a piece about a GMO tomato that turned up in supermarkets way back in 1994. People went hysterical over the Flavr Savr tomato, either as an evil plot or the salvation of our food supply. After the cameras were shut off and the reporters went away, the company that made the tomato struggled to make a business out of it and quietly sold their patent off to GMO giant Monsanto, which then quietly shut the project down–arguably because it was boldly labeled in stores as genetically modified. Since then, Monsanto has gone on to make big profits on GMO plants by making farmers their customers, not consumers.
Here’s another piece, called Crack Babies. In the 1980s, people got frantically worried that crack-addicted women would give birth to a generation of brain-damaged infants. The idea–based on some preliminary research–turned out to be wrong. Yet it became a wildly successful meme, perhaps because it involved a then-new drug and perhaps because crack addicts were mostly poor blacks. Retro Report rightly asks why we never talk about the threat of “Booze Babies,” when alcohol is more harmful during pregnancy than crack.
These videos remind us forcefully that the real meaning of stories about science takes time to unfold. That is very hard to remember, because there’s something intoxicating about a new science story. Suddenly some great truth about the world seems to be unveiled. That truth can be terrifying, or elating. I can’t count all the emails I’ve gotten when I’ve written a story about some very preliminary research on a disease, from people who suffer from the disease and want to know where they can go to get cured.
In reality, a lot of science-related conclusions fall apart or have to be revised in later years. Science itself is starting to grapple with its flaws, with papers like “Most Published Research Findings Are False.” On the other hand, some findings gain strength over the years, as more and more evidence supports them. But those studies pile up like sand grains, and so it’s easy for journalists to overlook them, even after they’ve grown into a mountain.
I hope Retro Report does more investigations into science. They’re wonderful history lessons, and they also help people think more realistically about today’s news.
Other science stories include:
–Summer of Fire: How this year’s massive forest fires are part of a 25-year trend, due in part to human activity.
–Biosphere 2, the sealed building that was supposed to become self-sufficient and instead went wrong in a fascinating way.
–Y2K, the computer bug that terrified the world in 1999 with the prospect of computers shutting down on New Years Day.
–Voyage of the Mobro 4000: an ill-fated voyage of a garbage barge that gave rise to the recycling movement.
(P.S.: Retro Report is a non-profit project. The New York Times, where I’m a columnist, distributes Retro Report, but I’ve not had any dealings with them aside from as a viewer.)
September 6, 2013
Where Plagues Come From: Making A Catalog of the World’s Viruses
In 1999, a new disease came to light–a brutal fever that sometimes led to fatal encephalitis. After the first outbreak in Malaysia, scientists traced the cause of the disease to a virus called Nipah. Although it was new to medicine, Nipah virus didn’t come out of thin air. It had replicated for generations inside Indian Flying Foxes, a common species of fruit bat in southeast Asia. The virus spilled over into humans, thanks to the fondness both species have for date palms. Now Nipah virus can spread from person to person.
This scenario sounds like it came from the pitch meeting for last year’s creepfest Contagion. Unfortunately, it’s all quite well documented. So is the emergence of many other viral diseases. (Check out David Quammen’s book Spillover for a sweeping view of these new diseases.)
In my Matter column this week in the New York Times, I take a look at a new way to battle these emerging diseases: by figuring out how many viruses there are in mammals that might spill over in the future. Scientists have taken the first step to such a virus catalog with a suitable species: the Indian Flying Fox. And unfortunately, it’s chockful of mammal viruses, most of which are new to science. Here’s the full story. (Also check out fellow Phenom Ed Yong’s report on the study for The Scientist.)
September 4, 2013
Understanding Our Moral Tribes
A decade ago, I traveled to Princeton to spend some time with a young philosopher who had decided to start scanning people’s brains. I was working on a book about the history of neurology, called Soul Made Flesh, and I was fascinated by how the study of the brain had emerged from a scientific attempt to save souls. I wanted to end the book with a look at how scientists study the brain 350 years later, and during my research I discovered the work of Joshua Greene. He was taking the arguments that moral philosophers had developed over many years and testing them out on flesh-and-blood brains, monitoring neural activity as people worked through moral problems.
In addition to putting Greene into my book, I ended up writing a profile of him called “Whose Life Would You Save?” for Discover (which you can also read in a collection of my articles available at Byliner). Here’s how it starts…
Dinner with a philosopher is never just dinner, even when it’s at an obscure Indian restaurant on a quiet side street in Princeton with a 30-year-old postdoctoral researcher. Joshua Greene is a man who spends his days thinking about right and wrong and how we separate the two. He has a particular fondness for moral paradoxes, which he collects the way some people collect snow globes.
“Let’s say you’re walking by a pond and there’s a drowning baby, ” Greene says, over chicken tikka masala. “If you said, ‘I’ve just paid $200 for these shoes and the water would ruin them, so I won’t save the baby,’ you’d be an awful, horrible person. But there are millions of children around the world in the same situation, where just a little money for medicine or food could save their lives. And yet we don’t consider ourselves monsters for having this dinner rather than giving the money to Oxfam. Why is that?”
Philosophers pose this sort of puzzle over dinner every day. What’s unusual here is what Greene does next to sort out the conundrum. He leaves the restaurant, walks down Nassau Street to the building that houses Princeton University’s psychology department, and says hello to graduate student volunteer Nishant Patel. (Greene’s volunteers take part in his study anonymously; Patel is not his real name.) They walk downstairs to the basement, where Patel dumps his keys and wallet and shoes in a basket. Greene waves an airport metal-detector paddle up and down Patel’s legs, then guides him into an adjoining room dominated by a magnetic resonance imaging scanner. The student lies down on a slab, and Greene closes a cagelike device over his head. Pressing a button, Greene maneuvers Patel’s head into a massive doughnut-shaped magnet.
Greene headed off to Harvard a couple years later, where he’s now an associate professor of psychology. Over the years other scientists have also taken up the study of moral neuroscience, but Greene still stands out among them thanks to the philosophical rigor with which he thinks about the nature of morality. Over the years, he’s expanded his research from the basic biology underpinning morality to the different ways that it gets played out in human societies–and how, paradoxically, different forms of moralities bring people into conflict.
So I’m very curious now to check out a book he’s written about his research and ideas, called Moral Tribes, coming out next month. The Edge has a sneak preview of Greene’s ideas in the form of a video talk by Greene and a transcript. Check it out.
August 29, 2013
Good Germs In the Womb: My New “Matter” Column in the New York Times on the Fetal Microbiome
I just went back and listened to this interview I did on “Radiolab” with Robert Krulwich a couple years ago. It’s about the life within us. I led Robert on a quick tour through our gut, stopping to describe a few of the many species that lurk inside our bodies.
It all still holds true, I think–except for one thing I say at 3:02.
It’s at that point that I say that in the womb, we’re sterile. Only as we’re being born, I inform Robert, do we start to getting inoculated with microbes.
I thought I was right at the time. Scientist after scientist told me that. I read it in scientific reviews.
But now a number of scientists are having some serious doubts about sterile fetuses. In fact, mothers might be seeding their babies in the womb, bestowing on them the friends that will help them get through pregnancy safely and get off to a good start in life.
If Krulwich ever asks me again about the microbiome, I’ve got something to add.
This fascinating new prospect is the subject of my new “Matter” column today in the New York Times. Check it out. (Here’s an alternate link if the recent NYT hacking woes are still causing grief.)
August 27, 2013
When Baby Snapping Turtles Reach the Edge of the World
Despite living in the carotid artery of Northeast traffic, I still share my property with a particularly prehistoric kind of wildlife. Each spring, monstrous snapping turtles emerge from the salt marshes and rip up our garden to lay eggs. Then in late summer the baby turtles hatch and crawl out of the mulch to head for the water again.
This was the scene outside our front door (I highly recommend setting the movie to full-screen). The baby snapping turtles, each the size of a jumbo chicken egg, crawled to the light one by one and clambered onto our stone steps. As this video demonstrates, baby snapping turtles deal with these unexpected situations without much hesitation. They climb to the edge of their world and keep going.
August 25, 2013
A Gathering of Giants (Science Ink Sunday)
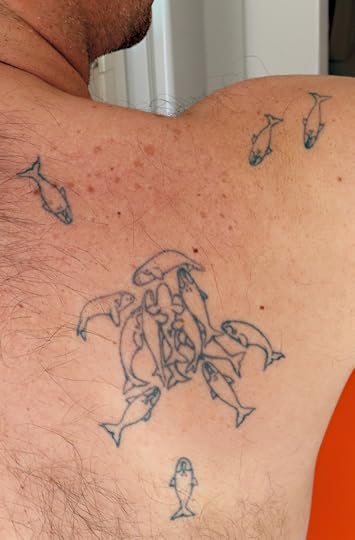
Luciano Valenzuela writes,
I got this tattoo after finishing my PhD at the University of Utah working on the ecology of southern right whales that visit the coast of Peninsula Valdes (northern Patagonia), Argentina. The tattoo depicts a Surface Active Group (SAG). SAGs are usually thought as mating groups or whales in apparent courtship behavior. At Peninsula Valdes the SAGs that we normally see are much smaller with only a handful of whales, but the energy displayed by these animals is just as impressive as the large groups seen in other populations or species. The tattoo is actually a modification of Figure 1 in Kraus and Hatch (2001) showing a SAG of North Atlantic right whales. I think once you see them from close proximity you can appreciate how powerful and gentle at the same time these huge animals can be.
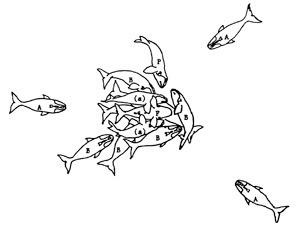 Here’s the original figure.
Here’s the original figure.
You can see the rest of the Science Tattoo Emporium here or in my book, Science Ink: Tattoos of the Science Obsessed.
August 23, 2013
And the Genomes Keep Shrinking…
Here are a few numbers about DNA–some big ones, and then some very small ones.
The human genome contains about 3.2 billion base pairs. Last year, scientists at the University of Leceister printed the sequence out in 130 massive reference-book-sized volumes for a museum exhibit. From start to finish, they would take nearly a century to read.
A typical gene is made up of a few thousand bases. The human genome contains about 21,000 genes that encode proteins. There are other genes in the human genome that encode molecules known as RNA, but how many of those RNA molecules actually do anything useful in the cell is a matter of intense debate. A lot of the human genome is made of neither protein- or RNA-coding genes. Much (maybe most) of it is made up of dead genes and parasite-like stretches of DNA that do little more than making copies of themselves.
As I wrote recently in the New York Times, 3.2 billion base pairs and 21,000 genes are not essential requirements for something to stay alive. E. coli is doing very well, thank you, with a genome about 4.6 million base pairs. That’s .14% the size of our genome. Depending on the strain, the microbe has around 4100 protein-genes. That’s about a fifth the number of protein-coding genes that we carry. The high ratio of genes to genome size in E. coli is the result of its stripped-down, efficient genetics. Mutations that chop out non-functional DNA spread a lot faster in microbes than in animals.
E. coli, in turn, has proven to be positively gargantuan, genetically speaking, compared to some other species. As scientists explore more of the microbial world, they find species with smaller genomes. In my column for the Times, I wrote about the record-holding tiny genome, belonging to a microbe called Tremblaya. Its genome is a mere 139,000 base pairs. That’s .004% the size of our genome. You could print the entire sequence in a single slim paperback you could slip in your pocket. And in that sleek genome are just 120 protein-coding genes–.6% of our own collection of protein-coding genes.
Whenever I report on such record-breakers, I try to stress that they are only breaking records at that moment. Tremblaya has the smallest genome known. Or, I should now say, it had the smallest genome known last month.
This month in the journal Genome Biology and Evolution, Gordon Bennett and Nancy Moran describe a new record holder, called Nasuia deltocephalinicola. It has a genome of just 112,000 base pairs. Imagine taking that slim novella and ripping off the last chapter. Ironically, Nasuia packs in more genes into its DNA than Tremblaya–137 protein-coding genes, Bennett and Moran estimate.
What’s really striking about all these current and former record-holders for small genomes is that they all live in a single exotic ecological niche. Without exception, they can be found inside plant-feeding insects. Tremblaya lives in mealy bugs, for example, while Nasuia lives in a leafhopper (Macrosteles quadrilineatus).
Inside those hosts, these microbes carry out chemical reactions on the food that the insects eat. The insects feed on sap and other fluids from plants, which contains few nutrients. But the bacteria can use the compounds floating in the fluid to build amino acids, which the insects can then assemble into proteins.
Leafhoppers, cicadas, sharpshooters, and other related insect species carry related versions of the same stripped-down bacteria. By drawing their evolutionary trees, Bennett and Moran have found that the insects got into a symbiotic relationship with the microbes over 260 million years ago. I’ve reproduced their tree below for those who want some gory details. The blue lines show Nasuia and related lineages of microbes. The insects also acquired another species of bacteria, known as Sulcia. Together, these two microbes split the work for millions of years. (In some insects, fungi also jumped into the mix.)
The ancestors of Nasuia started out as free-living microbes that had genomes on par with E. coli. But once they got inside a host, they were able to lose DNA without paying a price. The insects gave them a stable home, building special organs to shelter them, and they even pass down the bacteria to their offspring. The bacteria cast aside many genes that might otherwise seem essential, such as a number of genes involved in generating energy. All they needed to do was continue to provide a service, by synthesizing some amino acids.
Nasuia holds the record now, but probably not for long. There are many other species of insects left to investigate. Moran had John McCutcheon of the University of Montana have done some back-of-the-envelope calculations to figure out how much smaller the genomes of those symbionts can get. All known insect symbionts share 82 genes in common. It’s possible those genes are absolutely required to survive as a symbiont. But a symbiont also needs to provide a benefit to its host, or its host will likely get rid of it. It takes at least 11 genes to synthesize a single amino acid. Those 93 genes, McCutcheon and Moran estimate, could fit in a genome as small as 70,000 base pairs.
It’s funny that these bacteria allow us to probe one of the most basic questions about life: how simple life can get and yet still qualify as being alive? While those who make fun of science for a living may consider such research a waste of time, studying these stripped-down organisms is also about as practical as science can get. The leafhoppers that house Nasuia, for example, are a nightmare for farmers, causing damage to a wide range of vegetables by spreading fungi and bacteria. Yet they would be helpless if not for their exquisitely simple lodgers. If we can understand how they survive with such tiny genomes, we may be able to stop them from enabling their hosts.

From Bennett and Moran 2013
August 22, 2013
Are We Making Animal Brains Bigger? My New “Matter” Column for the New York Times
The Stanford biologist Stephen Palumbi wrote an excellent book some years ago called The Evolution Explosion, in which he argued that humans have become a powerful force in the evolution of life. We’ve altered the whole planet, so that now many species are traveling on new evolutionary trajectories. (For more, here’s a review of the book I wrote for the New York Times Book Review.)
Over the years since Palumbi’s book came out, scientists have documented more examples of our effect. This week, I was intrigued to come across a new study that we may be even altering the brains of animals. It’s the subject of my new Matter column for the New York Times. It’s only a preliminary study, of course, but it does raise some fascinating questions about the mental challenges animals now face as they navigate a human-dominated world. Check it out.
August 16, 2013
Experimental Evolution And The False Solace of “They’re Still Bacteria”
When Charles Darwin developed his theory of evolution, he resigned himself to only seeing its effects. Evolution happened so slowly, he was convinced, that we couldn’t see life changing from one generation to the next by a mechanism such as natural selection. So he found other ways to amass evidence for evolution.
He pointed out, for instance, that natural selection was a logical–even inescapable–fact of life. Individuals varied in their traits. Some of those variations influenced how many offspring they had. And those traits could also be passed down to offspring. Under such conditions, natural selection just happens.
Darwin also looked back over the history of life and showed how powerfully evolution could explain its large-scale patterns. He couldn’t account for every jot and tittle over the past four billion years, of course. But he could, for example, account for how groups of species shared sets of traits: because they descended from a common ancestor that lived millions of years ago.
Starting in the mid-1900s, however, evolutionary biologists began documenting measurable evolution over the course of years, not millennia. As chronicled by Jonathan Weiner in The Beak of the Finch, for example, Peter and Rosemary Grant have measured changes in the beaks of Darwin’s finches over the past four decades.
Microbes–which breed much faster than animals and acquire mutations at a faster rate–are also opening new lines of research into evolution. Scientists like Richard Lenski and Paul Turner are tracking the evolution of bacteria and viruses over a matter of weeks, or even days.
This week in my “Matter” column for the New York Times, I took a look at a new study on experimental evolution. Bacteria living in Petri dishes evolved extra tails, which allowed them to swim faster and take over their populations. The experiment is fascinating in many ways–from its potential applications to medicine to what it says about the predictability of evolution. Plus, it comes with cool videos.
While the response to my column has been generally enthusiastic (thanks), I have gotten some negative comments that echo an old refrain I often hear when I write about experimental evolution. Basically: they’re still bacteria.
@_NIKD_ @carlzimmer So the bacteria……remained a bacteria.
— V. Hugo (@HugoPush) August 15, 2013
@Crispybroccoli What NEW traits were created? Getting an extra finger, etc..are mutations of existing genes. — V. Hugo (@HugoPush) August 15, 2013
Here’s a related chain of tweets…
@carlzimmer @nytimesscience Short-run experiment doesn’t allow time long enough to separate species, e.g., ape and human. — NEVATHIR (@A_NEVATHIR) August 15, 2013
@carlzimmer @nytimesscience That’s not biological evolution, but rather pattern formation: Dogs’ offsprings’ color change, right?
— NEVATHIR (@A_NEVATHIR) August 15, 2013
@carlzimmer @nytimesscience You are all pseudo-scientists.
— NEVATHIR (@A_NEVATHIR) August 15, 2013
Opponents of evolution often like to decree what evolution really is. That way, when scientists study evolution, they can declare, “That’s not evolution.”
Nevathir, for example, claims that that what happened in this experiment is just “pattern formation,” which apparently refers to how dogs give birth to puppies that have different color patterns. (That’s not actually called pattern formation, but I have to guess here.)
Puppies get different color patterns because (among other reasons) they inherit different combinations of genes from their parents. The experiments I wrote about this week are not “pattern formation” in this sense of the phrase. They started with genetically identical bacteria, which divided, producing identical clones except when new mutations arose. Those mutations were then passed down to their descendants. Mutations to one gene in particular led to the emergence of “hyperswarmers.” Hyperswarmers were genetically programmed to make more tails, which allowed them to swim faster than their ancestors. And they quickly drove slower bacteria extinct as they came to dominate the population.
That is evolution–evolution in under a week, in fact.
V. Hugo asks what new traits were created. Apparently acquiring a number of new tails is not a new trait, in the same way that a mutation in people can lead to the development of an extra finger on the hand. And apparently changes can only be called evolution if they involved the evolution of a new trait.
It’s very hard for me to see how evolving from a single tail to up to half a dozen tails–all of which work together rather than getting tangled up with each other–is not a new trait. But even if we go along with V. Hugo this far, his sort of argument still fails, because it’s not an argument at all. He’s just creating a personal definition of evolution in order to scoff at scientific research. The origin of new traits is part of evolution, but so is the spread of beneficial mutations due to natural selection.
I suspect that Nevathir and V. Hugo aren’t satisfied with this experiment because it isn’t a large-scale episodes of evolution–the split between species, for example, or the origin of an eye or a hand. (I’m guessing here, but it’s a guess educated on many previous such comments.) Large-scale episodes take time, typically stretching across thousands or millions of years. The scientists who study bacteria over the course of a few weeks don’t expect to witness such transformations. Instead, they are finding that they can dissect the mechanisms of evolution. They can even document the emergence of new genes, as mutations accidentally duplicate stretches of DNA, which can then begin to take on new functions.
And then there’s the “They’re-still-bacteria” remark. I hear variations of this refrain many times, which makes me assume that it gives opponents of evolution great comfort. Bacteria are one “kind” of life form, and since these experiments don’t show them evolving into another “kind”–like a dog–then they reveal nothing.
Such a remark isn’t just wrong-headed about evolution, though. It reveals a misunderstanding of bacteria. Bacteria originated about 3.5 billion years ago and have been diversifying into many different forms ever since. Some bacteria float in the ocean, turning sunlight into carbon. Others breathe iron. Others make squid glow. Watching bacteria evolve in a Petri dish helps us to understand not just evolution in general, but bacteria in all their particulars.