Carl Zimmer's Blog, page 18
December 19, 2013
An Up-to-Date Neanderthal Genome Fits Into the Web of Humanity
There’s more news on the ancient human DNA front: as I report in my new “Matter” column in the New York Times, scientists have now reconstructed the genome of a Neanderthal with exquisite accuracy. Their genome sequence is as good as what you’d get if you had your own genome sequenced with the finest equipment available today. And yet the DNA comes from a fossil that’s approximately 130,000 years old.
You can read more about this remarkable feat–and what it implies–in my column. But there’s something more that I didn’t have room to discuss that I found really intriguing. Here’s the tree of human evolution that scientists have generated from the Neanderthal genome in comparison with other human DNA:

Nature
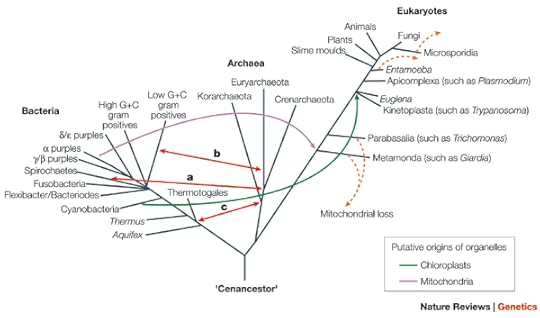
Now zoom out to the tree of all living things*:
Brown, Nature Reviews Genetics http://www.nature.com/nrg/journal/v4/...
Evolution is a mixture of flow–the cascade of genes from parents to offspring, and the criss-cross movement between populations and species. It has made us who were are, over just the past 60,000 years and over the past four billion.
[Note: The image at the top of this post comes from the Neanderthal Museum in Germany. I have never been there, but I can only guess that it's fantastic.]
[*This tree is somewhat out of date. Eukaryotes now look to be just one branch of the Archaea, for example, rather than a third domain. But the criss-crossing remains.]
December 18, 2013
A Phenomena(l) Year!

M.C. Escher http://www.wikipaintings.org/en/m-c-e...
A year ago today, Phenomena was launched, and I just wanted to take a moment to thank all of you for reading the work of Virginia Hughes, Brian Switek, Ed Yong, and myself over these past 365 days. The Loom has seen a lot of homes in its ten years, but Phenomena has been the best, I must say, from its delightful design to the support of people at National Geographic such as Jamie Shreeve and Brian Howard.
In case you’re curious, here are the ten most-read posts I wrote here over the past year:
The Norovirus: A Study in Puked Perfection
Tongue-Eating Fish Parasites Never Cease to Amaze
When You’re A Naked Mole Rat, Why Stop At One Weapon Against Aging?
The Weird Youth of the Animal Kingdom (Slide Show)
Charlemagne’s DNA and Our Universal Royalty
The Brain-Chilling, Shrimp-Caressing, Lamppost-Sized, NSFW Organ Hiding In A Whale’s Mouth
An Open Letter to Science Students and Science Teachers
The Guinea Worm: A Fond Obituary
On to another year of cavities, noroviruses, Charlemagne, and other strange wonders!
December 12, 2013
In Search of the First Animals
All animals–from corgis to Greenland sharks, from dog ticks to toucans to you–descend from a common ancestor. The fossil record of animals, which runs back over 600 million years, can help us travel back some of the way through animal evolution towards the origin of the kingdom. But those early rocks contain precious few remains of animals, and so fossils alone can’t tell us what our common animal ancestor looked like.
Scientists can add to their supply of clues by studying living animals. And it now looks as if some of the most important clues to how animals got their start come from a beautiful creature called the comb jelly. This video from the Monterey Bay Aquarium is a good introduction to their luminescent loveliness.
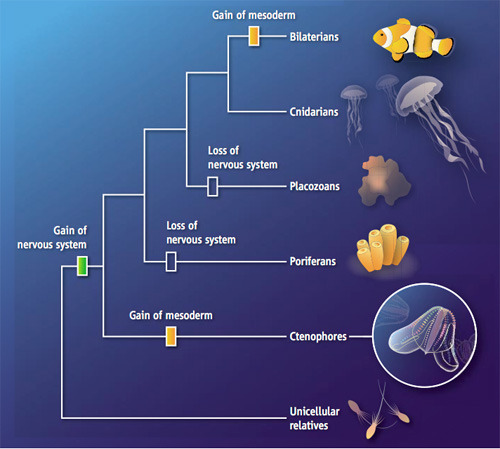
In the past, a lot of scientists would not have put so much importance on comb jellies. If you asked them (as I did) how animals evolved, they’ve sketch out a version of events that runs like this:
1. Before multicellular animals evolved, their ancestors were single-celled protozoans that may have formed colonies. Our DNA shows that our closest non-animal relatives are critters called choanoflagellates. I wrote about our single-celled cousins in the New York Times.
2. Our ancestors then crossed the line from colonial life to life as multicellular creatures. They became the first animals.
3. The animal lineage then started to split into new branches. Many branches are now extinct. Among living animals, the first split divided the ancestors of today’s sponges from all other species.
4. That could tell you something important about what the earliest animals were like. Sponges have no nervous system and no muscles, for example. All other animals, from comb jellies to starfish to clams to us do. So the early animals didn’t have muscles and neurons yet, and didn’t evolve them until after sponges split off on their own. You might even go so far as to say that our own direct ancestors were sponges–animals anchored to the sea floor, filtering food through small pores.
5. Later, the common ancestor of the non-sponge animals evolved muscles and neurons. These animals began to move around–as pulsating jellyfish, crawling worms, and, eventually, swimming fish.
6. Later still, the muscle-and-neuron carrying animals split apart into two main lineages. Jellyfish belong to a lineage called cnidarians. The other branch is known as bilaterians. It includes all the animals with heads, brains, and tails, from insects to mammals.
Comb jellies seemed to many researchers to simply be a cousin lineage to cnidarians. And that meant they weren’t important to understanding how animals first evolved.
But a few years ago something weird started to happen. When scientists compared the DNA from more and more species, some of them would end up with animal trees in which the comb jellies split off first, not the sponges. (Here’s a piece I wrote about that work for the Boston Globe in 2008.)
A lot of debate ensued. Drawing evolutionary trees is no simple task, especially when you’re looking at branches that split off from each other hundreds of millions of years ago. Teasing apart the order of the branches can be as tricky as teasing apart the stars in a distant corner of the galaxy. So evolutionary biologists built themselves a better telescope.
A new paper today in Science is that telescope. In the past, scientists have compared limited segments of DNA from different species to work out the animal tree. A team of researchers at the National Institutes of Health and elsewhere decided that a more powerful view of the tree might come from looking at the entire genomes of many animals. And it just so happened that of all the major groups of animal species (known as phyla), only the comb jellies were without a sequenced genome.
The scientists rectified the matter, sequencing the first comb jelly genome, belonging to a species called Mnemiopsis leidyi. They then compared the genomes of 13 species, and then did a second comparison of smaller pieces of DNA from 58 species. The studies all pointed in the same direction: comb jellies, not sponges, split first. And the statistical support for that split was very strong.
This diagram, published in Science, sums up the findings nicely:
Having sequenced a comb jelly genome, the scientists could also march through its catalog of genes to see how many genes for different functions it shares in common with us. Despite the fact that comb jellies have muscles, they lack many of the genes essential for muscles in other animals. This would suggest that muscles evolved twice in the animal kingdom.
On the other hand, comb jellies have a lot of nervous system-related genes in common with bilaterians and cnidarians. It’s therefore possible that the common ancestor of all living animals had a simple nervous system. Sponges lost their nervous system and muscles entirely as they adapted to a quiet existence as filter feeders. (The placozoans shown on this tree are an obscure group of weird animals that are just tiny sheets of cells that creep across the sea floor. If the new study is right, they lost their nervous system and muscles too.)
I asked Antonin Rokas, an expert on animal relationships at Vanderbilt University, what he thought of the research. ” I think it’s a step in the right direction,” he told me, “but I doubt that it will silence those who have championed sponges as the earliest branching animal phylum.” The idea of comb jellies as belong to such an ancient lineage runs counter to a lot of thinking in zoology for over a century. It may take studies of more genomes to convince the tougher skeptics.
Aside from basic curiosity, there are other good reasons to settle the debate–and they help explain why the National Institutes of Health spearheaded this study. The NIH sponsors research on animals as models for human diseases. That’s because we share a lot of genes in common with them. Of particular interest to health researchers are so-called “disease genes”–human genes that are associated with diseases if they acquire mutations.
It turns out that over half of known disease genes are present in comb jellies–including many that are missing from species such as fruit flies that scientists use a lot to study human diseases. A lot of our diseases may arise from damage to the fundamental system for building an animal that evolved some 700 million years ago.
(For more on comb jellies, read this feature from earlier this year by Amy Maxmen in Science News.)

Stefan Siebert, Brown University
What the Evolution of Vitamins Means For Human Health

Leeds Museums and Galleries, Creative Commons. http://bit.ly/1h2MRO7
On Tuesday I wrote a feature for the New York Times about the four-billion-year history of vitamins on Earth. Today, in my “Matter” column for the Times, I look at the lessons that history can teach us for improving human health. My favorite one is learning how to use vitamins as weapons against our invisible enemies. Check it out.
December 11, 2013
The Smell of Evolution
Evolution drives relentlessly forward, leaving behind a messy wake. One of the best places to survey its sloppy creativity is inside your nose.
When you smell a lily or a cigar or a jug of spoiled milk, you are grabbing their molecules out of an ocean of air. You have exposed nerve endings dangling deep inside your nostrils, each of which is studded with proteins called olfactory receptors. Each neuron is covered in one type of receptor, the shape of which allows it to grab tightly onto certain odor molecules and weakly to others, while letting many others drift by.
Snagging a molecule causes the receptor to squirm, leading to a falling-domino-like series of reactions that ends with the neuron firing an electric signal into your brain. The brain gets signals from thousands of neurons in our noses, creating a distinctive signature for each kind of smell we perceive.
We can distinguish between a vast number of different odors, thanks in part to the vast number of olfactory receptor genes our neurons can choose from. So far scientists have identified 390 different genes in the human genome that encode olfactory receptors.
But they’ve also found something else that’s rather stunning. The human genome contains another 468 olfactory receptor genes that neurons cannot use to make a receptor. They’re known as pseudogenes (“false” genes). While their sequence is overwhelmingly similar to working olfactory receptor genes, these pseudogenes carry mutations that make it impossible for a neuron to translate their sequence into a protein.
To figure out how the human genome could end up with more pseudogenes genes for olfactory receptors than working ones, scientists have looked back at their evolutionary history. They can get in a time machine by comparing the olfactory receptor genes in humans with those in other species. We share a lot of these genes in common with chimpanzees–both working and broken. Scientists can track this kinship back further in time by looking at more distant relatives–other mammals, reptiles, amphibians, and even fish. Eventually, they can rebuild the rough outlines of half a billion years of sniffing.
The most distantly related animals that share our olfactory receptors are known as lancelets. They look a bit like sardines with their heads cut off. They split off from our own ancestors about 700 million years ago, not long before the evolution of the brain and eyes. As a result, lancelets can give us a glimpse at what our invertebrate ancestors were like. Lancelets don’t have anything like a nose, but they do have about 40 olfactory receptor genes. It’s possible that they turn their whole body into a nose, using receptors scattered across their flanks to pick up odor molecules from the water surrounding them.
Over the next 200 million years, full-blown fish evolved. They evolved something more like our nose, although these were just dead-end holes in their heads. As fish swim, water rushes into these holes, and the animals can sample the molecules in the water with about 100 different olfactory receptors.
Our kinship is even clearer when scientists look up the noses of amphibians. There are even more olfactory receptor genes that match closely to our own. What’s more, some of the genes have changed structure, so that instead of capturing water-soluble molecules, they can capture airborne ones. (Amphibians still make receptors for grabbing water-soluble ones, since they spend time in water.) Mammals, to which we’re even more closely related, have many more olfactory receptor genes, which show even closer kinship to our own.
As scientists trace olfactory receptor genes along this tree of life, they can see two forces at work in their evolution: an evolutionary version of birth, and one of death.
Olfactory receptor genes are located in regions of our chromosomes that are especially prone to copying mistakes. From time to time, an olfactory receptor gene’s DNA gets copied out twice, creating a duplicate of the original.
After the duplication, one of the two identical genes may mutate. Sometimes the mutation disables the gene, turning it into a pseudogene. Losing the gene isn’t a catastrophe, however, since the other copy of the gene still survives. Sometimes a copying error may delete the pseudogene altogether.
In other cases, the new copy mutates, but not fatally. It may continue making the same olfactory receptor, despite the slight change its DNA. Or the receptor’s structure changes. Its different shape alters its grip on odor molecules. That change may trigger a subtle shift in the smells an animal can sense.
Run this operation over and over again for millions of years, and you end up with the diversity of smelling genes found today in living creatures. Like humans, other mammals have have hundreds of working genes and hundreds more pseudogenes. Functional or not, the genes in different species belong to different “families” that originated in duplications millions of years ago. The fact that pseudogenes are “cousins” to functional genes is evidence that they once functioned.
Animals that depend heavily on smell often have huge numbers of olfactory genes, and relatively few pseudogenes. Rats, for example, have 1207 working olfactory receptor genes, for example, and only 508 pseudogenes–roughly a quarter knocked out. Humans have far fewer olfactory receptor genes, and about 55% of them no longer make receptors. Some other species have suffered even greater losses. Chickens have just 554 genes, of which 476 (86 percent) are pseudogenes. Sperm whales, whose ancestors returned to the sea, lost all their working olfactory receptor genes. They only have pseudogenes left.
The churning evolution of smell has not stopped, however. In a new study just published in Nature Neuroscience, for example, a team of scientists took a close look at the genes we living humans use to build olfactory receptors. Our olfactory receptors vary tremendously from one person to the next. Some people have extra copies of olfactory receptor genes, for example. Many have mutations in some of their genes.
To see if these differences have an effect on how people smell, the scientists developed a way to analyze olfactory receptors on an industrial scale. They created hundreds of lines of cells, each of which produced one type of receptor on its surface. They could then expose the cells to a collection of odor molecules and see if any stuck.
Once they found these molecules, the scientists then tested out the different variants of olfactory receptors that are found in human populations. In almost two-thirds of the cases, the scientists found that olfactory receptor genes had variations that changed how they grabbed onto odor molecules. On average, any two people in the study had functional differences in over 30% of their odorant receptor genes.
To see if these chemical differences translated into a different experience of smells, the scientists zeroed in on one gene, called OR10G4. The OR10G4 receptor can bind to a few different molecules, including vanillin and a molecule called guaiacol that has a flowery smell. The scientists found that one common mutation to OR10G4 influences how strongly the receptor binds to guaiacol, but not to vanillin. It turns out that people with the mutation perceive the pleasantness and strength of guaiacol differently too. But there’s no difference in their perception of vanillin.
This study represents one very small, very preliminary survey of how our olfactory receptors give rise to our rich sense of smell. There will be many such studies in years to come. But we can already see in this research how we are not separate from the evolutionary changes that made us who we are. We are part of the turbulent, fragrant flow.
December 9, 2013
Vitamins: The First Four Billion Years
Vitamins are one of those features of life that we take for granted. For some odd reason, we must obtain trace amounts of a dozen or so tiny molecules, or we will get very, very sick. To understand why this is so, you have to look back at the history of vitamins. And that history stretches back pretty much to the origin of life, a history whose traces we can see in our own DNA, and one that has shaped the balance of nature. For more, check out my feature in tomorrow’s New York Times (I’ll have more to add in my “Matter” column for the Times on Thursday).
December 4, 2013
An Ancient, Mysterious Scrap of Human DNA
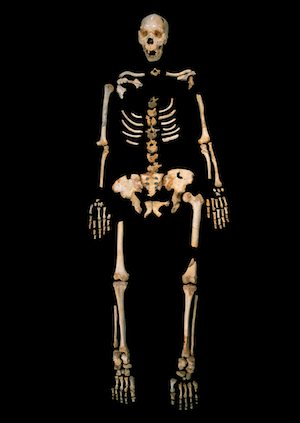
A 400,000-year-old skeleton from Sima de los Huesos in Spain.
I can still remember back in 1997 being shocked that a team of scientists had managed to extract a few hundred bases of DNA from a 40,000 year old Neanderthal fossil. Neanderthal DNA! In the years that followed, scientists made huge advances in recovering ancient DNA, with the entire Neanderthal genome published in 2010. But for all that amazement, I had to learn to be resigned that scientists probably wouldn’t get human DNA older than about 100,000 years. Beyond that vintage, the DNA was just too busted up to be recoverable.
Wrong. Very wrong.
In the New York Times, I report on how scientists have now extracted 400,000-year-old DNA from a human fossil. That’s ten times older than the first pieces of Neanderthal DNA.
I’ll point you to the newspaper for the straight story. But there is, of course, a lot more to the tale than can fit into 1000 words. Let me lay some of the extra stuff out in Q/A form:
So, how did they manage this now?
One problem with retrieving DNA is that it fragments into tiny pieces in fossils, and the older it gets, the tinier the pieces become. But scientists have made big advances in gathering tiny DNA. Some of this is pretty straightforward, like tweaking the recipe of chemicals used to pull DNA out of bone powder so that the smallest pieces of DNA don’t get washed away with impurities. It also involves finding clever new ways to compare fragments of DNA to sequences of related humans that have already been sequenced.
Did they get the whole genome of a 400,000-year-old human?
Far from it. They only got DNA from the mitochondria of the human in question. Quick mitochondria refresher: these are blobs in our cells that generate fuel for us. They carry their own genes because they were once free-living bacteria. Each cell has hundreds of mitochondria, each with a nearly identical collection of DNA. Sperm don’t pass mitochondria to eggs in fertilization, so we all get mitochondria from our moms.
Mitochondria is an easier place to look for ancient DNA than the nucleus, where we keep the vast majority of our DNA, because every cell has hundreds of copies of mitochondrial DNA and just one of the nuclear genome. The scientists got the entire mitochondrial genome from the human fossil. But that’s just 16,000 base pairs–tiny compared to the 3.2 billion in the nuclear genome.
What’s the family tree they got from comparing mitochondrial DNA from different kinds of humans?
Behold:

Meyer et al Nature 2013
To make sense of this tree, let me point out a few things.
1. The branch lengths show how many mutations have accumulated on each branch. Roughly speaking, the longer a branch, the more time has passed. (Only roughly, of course–all the Africans, Asians, and Europeans are alive today, despite their different branch lengths.)
2. Denisovans are known from a single cave in Siberia, where 80,000 year old fossils have preserved their genome. They have sometimes been called “eastern Neanderthals.” They interbred with ancestors of living humans–people now found in Australia and elsewhere.
3. Sima de los Huesos humans were believed to be Neanderthal forerunners because their fossils have Neanderthal features, like a somewhat beak-shaped nose and upper jaw, as well as peculiar spaces in the back of their lower jaw.
So…what’s going on?
Hard to say at the moment!
The simplest interpretation of this tree on its own would be that Sima de los Huesos people are an early offshoot of Denisovans. The problem with that is that they are older (400,000 years) than the estimated split between Neanderthals and Denisovans (300,000). Plus, if these Denisovans were spread across Spain to Siberia, you’d think we’d have known about them before the past couple years.
So now we get into the trickier ideas–the ideas that are based on the fact that genes can take funny courses through history.
1. Imagine that the ancestors of Neanderthals and Denisovans have many different mitochondrial DNA variants. That’s natural–we humans have many variants, too. As Neanderthals and Denisovans diverge, each population acquires some of those variants. After a while, some variants just disappear. That’s natural, too. If a woman has ten sons and no daughters, her mitochondrial DNA goes extinct. Perhaps at Sima de los Huesos, scientists have found one of those ancient variants that disappeared later in Neanderthal evolution, and which survived in Denisovans. So these people have Neanderthal-like bodies, and a little DNA that is Denisovan-like.
2. Or maybe the people of Sima de los Huesos are very different from what we thought. What if they belongs to another species–the enigmatic Homo erectus that I in the Times? Perhaps Denisovans mated with Homo erectus in East Asia and acquired their mitochondrial DNA.
There are a number of other possibilities.
How can scientists test these possibilities?
With more DNA!
From where?
From the same fossil they got the first batch from, for starters. Their methods may not let them recover a whole nuclear genome, because they’re dealing such tiny fragments. But even if they got a million bases of nuclear DNA, that would be great. They could draw a second family tree by comparing the 400,000 year old DNA to the corresponding segments in other humans and see if the tree looks the same. It might not (we’ve seen this mismatch happen before).
Then scientists could look at the other human skeletons at the same site for more DNA.
Does this usher in a whole new age of ancient human DNA studies?
I sure hope so, but it’s worth remembering that Sima de los Huesos is a unique place. Around 30 people were buried there–perhaps in an underground graveyard. In a deep shaft, their bones were maintained at a cool temperature and damp conditions for 400,000 years. That may be required for preserving ancient DNA for so long. But even if this become our only window into early human genetic evolution, we can still learn a lot.
Update:
Harkanwal Hothi asks: How far do you think we can go in terms of explaining the past with the help of DNA fossils? Obviously, this is only going to improve with technology, as it has since 1997. But what could be the absolute limitations for our understanding of human history?
We don’t know what the absolute limitations are going to be. Svante Paabo, the scientist who sequenced that first Neanderthal DNA–and who led the latest project–has actually stated on the record in the past that we can’t go far older than 100,000 years with ancient DNA. If Paabo can be wrong, then I don’t dare setting limits! Even if we don finally reach ancient DNA limits, we will still learn a lot, especially about how variations in genes flow down through lineages, and between them.
Samantha asks: Will this discovery change our views on Neanderthals in relation to the human evolution? (ie: Neanderthal’s are direct ancestors)
Not that I can see. The combined evidence from fossils and DNA suggests that Neanderthals, Denisovans, and Homo sapiens share an ancestor that lived in Africa about 500,000 years ago. Our ancestors stayed in Africa while the ancestors of Denisovans and Neanderthals moved out to Europe and Asia. Homo sapiens evolved in Africa about 200,000 years ago, and then humans expanded out of Africa 60,000 years ago, after which they interbred with Neanderthals and Denisovans. So, yes, many people on Earth today are have direct ancestors that were Neanderthals. Some have direct ancestors that were Denisovans. But in both cases, most of these people’s ancestors were Homo sapiens.
This new DNA (or, rather, this really old DNA) is from long before Neanderthals and Denisovans made contact with humans–but probably after their ancestors split from ours.
Mike Lewinski writes: I’m unsure about this point and think it would make a fascinating followup story some time:
“Sperm don’t pass mitochondria to eggs in fertilization, so we all get mitochondria from our moms.”
I’ve read in Nick Lane’s book “Oxygen: The Molecule that Made the World” that sperm probably do contribute some mitochondria, but that they’re quickly diluted out of existence because they’re old and worn out and can’t reproduce nearly as fast as maternal mitochondria (which in any case vastly outnumber them).
We do know women with mitochondrial diseases are infertile, suggesting that the paternal contribution is in any event too small and/or too damaged to sustain a growing fetus.
One benefit of this uniparental inheritance mechanism (however it is accomplished) is that the two mitochondrial lineages won’t be in conflict. Natural selection might drive their competition in ways that benefit them but are detrimental to us.
I’ve also read that they’re actively eliminated in the egg. In any case, mitochondrial DNA remains a way to track maternal inheritance.
Richard Jowsey asks: John Hawks: “The difference between Sima and Denisova [mtDNA] sequences is about as large as the difference between Neandertal and living human sequences. It would not be fair to say that Denisova and Sima represent a single population, any more than that Neandertals and living people do.” Splitter or lumper? Are Sima and Denisova different hominid “species”, but could still interbreed?
Scientists who study recent human evolution don’t like to talk firmly about species. Certainly, when I spoke to Svante Paabo and his colleagues about their discovery of the Denisovan girl, I asked them if she belonged to a new species. They wouldn’t settle on a yes or no. There’s just not enough information to really say. If reproductive barriers are how we define species, then they’re not separate species, because clearly we can interbreed. If a distinctive selective pressure on a population is our definition, then we don’t know enough about how Denisovans lived. We’ve just got a genome from a finger bone and some mitochondria DNA from a tooth. As for interbreeding, that’s made even trickier to answer because of time: the Sima people lived 400,000 years ago, and the Denisovans we know of lived 80,000 years ago.
Steve Ferry asks: Is it fair to say that early humans/middle Pleistocene hominids were far more mobile than we thought? That they did not have ‘ranges’, east or west Eurasia for a particular group or species, but instead a particular region would have had representatives from many groups. For example Denisova had three ‘species’ of human around, perhaps not at exactly the same time.
It would be interesting to see how much genetic diversity there is in the group from Sima de Los Huesos.
No, I don’t think that this argues for more mobility than we thought. There is a 320,000 year gap between the Denisova DNA in Siberia, and the Denisova-like DNA in Spain. It is true that Denisovans, Neanderthals, and humans all used the Denisova cave, but they may have used it thousands of years apart from each other. They weren’t subletting together.
Jay Stern asks: Are we now firm in the belief that mitochondria were once free-living organisms themselves that became symbionts to multi-cellular organisms? But if that is so, what would prevent more than one species of these organisms from being taken up by multicellular organisms? In other words, why couldn’t the mitochondria in a given cell be of multiple origins and possess different DNA themselves?
The evidence is very strong that bacteria became symbionts inside the cells of our ancestors–not in our animal ancestors, but in our single-celled protozoan ancestors a couple billion years ago. This is based on their presence in all eukaryotes, and the strong similarity of their genes to certain kinds of bacteria. It is interesting that it only happened once, no doubt. Maybe it could only happen in certain circumstances, and once it did, the mitochondria-fueled eukaryotes could outcompete any new rivals. On the other hand, many species of eukaryotes have taken up other bacteria as symbionts–most spectacularly, the algae ancestors of plants, which swallowed up photosynthesizing bacteria.
Kirk Maxey asks: I’m curious why you have placed modern Africans on the top branch of your modern humanity tree, closest to Neanderthals, when one of the clear facts emerging from this story is that Africans never interbred with them. On the other hand, Eurasians have a distinct (3-5%) crosslink to the Neanderthal line, and the Denisovans are even more richly interbred with them as well. To be accurate, shouldn’t you pivot your maize bar and place it above your royal blue bar? and then, add some dotted lines between all the branches except the African one?
The tree is from the paper published this week in Nature–it’s not mine. The tree shows the relationship of mitochondrial DNA in different humans only. Africans are not any closer related to Neanderthals in their mitochondrial DNA than Europeans or Asians. All living humans share the same common ancestor when it comes to mitochondrial DNA, a common ancestor that lived after the split of the lineage that led to Homo sapiens and the one leading to Neanderthals. The authors of the paper could have shown the same results by rotating the African+Eurasian branch 180 degrees so that the Africans appear at the bottom rather than the top. When it comes to mitochondrial DNA, Eurasians are NOT closer to Neanderthals than Africans. The few percent of DNA from Neanderthals and Denisovans lie on the nuclear genome.
Richard Jowsey returns to ask: Today I was trying to explain the nuances of ancient hominid “speciation” and “varieties” to a couple of smart 15 yr-olds, while discussing your article. Yikes! Science communication is hard!! Could you please explain what you mean by “distinctive selective pressure on a population” with respect to Homo erectus evolution, and our labelling of branches on the tree?
One feature of many species is that they can’t interbreed easily with other species. But that’s not an absolute rule. Scientists often find two sets of animals that look distinct, that have different ecological niches, have been reproductively isolated for the most part, and yet sometimes interbreed and produce viable hybrids. These may be distinct species, if you look at species as groups of organisms that belong a single lineage that are adapted to the same ecological niche. See, for example, this research on fish that share a lake. Unfortunately, paleoanthropologists can’t study ancient humans like living fish in a lake.
Lisa Strohlein asks: Dear Carl, I read your article with great interest. Maybe my questions are a bit naive but I wonder about the following to aspects:
1) How can scientists reconstruct the DNA sequence from all those tiny pieces being sure, that it matches the original sequence? Is it sufficient to compare them to related species?
2) Does the mitochondrial sequence say anything about the nuclear sequence? Would an analysis of nuclear sequences possibly lead to completely different results?
Not naive at all! These are the basic questions any science writer should ask scientists. They matter.
1) There are many steps to reconstructing DNA sequences from fragments. Here are a couple key ones. First is the sequencing of the fragments. Because there are so many copies of mitochondrial DNA in a single cell, scientists can get lots of fragments of mitochondrial DNA from a fossil (if they use the right chemistry). Some of those fragments will overlap. For example, you might find one fragment that runs from position #1 to position #50 in the fossil DNA sequence. Then you find another segment that runs from #25 to #75. A computer can line up those fragments based on the overlap. Then you can compare the sequence you build up to similar sequences from relatives–in this case, humans and Neanderthals and Denisovans. If the fragments have been assembled correctly, you’ll expect them to share long stretches of identical DNA.
2) The mitochondrial sequence does not say anything about the nuclear sequence–except that the two sequences had to end up in the same human. The nuclear sequence might very well produce a different tree. That’s what happened when scientists discovered the Denisovans. They started with the mitochondrial tree, which indicated Denisovans split off from the common ancestor of Neanderthals and humans (i.e., Neanderthals and humans are siblings, and Denisovans are cousins, as it were). But the nuclear tree showed Denisovans were “siblings” of Neanderthals. Both findings can be true, because each tree only shows the history of a particular chunk of DNA.
Bigfoot Party asks: What was the climate like in the area the fossils were recovered from, 400,000 years ago? Was there a land bridge at Gibraltar during this time? or was it closer to Africa? Did the Neandertal migrate through Suez, and these people cross at Gibraltar?
The climate was in the midst of the Ice Age cycles that have dominated the planet for over 2 million years. There definitely were humans in North Africa at the time (see this pdf for details). But there wasn’t a land bridge at Gilbraltar at the time. Unless humans were sailing at the time (unlikely), the evidence indicates that the common ancestors of Neanderthals and Denisovans expanded out through Sinai, as other human lineages did before and after. (See this pdf for more on this.)
–I can update this post with more Q & A’s if you leave them in the comments.
November 30, 2013
Genetic Secrets in Our Medical Records: My New Column for the New York Times
Many hospitals and medical practices are shifting from paper to computers, as they convert to electronic medical records. While the technology has been touted mainly as a way to cut costs and improve medical care, it turns out it has an unexpected side benefit: scientists can probe electronic medical records to find hidden connections between genes and diseases. This week in my “Matter” column for the New York Times, I look at this new tool for exploring to our DNA.
(Some readers have asked me about the issue of privacy and consent. When it comes to electronic medical records, these are very important issues–as illustrated by the shocking case of a Canadian woman who was barred by U.S. Homeland Security from entering the United States because they had looked at her medical records and found that she had been treated for depression. Ah yes, those depressed terrorists…Anyway, the researchers I write about, known as the eMERGE Network, do get consent for their research from patients and then go to great lengths to make the data anonymous. Details are here and here [pdf])
November 26, 2013
Same-Sex Mothers: Letting Albatrosses Be Albatrosses
There was a time when the Laysan albatross might seem a perfect icon for the virtues of marriage. When naturalists visited the bird’s nesting grounds in the Pacific, they’d find males and females bonded in pairs for life. Each breeding season the pairs of birds would nuzzle their heads together and perform other adorable courtship rituals. After they mated, the female would lay an egg. Both the male and female would take turns sitting on the nest to incubate it, taking three week shifts. After the chick hatched they’d rear it together until the end of the breeding season. The birds would then fly out to sea in different directions, but they’d return the following year and start up their partnerships all over again. The albatrosses would repeat this behavior for life–which, in their case, can last for many decades.
But then scientists realized they weren’t seeing the birds correctly. It turned out that sometimes a pair of albatrosses were both females. On the Hawaiian island of Oahu, for example, 31 percent of the pairs are same sex couples.

Two female Laysan albatrosses. Photo by Eric VanderWerf
Lindsay Young, a biologist who has been conducting a study on Laysan albatrosses for a decade, bristles when people try to use her research as a weapon in cultural debates. “‘Lesbian’ is a human term,’” she told the writer Jon Mooalem, who wrote a wonderful feature about same-sex animal couples for the New York Times Magazine in 2010. “The study is about albatross. The study is not about humans.”
I was reminded of Young’s words when I read her latest report on the birds, which appears today in the Proceedings of the Royal Society. Young and her colleague Eric VanderWerf analyzed ten years of field notes for every female Laysan albatross on Oahu–all 145 of them. In that data, they searched for an evolutionary explanation for why so many of the birds are in same-sex pairings. The answer they’ve reached is an intricate one–and it hinges on what it means to be an albatross, not a human.
Over the course of the study–from 2003 to 2012–Young and VanderWerf observed a number of same-sex pairs sticking together for years. Each breeding season, the females would find male albatrosses to mate with. Then they’d return to their own nest to lay their eggs. Like male-female pairs of albatrosses, they would take three-week shifts. But a pair of albatrosses can only incubate a single egg, and so when both females laid one, one of their eggs died. From year to year, it appears, the females alternate between which bird gets to lay an egg.
From an evolutionary point of view, Yong and VanderWerf found, the numbers are bleak for a female albatross with a female partner. At best, she’ll get to lay one egg every two years. That’s half the maximum rate that a female with a male partner can hope for. In the real world, however, all the birds fall far short of their upper limit. For one thing, albatrosses sometimes skip a breeding season. For another, many albatross chicks die in their first year.
But same-sex pairs have worse luck than male-female pairs, Young and VanderWerf’s research shows. An average female with a same-sex partner produces 80 percent fewer chicks a given year than one with a male partner. Over the ten years of the study, a female in a same-sex pair ended up with just one offspring on average. Her counterpart in a male-female pair ended up with 2.14 of them.

Two female albatrosses. Photo by Lindsay Young
There may be many reasons that same-sex albatrosses have fewer offspring. One may be that the females themselves are at a greater risk of dying. Young and VanderWerf point out that males take over the first three weeks of sitting on the nest after a female lays an egg. In same-sex pairs, the female that lays the egg goes straight into an incubation shift. Unable to return to sea to find food, she starves for three weeks and puts her health at risk.
Young and VanderWerf’s study is impressive for having stretched across a decade, but that’s just a small fraction of the fifty years during which a Laysan albatross can lay eggs. The differences that Young and VanderWerf have documented between same-sex and mixed sex pairs probably expand drastically over the entire lifetime of the birds.
The scientists also found that the albatrosses weren’t completely locked into their relationships. At the start of each new breeding season, a few females switched from a male partner to a female one. Likewise, females abandoned female partners for males. These switches illustrate just how much better male-female pairs do than same-sex ones. When a female bird switches from a female to a male partner, her productivity doubles.
What’s also striking about these switches is that they are far from random. Females that fail to produce a chick are three times more likely to end up with another female the next year than females that succeed.
Young and VanderWerf also found that females are much more likely to switch from females to males than from males to females. But they only switched to males if they had a successful breeding season the year before. Not a single female albatross that failed to reproduce one year went on to switch to a male partner the next year. (The full complexity of a female albatrosses’s life is mapped out in the figure at the bottom of this post.)
Young and VanderWerf sketch out an explanation for all of these different patterns–same-sex pairs included–that starts with one of the most important facts about albatrosses: they’re sharing the ocean with us.
Laysan albatrosses soar across the ocean for fish. Female and male albatrosses go their separate ways to go fishing, and the males appear to be more likely to go after the bait set out by long-line fishing boats. The deaths of male albatrosses may account for the fact that, at some albatross colonies, the majority of birds are females. At the site on Oahu where Young and VanderWerf do their research, for example, 60 percent of the birds are females.
In these places, there simply aren’t enough males to go around. A female will have her highest possible reproductive success if she can find a male partner, but there’s a fair chance that she won’t. If she can find a female partner, on the other hand, she may only be able to produce one chick in a decade. That’s pretty paltry compared to females with male partners. But it’s infinitely better than the zero chicks a female albatross will produce if she has no partner at all.
This scenario can also account for the behavior of the males. In many species, males compete intensely with each other for the chance to mate with females. They may also put on an elaborate courtship to gain a female’s favor. The females can then choose which males they will mate with. But this arrangement evolves because the females invest a lot of time rearing their young. At any moment in these species, there are a lot of available males and relatively few females.
Among the Layson albatrosses on Oahu, the situation is flipped. There are many females and relatively few males. The fact that females only switch to males after a successful year may be the result of males choosing among many potential mates. They’re keeping an eye on other albatrosses, and they prefer fertile females. The males also take advantage of the abundance of females by serving as sperm donors. By mating with same-sex female birds, they can have several offspring, while only putting in the effort to raise one chick.

From left to right: soda can, albatross egg, and chicken egg. From Albatrosses at Work: www.wfu.edu/biology/albatross/atwork/...
For albatrosses, same-sex partnerships aren’t a genetically hardwired adaptation implanted in the brains of a minority of birds. Instead, they’re just one part of a flexible set of behaviors that the birds can switch between to make the best of the situation they find themselves in.
What does any of this mean for same-sex marriage laws? I’ll only answer that question if you lay an egg the size of a soda can first.

Probabilities of different transitions in the life of a female Laysan albatross. From Young and VanderWerf
November 22, 2013
The Future of Fighting the Flu: My Feature in The Atlantic
A few weeks ago I went to my local drug story a got a flu vaccine. So far <knock on lab bench> I’ve had a pretty healthy flu season. But there’s a fair chance I may get the flu anyway this winter, because flu vaccine effectiveness is modest compared to vaccines for many other diseases. What’s more, I’ll need to head back next year to the store to get another shot. That’s because flu vaccines today are still based in some fundamental ways–in their production in chicken eggs and in the molecules they target on viruses–on World War II-era science.
I’ve written an article for the December issue of the Atlantic about how we got into this strange situation, and how scientists are trying to bring our fight against flu into the twenty-first century. Check it out.