Carl Zimmer's Blog, page 17
January 16, 2014
Forecasting the Future of Flu
Influenza strikes every year, but every flu season is rife with uncertainty. In other words, it’s a lot like the weather–important to our lives, and hard to predict. For my new “Matter” column for the New York Times, I take a look at how flu researchers are borrowing the tools of weather forecasting to look into the future–with increasing accuracy. Check it out.
January 13, 2014
How We Got On Land, Bone by Bone
Travel back far enough in your genealogy, and you will run into a fish.
Before about 370 million years ago, our ancestors were scaly creatures that lived in the sea, swimming with fins and using gills to get oxygen from the water. And then, over the course of millions of years, they began moving ashore, adapting to the terrestrial realm. They became tetrapods, a lineage that would eventually produce today’s amphibians, reptiles, birds, and mammals. As scientists have unearthed fossils from those early days, one lesson has come through ever more loud and clear: the transition was not a single leap. Instead, it was drawn out and piecemeal.
One of the most important of these fossils came to the world’s attention in 2006. Digging in the Arctic, a team of scientists found a 370-million-year-old creature they dubbed Tiktaalik. As I wrote at the time on the Loom, Tiktaalik belonged to a lineage of aquatic vertebrates called lobefins–a group that today includes lungfish and coelacanths. A number of anatomical features set lobefins apart from other fish, and show them to be more closely related to us and other tetrapods. They generally have stout fins that contain bones corresponding to the upper bones of our arms and legs. Some fossils of lobe fins don’t just have a bone corresponding to the humerus–the long bone attached to the shoulder–but the radius and ulna, too.

The front half of Tiktaalik. Photo © Ted Daeschler
But even among lobefins, Tiktaalik was remarkably tetrapod-like. It had a distinct neck, for example, and its fins had additional limb-like bones. Along with bones corresponding to a humerus, radius, and ulna, it even had wrist-like bones that functioned as a joint, as they do in our hands. Without digits, Tiktaalik couldn’t grasp a branch with its fins. But it could do a decent push-up in the muddy shallows that it called home in the Devonian Period. (Neil Shubin, one of the discoverers of Tiktaalik, told the creature’s story in his 2009 book Your Inner Fish.)
The bones that Shubin and his colleagues described in 2006 came from the front half of Tiktaalik. Only now, eight years later, have Shubin and his colleagues unveiled the other half of this remarkable beast. And they’ve now stretched out the transition from fish to tetrapod even more.
An eight-year delay is hardly unheard of in the world of paleontology. Unearthing and analyzing fossils is a very slow business. When Shubin and his colleagues first discovered Tiktaalik in the Arctic in 2004, they didn’t try to extract the bones then and there. Instead, they hacked out a three-foot wide hunk of rock that contained the fossils, which they would then bring back to the University of Chicago. There, they could carefully extract the fossils in the comfort of a lab.
But before they could put the rock on a helicopter to start the journey home, they had to protect it by covering it in plaster. Unfortunately, they hadn’t expected to end up with such a massive boulder. Its immense weight would require a thick plaster jacket, and they didn’t have enough plaster at their camp for the job. Instead, Shubin and his fellow paleontologists realized, they’d need to split the rock in two and wrap the two hunks in thinner jackets.
The two rocks made it safely back to Chicago. The scientists began work on the rock that contained the skull and other bones from the front half of Tiktaalik. By the time they were done, they had isolated bones from three different individuals in the rock. Once they had analyzed the bones and written up detailed descriptions of Tiktaalik’s anatomy, they turned their attention in 2008 to the other rock, which had been sitting untouched for four years.
Chipping away, they started to come across bones. Some were fin rays from the pelvic fin. Some were ribs from the back half of the animal. And nestled in the rock was an especially valuable bone: a pelvis.
It was not what Shubin and his colleagues were expecting. The closest lobe-fin relatives of tetrapods had tiny pelvises, which only served to attach muscles that controlled the pelvic fin during swimming. Tiktaalik had a massive pelvis–as big as those of the earliest true tetrapods with legs and digits. And like us, it also had a massive scoop carved out of the side, where the ball of the femur could fit.

Image courtesy of John Westlund, University of Chicago.
The discovery prompted Shubin and his colleagues to look back at the thousands of other fossil fragments they had found at the Tiktaalik site over the years, many of which remained puzzling to them. They compared the new Tiktaalik bone to those unclassified fossils and found that they had unwittingly found five other Tiktaalik pelvises. Until they knew what a Tiktaalik pelvis actually looked like, they didn’t know what they had.
All those hip bones have brought Tiktaalik into sharper focus. For one thing, they show that the creature could get big. The largest pelvis bones they’ve found suggest that Tiktaalik could grow up to nine feet long. Our ancient relatives, in other words, were the size of alligators.
Not only was its pelvis big, but its pelvic fin was big, too. Shubin and his colleagues envision Tiktaalik using massive muscles anchored to its pelvis to power its hind fins–not just to swim, but to walk underwater or push its way across muddy flats.

Adapted from image courtesy of John Westlund, University of Chicago.
While Tiktaalik had hips that were tetrapod-like in size, they were still fish-like in anatomy. Our own hips are tightly fused to our spine. It would be catastrophic for them to be floating free in our bodies, because we wouldn’t be able to hold up our torsos against the force of gravity, nor could we transmit much of the force generated by our legs to the rest of our body. That is true of most other tetrapods, all of which are adapted for moving on dry land rather than being supported by water. By 360 million years ago, early tetrapods had evolved attachments from the pelvis to the spine.
But their forerunner Tiktaalik still had free-floating hips. IN other words, Tiktaalik shows that 370 million years ago the tetrapod body plan was still very much a work in progress–from head to tail.
(For a pre-Titaalik history of this research, see my book At the Water’s Edge.)
Reference: Neil H. Shubina, Edward B. Daeschler, and Farish A. Jenkins, Jr., “Pelvic girdle and fin of Tiktaalik roseae,” Proceedings of the National Academy of Sciences. 2014. http://www.pnas.org/cgi/doi/10.1073/p...
January 12, 2014
Where Is This Place You Call Earth? (Science Ink Sunday)
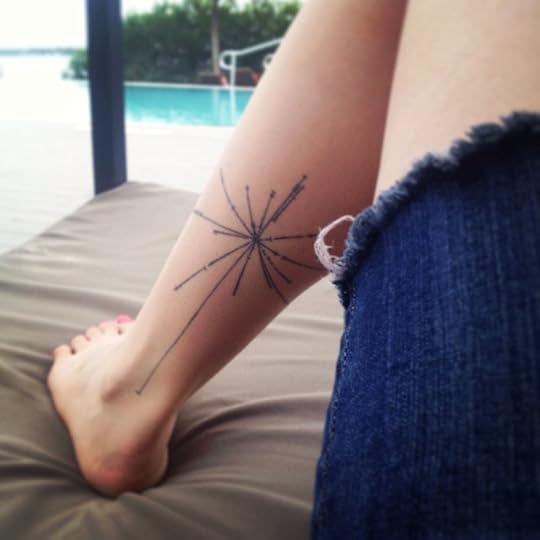 Writer Jaime Green writes, “Here is my contribution to the collection, my tattoo of the pulsar map from the Voyager golden record, tattooed by the awesome Joseph Ari Aloi. A high point of my life was getting to show it to Frank Drake.”
Writer Jaime Green writes, “Here is my contribution to the collection, my tattoo of the pulsar map from the Voyager golden record, tattooed by the awesome Joseph Ari Aloi. A high point of my life was getting to show it to Frank Drake.”
You can see the rest of the Science Tattoo Emporium here or in my book, Science Ink: Tattoos of the Science Obsessed. (The paperback edition comes out in May; you can pre-order here.)
January 9, 2014
After Seven Hundred Years, Crustaceans Rise Again to Show Us How We Steer Evolution
On Monday I wrote here about how scientists could retrace the history of evolutionary change in bacteria they raised in their lab by thawing out ancestors and comparing them to their descendants. That’s a much harder thing to pull off in the wild, but under the right conditions it can be done.
For my column this week in the New York Times, I write about a record-setting study of crustaceans that live in a Minnesota lake. Scientists hatched animals from eggs as old as 700 years that were buried in the mud at the bottom of the lake. And by comparing these resurrected water fleas over the centuries, they discovered a big evolutionary shift in the animals–which was probably caused by us. Check it out.
January 6, 2014
Evolution Hidden in Plain Sight
It’s hard to believe that Escherichia coli could have any secrets left.
For over a century, scientists have picked the microbe apart–sequencing its genes, cracking its genetic code, running experiments on its metabolism, earning Nobel Prizes off of it, and turning it into, arguably, the most-studied organism in history.
But as deep as scientists dive, they have yet to touch bottom. That’s in part because Escherichia coli is not fixed. It continues to evolve, and even in the most carefully controlled experiments, evolution leaves behind a complicated history.
Twenty-five years ago, Richard Lenski used a single microbe to seed twelve lines of bacteria. He fed each line a meager diet of glucose, and the bacteria have been adapting to this existence in his lab at Michigan State University ever since. (Here I’ve gathered together a few pieces I’ve written over the years about the 58,000-generations-and-counting Long-Term E. coli Evolution Experiment.)
In 2003, Lenski’s team realized that something utterly unexpected happened. One of the hallmarks of Escherichia coli as a species is that when there’s oxygen around, it can’t feed on a compound called citrate. But one day a flask turned cloudy with an explosion of E. coli that were doing just that. The change was so profound that it may mean these bacteria had evolved into a new species.
For the past 11 years, the scientists have been trying to figure out how the bacteria gained this ability to feed on citrate. Thankfully, Lenski decided at the outset of the experiment to freeze some of the evolving bacteria every 500 generations. As a result, he and his colleagues can resurrect ancestral microbes, sequence their genomes, and probe their biology for clues.
After sifting through the frozen history of citrate feeding for a couple years, the scientists discovered an important step in this evolution. It involves a gene called citT.
The citT gene encodes a protein that lets E. coli feed on citrate when oxygen levels get low. The protein sits in the microbe’s membrane and helps pull in citrate molecules from the environment. As it draws citrate in, however, it pumps another molecule–succinate–out. The pushing and pulling of these two molecules helps keep the chemistry of the cell in balance.
A small segment of DNA next to citT serves as a switch. If the microbe detects oxygen, a protein grabs onto the segment and shuts citT down. The microbe no longer feeds on citrate, instead feeding on better sources of energy, such as glucose.
The scientists found that around generation 31,500, a microbe that was copying its DNA in order to divide made a big mistake. It accidentally made an extra copy of a segment of DNA. That segment, it just so happened, contained citT. The microbe inserted the copy next to the original one, so that one of its daughter cells now had two copies of citT.
This sort of gene duplication happens from time to time in all living things. Human DNA regularly gets copied, too. And it can lead to important changes, because the two copies can start to do two different things. And that’s what happened to the E. coli. In Lenski’s experiment, the new copy of citT ended up near a new bit of DNA that controlled genes in a different way. Instead of shutting down genes in the presence of oxygen, it keeps them always switched on. Thanks to this mutation to citT, the bacteria could start feeding on citrate in Lenski’s oxygen-rich lab.
But the scientists found that this mutation was only one part of the story. The citT mutation allowed the bacteria to grow on citrate, but only slowly. Only after 1500 more generations had passed did the citrate-feeding bacteria begin growing quickly enough to dominate their flask.
During those 1500 generations, the scientists found, the bacteria made more copying mistakes, turning the new citT gene into four duplicates. Those extra copies enabled the bacteria to make more citrate-pulling proteins. But other mutations arose from generation 31,500 to 33,000, and the scientists had no way of knowing if they were important as well.
The story also turned out to have an earlier chapter. The scientists went back through the frozen archive to the very beginning and thawed out some microbial ancestors. They inserted the evolved citT genes into the ancestors, and found that the microbes could not feed on citrate. So the evolved citT gene alone was not enough to turn a microbe into a citrate-feeder.
The scientists did the same thing to bacteria from generation 20,000 and got a different result. When those more evolved bacteria got the citT gene, they could feed on citrate. Results like these suggested that early in the evolution of the bacteria, they picked up mutations that would later make it possible for the citT mutation to turn them into citrate feeders.
So, to recap: the scientists now had a story in three parts. Up to 31,500 generations, it was a story of groundwork mutations. Then came the big citT duplication. And after that came refining mutations, leading to world domination by generation 33,000. (The world, in this case, being a shot-glass-sized flask.)
In order to read this story in its full details, the scientists would need to understand the order by which every mutation arose, step by step. And they’d have to understand how each mutation helped produce a new kind of organism.
Despite the carefully controlled conditions of the experiment, this was a fiendishly hard problem. By the time the bacteria had evolved into full-strength citrate feeders at generation 33,000, they had acquired 79 mutations not found in their ancestor. Many of those mutations probably had nothing to do with citrate feeding. They may have helped the early bacteria grow better on glucose. Some might have had no effect on the bacteria one way or the other.
One of the scientists studying the citrate eaters was post-doctoral researcher Jeffrey Barrick. In 2011, he moved to the University of Texas to set up his own lab, and there he continued to study the citrate eaters, developing new methods to tease apart the evolutionary history of the citrate feeders.
He and his colleagues developed a new method of engineering bacteria in order to identify the mutations that were absolutely essential for full-blown citrate feeding. They combined portions of the citrate-feeding genome with that of the ancestral genome and then dropped these hybrids into dishes with only citrate to feed on.
Most starved to death. But a few grew. The scientists then plucked out the surviving hybrids and put parts of their DNA into other ancestral bacteria. Round after round of experimenting let them zero in on the essential segments for growing on citrate. Eventually, they could pinpoint the specific mutations.
Their results were weirdly few.
One result was no big surprise. Barrick and his colleagues found that in order to feed on citrate with maximal gusto, bacteria needed extra copies of the rewired citT genes.
But, as Barrick and his colleagues reported in a recent paper, they found just one other essential mutation.
This mutation affects a gene called dctA. When the scientists inserted the evolved versions of citT and dctA into an ancestral microbe, it became a full-blown citrate feeder. Neither gene on its own could achieve the same result. And no other genes were required for the metamorphosis.
This discovery prompted the scientists to look closely at the dctA gene. It encodes another membrane protein that’s responsible for pumping molecules in and out of the microbe. While citT pumps succinate out of the microbe, dctA pumps it in.
Barrick and his colleagues suspect that the evolution of a new kind of dctA gene allowed the bacteria to keep up a supply of succinate, which they needed on hand in order to feed on citrate. Together, the mutations to citT and dctA turned the mutant microbes into winners.
Which leaves the role of all the other mutations shrouded mystery. In the new study, none of the mutations that came before generation 31,500 proved to be vital for being a full-blown citrate feeder. They didn’t lay the groundwork in any essential way. And yet the previous research clearly indicated that things were afoot before generation 31,500.
Given the new results, Barrick and his colleagues have a few ideas for what was going on before then. It’s possible that some of the early, mysterious mutations were favored by natural selection because they helped the bacteria grow on their regular diet of glucose. And as a side effect, they helped build up a small supply of succinate. That succinate turned out to be a big benefit later on, when citT mutated. Now the bacteria had enough succinate (or some related molecule) to push out as it pulled citrate in. If the citT mutation had arisen before those mutations, the bacteria might not have been able to feed on citrate. And then later on, the dctA mutation arrived, kicking the citrate feeding into overdrive.
I contacted Lenski, who was not a co-author on Barrick’s new study, to see what he thought of the results. “I love the fact that this paper shows just how complex evolution can be,” he replied, “even for one little species in a tiny flask world for just a couple of decades.”
(For more on E. coli’s strange scientific history, see my book
Microcosm
.)
January 3, 2014
Does a Woolly Mammoth Need a Lawyer?
Shelley Estelle, Presidio Trust. via Flickr Click on image for link
In 2009, a biologist named Daniel Gluesenkamp was driving through San Francisco when he saw a ghost. Draped over a bluff by the side of the road was a twenty-foot wide shrub festooned with cream-colored flowers. Gluesenkamp immediately recognized the plant as Franciscan manzanita, or Arctostaphylos hookeri franciscana. He was astonished, because it had been considered extinct in the wild for decades. The last known wild Franciscan manzanita had been bulldozed in a graveyard in 1947.Before 1947, a few clippings of Franciscan manzanita had ended up in nurseries. Today you can buy the plant online. But the nursery form is the result of hybridization and extreme breeding; it’s now about as much like wild Franciscan manzanita as a German shepherd is like a wolf. It’s unlikely it could survive in the wild anymore. For thousands of years, wild Franciscan manzanita had grown luxuriantly in the prairies that carpeted much of the California coast. Now the wild plants were all gone–or almost, it turned out.
Before Glusenkamp’s discovery, the U.S. government officially listed Franciscan manzanita as extinct in the wild. But then three organizations–the Wild Equity Institute, Center for Biological Diversity, and California Native Plant Society–petitioned the U.S. government to change its status. In 2012, the Fish and Wildlife Service agreed to the request and reclassified Franciscan manzanita from extinct to endangered. Its known wild population was precisely one.
This wasn’t exactly the original plan for the Endangered Species Act when it was enacted in 1973. It was intended to protect species that were moving in the other direction, from healthy populations towards extinction. But once the single wild Franciscan manzanita plant came to light, the government decided that it deserved protection, too.
If that single surviving plant remained where it was, it would soon be destroyed in a highway renovation project. And so the plant was hoisted out of the ground and moved to a city park. Cuttings from the plant are now growing in nearby botanical gardens. The Fish and Wildlife Service has identified hundreds of acres in the San Francisco area as critical habitat for Franciscan manzanita. Eventually, the cuttings may be reintroduced into those fragments of its former range.
The Franciscan manzanita’s story isn’t just a tale of fortunate rescue. It may also become a legal precedent for how the government deals with a more science-fiction-like situation. As I wrote in National Geographic in April, scientists are actively working on ways to bring extinct species back to the planet. If the Franciscan manzanita can gain government protection, then what about passenger pigeons? What about mammoths? What would the legal status of a saber-toothed tiger be?
In the January 2014 issue of The Stanford Environmental Law Journal–normally a place where you can read sober articles on fracking regulations and forest health–three lawyers explore this prospect. Their article is entitled, “How to Permit Your Mammoth: Some Legal Implications of ‘De-Extinction.’” (The authors are led by Norman F. Carlin, who has a Ph.D. in evolutionary biology and now practices environmental law.)

Courtesy of Mike Tyler
How the government deals with de-extinction may depend on how scientists bring it about. At the moment, there are a few different methods on the drawing board. The most straightforward one–at least in the view of Carlin and his colleagues–would involve cloning. As fellow Phenom Ed Yong explained in March, Australian scientists are trying to bring revive a vanished species of frog by thawing frozen cells that were taken from the animals before their extinction in the early 1980s. The scientists are putting the extinct frog’s DNA into eggs from closely related species and coaxing the eggs to develop into embryos. If they can get the frogs to develop into adults, the animals could start reproducing and build a growing population.
Carlin and his colleagues think that a population brought back from oblivion this way would immediately deserve to go onto the list of endangered species. “The central purpose of the statute is to identify, protect, and promote the recovery of precisely those species facing the greatest risk,” they write. “None has faced greater risk than a species that actually has gone extinct.”
But in order for a revived species to survive, it will need help. You couldn’t just toss a few tadpoles down the nearest sewer grate and expect them to handle things from there. They’d need protected land, safeguards to defend them against devastating diseases, and perhaps a long-term captive breeding program to deliver enough new animals to get the population off the ground. Otherwise, a revived species could quickly suffer an unprecedented fate: a second extinction.
In theory, a species could gain this official protection as soon as scientists produced a single individual. A single individual may not seem to warrant being called a species. After all, a healthy species is made up of thousands of individuals and has lots of genetic variation. But if the Franciscan manzanita serves as an example, Carlin and his colleagues argue, the government could grant protection to a single individual.
Even if a revived species did gain official protection, however, it may not necessarily see the outside of a laboratory. The government would need to judge the risk of releasing it into the wild. This would not involve a government official played by Jeff Goldblum standing up and shouting, “Hasn’t anyone seen Jurassic Park?” The serious risks that de-extinction might pose would be for other animals and plants, not people.
Carlin and his colleagues suggest that regulators could follow the example of the Florida panther. When conservation biologists set out to save the dwindling population of panthers in Florida, they decided to bring panthers from Texas to increase their genetic diversity. Before the biologists could carry out this plan, though, the government first had to determine the risk of such an introduction. Would the Texas cats bring a disease that would kill the ones in Florida? The answer, regulators decided, was no. The Texas panthers were delivered, and they’ve been a boon to the Florida population.

Passenger pigeon. Image from Wikipedia.
In some cases, in other words, the existing rules would be good enough for the government to make decisions about de-extinction. But their decisions will get tougher if species are revived in less direct ways than cloning. Nobody froze a passenger pigeon before the species became extinct in 1914, for example, and so Ben Nowak of the University of California at Santa Cruz and his colleagues have proposed reverse-engineering a closely related pigeon species instead.
The first steps in this process are already underway. Nowak and his colleagues are gathering fragments of DNA from preserved museum specimens and combining their sequences to reconstruct much of the passenger pigeon genome sequence. They can then compare its genome to those of close relatives, like the band-tail pigeon, to get a sense of how the passenger pigeon evolved to become so distinctive.
Even if Nowak and his colleagues created a perfect copy of the passenger pigeon genome, it wouldn’t be possible to synthesize a matching DNA molecule. Technology today only offers the possibility of synthesizing segments of DNA and inserting them into the genome of a related bird. If scientists identified the key segments that made passenger pigeons distinct from other species, they could, theoretically, alter a bird like a band-tailed pigeon so that it became in effective passenger pigeon.

http://www.pinterest.com/pin/23622794...
Carlin and his colleagues question whether such “facsimiles,” as they call the hypothetical creatures, deserve to be called passenger pigeons. If genetic authenticity is required, then they may not make the grade. In fact, government regulators might look at them instead as genetically modified organisms–a banded pigeon engineered with passenger-pigeon qualities. If that’s how things turn out, then a different set of laws will come into play–ones that are used to evaluate everything from bacteria that churn out human insulin to zebrafish that glow with jellyfish genes.
The U.S. government can set different levels of control for a genetically modified organism. It may be intensely regulated, for example, or the government may decide that there’s no risk to the environment if it gets into the wild. Today, for example, the U.S. Department of Agriculture released a draft environmental impact statement for genetically modified seeds from Dow AgroSciences. Part of their Enlist Weed Control System, these corn and soybean plants are engineered to withstand a weed-killer called 2,4-D. Today’s report could open the way to deregulating the seeds, which would lead to their commercial development. Farmers could plant them without worrying about keeping the plants from spreading in the environment. Carlin and his colleagues suggest that the same process could be adapted to evaluate de-extinction facsimiles.

Carolina parakeet. Wikipedia
There’s another way in which Enlist seeds could be relevant to de-extinction: they’re patented. An environmental group that wants to bring back an extinct sea cow to the Pacific may not have any interest in patenting the animal. But there might well be corporations that get interested in doing so. Carlin and his colleagues suggest, for example, that bird-lovers might pay top dollar for their own Carolina parakeet, a gorgeous bird that became extinct by the 1920s. Two prominent advocates have already formed a de-extinction company, called Ark Corporation that might use technology developed for bringing back extinct species to devise new livestock breeds.
Can you patent a mammoth? Carlin and his colleagues find this to be a very tricky question, thanks to the trickiness of the patent system. Congress has stated that “anything under the sun that is made by man” should be patentable. That statement has a nice poetic ring, but it leaves us to decide what “made” means. The Supreme Court has ruled that genetically engineered organisms can be patented if they have “markedly different characteristics from any found in nature.” If a company makes woolly mammoths by reverse engineering an Asian elephant, they might be able to argue that it’s so unnatural that it can be patented. But if scientists found an exquisitely preserved frozen mammoth egg and reared it into a full-grown mammoth (dream with me here, people), then it might not be patentable. It would by revived by man, not made by man.
While Carlin and his colleagues delve into fascinating detail about the legal future of de-extinction, they make it clear from the outset that they’re not dealing with the ethics of it. As intriguing as de-extinction can be in theory, critics have attacked it as a foolish distraction from the true horrors of extinction going on right now around the world. (I’d recommend reading this strongly critical post by fellow Phenom Brian Switek.) These critics may find a discussion of the laws governing de-extinction to be an equally pointless distraction. But just because they don’t like de-extinction doesn’t mean that someone else won’t try it. And it’s those conflicts that laws are supposed to sort out in a democracy. “How to Permit Your Mammoth” is a first step towards preparing for the conflicts that de-extinction may well bring.
January 2, 2014
What Doesn’t Kill You Makes You Speciate: My First New York Times Column of 2014
Insects and tropical plants are locked in an endless battle, featuring daggers, ant mercenaries, and chemical weapons. But as ugly as the fight can become, there may be a lovely consequence. Some researchers argue that the pests in the tropics play a major role in fostering the overwhelming diversity of the rain forest. For more details, check out my “Matter” column this week in the New York Times.
(Thanks to Elizabeth Kolbert for the headline!)
December 30, 2013
The Scientists We Lost in 2013
A couple weeks ago, All Things Considered asked me to talk about the deaths in 2013 of three Nobel-prize winning scientists: Francois Jacob, Frederick Sanger, and David Hubel. I had blogged about Jacob’s death in April, and reflecting on his career in conjunction with those of Sanger and Hubel was a thought-provoking experience. In some ways, these three scientists seemed worlds apart–Jacob poring over bacteria feeding on sugar, Sanger tearing apart insulin molecules, and Hubel using electrodes to eavesdrop on neurons in the brains of cats.
But what unites them all, I think, was their ability to use the very simple scientific tools available to scientists in the 1950s to open up vast realms of biological complexity–from the orchestral activity of the genome to the reality-building network of cells in our brains.
Here’s the story that NPR producer Rebecca Hersher put together for last night’s show. I’ve embedded it below:
Of course, there would have been plenty to say about many other troikas of scientists who passed away this year. On Twitter, ecologist Jacquelyn Gill reminded me of the pioneering ecologist Ruth Patrick, for example. Neuroscientist John Kubie pointed me to his homage to Robert Muller, who did ground-breaking work on memory. The Scientist has a longer list on their blog. While we mourn their loss, science preserves their memory in the research that goes on today, made possible by their earlier work.
December 27, 2013
Untethering the Brain
For my new ”Matter” column for the New York Times, I take a look at a new idea to explain that mystery between our ears. Our brains are enormous for our body size, and our minds are capable of extraordinary feats of cognition. Two neuroscientists have offered up a hypothesis that links these two facts, suggesting how an increase in brain size could have led to a change in how the brain is networked. Check it out.
You may also want to check out P.Z. Myers’s critique of the “tether hypothesis” on his blog Pharyngula. He raises some important questions about the idea, based on his own experiences as a neuroscientist. I’m puzzled, though, why he decided to kick it off with this swipe at me:
What does it take to get Carl Zimmer to review your research in the New York Times?
I suppose it helps to be at Harvard. It also helps to have a combination of subjects — evolution and the human brain — that Zimmer has written about in the past. It helps to have a paper with lots of very pretty diagrams — the authors’ hypothesis is professionally illustrated. It’s also a good idea to have a vast sweeping explanation for the exceptionalism of the human brain…You know what you don’t need? Data, or a hypothesis that makes sense.
I had no idea that Harvard had such a power over my feeble powers of judgment. Or that I am so vulnerable to pretty pictures.
What I thought happened was this: the tether hypothesis comes from Randy Buckner and one of his postdoctoral researchers, Fenna Krienen. I was long familiar with their work on mapping human brain networks, having visited them a few years ago when I wrote a story about the aging brain. Buckner was new to Harvard when I visited him, having made a name for himself beforehand at Washington University–which mysteriously failed to prejudice me against him.
After my visit, Buckner and his colleagues went on to do other important studies on the structure of the human brain, which they published in leading neuroscience journals. When I saw Buckner and Krienen’s new paper in the journal Trends in Cognitive Sciences, I did not, in fact, say, “Ooh, pretty pictures, ooh Harvard!” I said, “Scientists with a proven track record expanding their work on human brains to a comparison to other species. Interesting.”
Since I’m a journalist and not a neuroscientist, I also contacted outside experts. For example, I contacted Chet Sherwood of George Washington University. Now, I suppose Myers would think I’d be scared away because Sherwood isn’t at Harvard, but I actually am capable of recognizing that he’s an expert on mammal brain evolution who is familiar with the tether hypothesis–and therefore someone whose opinion should matter to me.
It turned out, as I mention in my article, that Sherwood found the tether hypothesis to be an exciting idea. He is intrigued by how it can potentially explain a lot about the anatomy and function of the human brain in a relatively simple way. That’s the sort of comment that makes me think that a paper would make for an interesting column.
It doesn’t surprise me that another scientist–in this case, Myers–disagrees. That’s how science works; recognizing that, I’ve included plenty of critics in my articles over the years. What does surprise me is that Myers would use a scientific critique to impugn my capacity as a journalist.
December 20, 2013
“The Dark Matter of Psychiatric Genetics”
The title of my blog post is provocative, I know, but I’m actually just lifting it from the title of a new commentary in the journal Molecular Psychiatry by Thomas Insel, the director of the National Institutes of Mental Health. In his piece, Insel expresses his excitement about a new way of thinking about how genes can contribute to our risk of psychiatric disorders such as schizophrenia. It’s based on an emerging understanding of the human genome that I explored in a recent story for the New York Times: each of us does not carry around a single personal genome, but many personal genomes.
When we start out as a single fertilized egg, we have a single genome. When the cell divides in two, there’s a tiny chance that any spot in the DNA will mutate. Over many divisions, the copies of that original genome accumulate mutations and become different from one another. Scientists only now have the tools to dig into this so-called mosaicism and see how different our genomes can become.
Scientists have long known that mosaicism can be important for cancer, but it’s only recently that experts on other diseases have thought about it. Insel clearly has turned his mind in its direction. As he notes in his commentary, a number of studies have implicated genes in the risk of conditions such as autism. But the picture is still murky, as reflected by the fact that among identical twins, it’s often the case that one sibling will develop a mental disorder and the other will not.
Part of the solution to this mystery, he suggests, is that the brain is a mosaic.
“The brain’s genome or more accurately genomes, may prove to be even stranger than we have imagined,” Insel writes.
What might be happening is this: when embryos are developing, the neurons of the brain are growing and dividing. A neuron may acquire a mutation, which it then passes down to daughter neurons. That new mutation alters how those neurons work and makes a person prone to developing a particular mental disorder. But you wouldn’t know that this mutation is playing a role if you just took a cheek swab from a patient and sequenced the DNA from the cells you retrieved. The mutation you need to see is locked away in the brain.
Scientists have already linked these late-arising mutations to a few brain disorders. One is hemimegalencephaly, in which one side of the brain becomes bigger than the other. Even though only a few percent of the neurons in the brain carry the mutation, they can still trigger large-scale changes to half of the brain. Some disorders seem to require a one-two punch, in which a child inherits a mutation from a parent, and then a new mutation arises on top of that in the brain.
Insel suspects that some mental disorders may have a similar origin. For example, males are more likely than females to develop most neurodevelopmental disorders. That may be because they’re especially vulnerable to late-arising mutations. While females have two X chromosomes, males have only one, the second X being replaced by a Y. If a mutation arises on the X chromosome as a male embryo develops, there isn’t a healthy back-up on another X chromosome to compensate.
As promising as this line of research may be, however, it won’t be easy to search for the brain’s mosaic. Cheek swabs won’t do. Scientists will need to look at individual neurons in the brain. As Insel notes, technology for probing single cells is improving enormously. But there’s still a needle-in-the-haystack quality to such a search. And the raw material for this kind of search is hard to come by. You can’t grab a few neurons from a living person with the ease that you can get cheek cells. You need autopsied brains donated to science.
So it’s unlikely that doctors would actually run a brain mutation test on patients to search for this mosaicism. Instead, understanding the mosaic brain could offer a more general insight: by identifying the late-arising mutations that lead to mental disorders, scientists will better understand their biology. And that knowledge could, some day, lead to better treatments.

![Shelley Estelle, Presidio Trust . via Flickr [Click on image for link]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1388889830i/7950291.jpg)


