Carl Zimmer's Blog, page 15
March 12, 2014
Seeing the Ocean With A Buzzing Nose
The challenge–and the pleasure–that evolutionary biologists face in their work is deciphering the history of nature, no matter how weird it gets. And nature doesn’t get much weirder than a beluga whale singing through its nose to see the ocean.
Ordinary vision doesn’t work as well in the ocean as it does on land, thanks to the way light travels through water. Sound, on the other hand, travels over four times faster through water than air. Belugas and other toothed whales (such as orcas and sperm whales) take advantage of its underwater speed by using echolocation.

Killer whales. Wikipedia/Robert Pitman en.wikipedia.org/wiki/File:Killerwhal...
The process by which they generate echoes is a complicated one. As mammals, toothed whales have to breathe air into their lungs. Other mammals can breathe through their mouth, or their nose, which sits right on top of it. In toothed whales, the nasal passage runs up above its eyes, creating a blowhole on top of its head. When it surfaces, it opens the blowhole to breathe. But underwater, toothed whales can use the air in their nasal passage to vibrate sets surrounding muscles. They keep their blowhole shut as they push this air around inside their heads, using a series of chambers to store and recycle it.
The vibrations are then guided by the bizarre anatomy of a toothed whale’s head. Behind the vibrating muscles, the skull rises to a ridge, preventing the sound from moving backwards. In front of the muscles is a large blob of fatty tissue, called the melon. It sits on top of the large, shelf-shaped upper jaw of the whale. As the vibrations pass through the melon, they become focused. The whale also has massive muscles anchored to the sides of its upper jaw and surrounding bones that let the animals squeeze the melon into different shapes–and thus direct the beam of sound in different directions.

Echolocation in dolphin head. Wikipedia
When the sound waves hit something in front of the whale–a coral reef, a fish, or some other object–some of them bounce back towards the whale. The whale boosts its hearing with its lower jaw, which contains a long cylindrical piece of fat running down each side. Vibrations that hit the jaw travel back to its ear, which can detect the sound. Studying dolphins in captivity, scientists have shown that they can recognize complicated shapes based on their echoes alone. They can even sense the texture with sound.
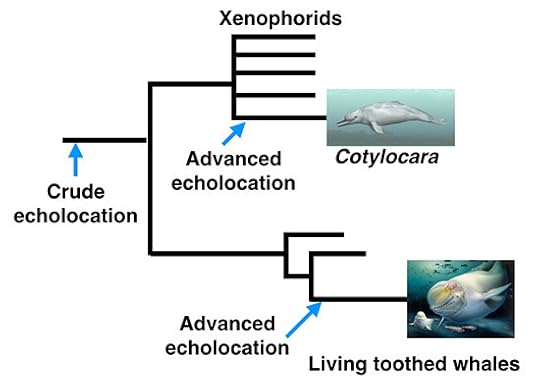
How did dolphins and other toothed whales get to this strange state? Fossils and evidence from DNA have helped scientists figure out how they evolved from land mammals. It’s a topic I first took up in my book At the Water’s Edge, and which I’ve been trying to keep up with ever since. Here’s a tree joining toothed whales to a selection of their living and extinct relatives. It comes from the recently-published second edition of my book The Tangled Bank. (You can see a bigger version here.)
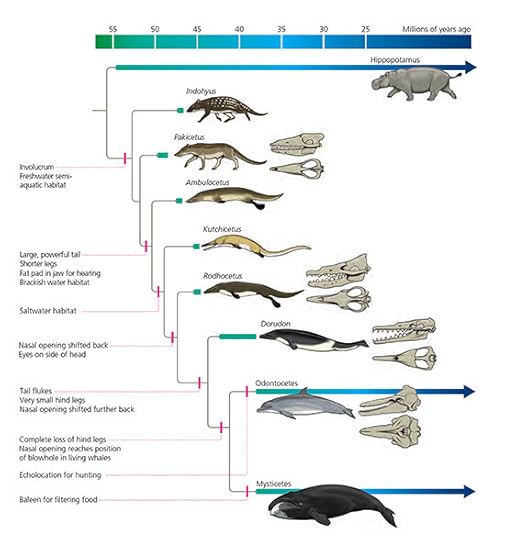
From The Tangled Bank (Second Edition) by Carl Zimmer
Whales evolved from land mammals, sharing a close common ancestor with hippos. Starting about 50 million years ago, they gradually lost their limbs, evolving a body dedicated to swimming rather than walking. Many lineages of whales evolved and thrived and eventually became extinct. All living whales descend from two lineages that split from each other about 40 million years ago, known as baleen whales and toothed whales.
While the back end of whales provide the most dramatic evidence of their evolution–turning from legs and a thin tail to flippers and a flukes–it’s the front end where much of the most essential adaptation took place. Whales needed to use their heads to sense their underwater world and to grab food from it. Early whales evolved long toothy snouts to catch prey. But then baleen whales and toothed whales evolved two different updates on that anatomy.
Baleen whales lost their teeth (although they still have broken genes for making teeth today). In place of teeth, they evolved fronds of baleen they could use to filter food from giant gulps of water they engulfed. As I’ve written on the Loom, paleontologists are finding fossils of early baleen whales that bridge the transition from a hunter to a filter feeder.
Toothed whales continued to catch prey individual prey, the way ancient whales had done before. But at some point they added on their extraordinary echolocation equipment–the head reflector, the air recycling chambers, the buzzing lips, the melon, and the rest.
Charting the evolution of echolocation has been tough because so much of this anatomy rots. That is, once a dolphin dies, its melon decomposes, along with its lips, muscles, and other organs essential for making sounds. All that scientists have to go on when they look at a toothed whale fossil is the skull itself. While scientists have been finding toothed whale fossils for a century, none of the older ones display many of the traits that you’d expect from an echolocator. Scientists have thus been left to wonder how long after the split between baleen whales and toothed whales this marvelous acoustic equipment evolved.
Today in Nature, Jonathan Geisler of the New York Institute of Technology College of Osteopathic Medicine and his colleagues offer details of a new fossil of a toothed whale, dating back 28 million years ago. It will go a long way to answer our questions about echolocation.

The skull of the 28-million-year-old Cotylocara macei. Credit: James Carew and Mitchell Colgan
The skull of the whale, dubbed Cotylocara macei, was found in a drainage ditch in South Carolina. It’s got lots of features that are found only in toothed whales, showing that it belongs to their lineage. And it also has a lot of traits that toothed whales use for echolocation. The hole where the nasal passage leaves the skull, for example, is surrounded by flanges that could control buzzing lips. The skull has cavities around the nasal opening that look like the chambers dolphins and other living toothed whales use to recycle air. The jaw bones are dense, perhaps allowing them to reflect sound waves into the melon. Its upper jaw forms a broad shelf, which would allow Cotylocara to anchor muscles for controlling its melon.
Taken together, Geisler and his colleagues write, these traits “make a compelling case that Cotylocara could echolocate.”

Cotylocara macei, reconstruction by Carl Buell
What makes Cotylocara even more interesting is how it’s related to dolphins and other living toothed whales. Short answer: not closely at all.
When the ancestors of toothed whales split from the ancestors of baleen whales, they then branched into new lineages. One of the earliest splits led to two branches. One branch eventually led to living toothed whales. The other branch led to a group of toothed whales, called xenophorids, which are all long extinct. Cotylocara belongs to that extinct xenophorid branch.
Scientists have dug up a number of other fossils of xenophorids in the past. If you look at any one species, it may have a few of the traits that we associate with echolocation, such as a broad upper jaw or cavities in its skull. But none of them come close to the new fossil, Cotylocara.
Yet the anatomy that Cotylocara might have used to echolocate is not identical to that of living toothed whales. Some of its cavities are located in places in the skull where you won’t find any in a dolphin, for example. It looks as if it evolved these traits on its own.
The explanation that Geisler and his colleagues favor is that echolocation had a complicated history. The ancestor of all toothed whales–living and extinct–had already evolved a crude sort of echolocation. Its descendants then branched off into new lineages. In at least two of those lineages, toothed whales evolved much more sophisticated muscles, bones, and various organs, giving them more control over the signals they sent out. While Cotylocara evolved some traits that were very much its own, it also evolved some of the same traits found in living toothed whales, such as dolphins. (This kind of evolution in parallel, which I’ve illustrated below, is called convergence.)
Geisler and his colleagues already know how they can test this hypothesis. If they’re right, then early toothed whales didn’t just have sophisticated anatomy for making sounds. They must have also had ears that were able to detect them. Cotylocara’s skull doesn’t preserve its ear region, so we can’t know yet how well it could hear. But Geisler and his colleagues predict that if paleontologists examine the ears of other early toothed whales, they should find signs that the animals were somewhat more adapted to hearing high-frequency echoes.
Not only does Cotylocara answer some questions we have about this weird bit of nature, but it tells us what new questions to ask in turn.
[Update: paper link fixed.]
March 10, 2014
Please Welcome Nadia Drake, the Newest Member of Phenomena
 Fifteen months ago, Virginia Hughes, Brian Switek, Ed Yong, and I joined National Geographic to form Phenomena. I’m delighted that our circle is now expanding. Starting today, science writer Nadia Drake will be writing “No Place Like Home.” I’ve followed Nadia’s work for the past couple years, but I’ve never had the chance to talk to her. To celebrate her debut, I asked her some questions about her past and future.
Fifteen months ago, Virginia Hughes, Brian Switek, Ed Yong, and I joined National Geographic to form Phenomena. I’m delighted that our circle is now expanding. Starting today, science writer Nadia Drake will be writing “No Place Like Home.” I’ve followed Nadia’s work for the past couple years, but I’ve never had the chance to talk to her. To celebrate her debut, I asked her some questions about her past and future.
Your father is Frank Drake, of the famed Drake Equation. What was it like growing up with a dad spending so much time thinking about life in the universe?
Grand cosmic questions loomed large in our home, in a good way. The walls were filled with astronomy related artwork, the shelves stuffed books about the stars; we have a rendering of the Pioneer plaque by the door, and a stained glass window depicting the Arecibo message. There’s a chunk of the meteorite that created Meteor Crater in Arizona sitting on the mantle. My parents used to host observing nights for my elementary school classes – in the backyard — and my sister and I would go with my dad’s college classes to look through the big telescopes at the Lick Observatory.
I learned a lot of astronomy by diffusion. Following dad to lectures or observatories, and tagging along to meetings overseas meant meeting a lot of very thoughtful scientists.
Dad is also hilarious, and exceedingly humble. We rarely knew when he was going to be on TV and often learned about it the next day from classmates and friends. But more than that, I learned by example that it’s OK to be interested in, and fascinated by, a variety of questions. It’s OK to cast a wide intellectual net. Our house wasn’t just filled by astronomy – my dad’s orchids and his wine-making and other projects were just as visible.
What was the path you took to becoming a science writer?
I took the long way. Started out planning on a professional dance career, then made a left turn and switched to academics when it was time for college. Later, that road would turn back on itself and I would end up dancing professionally after all, which was a pleasant surprise and one of the most fulfilling (and hardest) jobs I’ve had.
Along the way, I seriously considered law school, but ended up ditching those plans and working in a clinical genetics lab at Johns Hopkins Medical School, looking for abnormalities in fetal chromosomes. After that, I went to graduate school at Cornell University, where I worked in an epigenetics lab and studied a gene that’s imprinted in neonatal mouse brain — in other words, copies of the gene are either turned on or off, depending on whether they were inherited from the father or the mother.
It was only after I finished my PhD that I finally returned home to Santa Cruz and enrolled in the Science Communication program at UCSC. That was one of the best decisions I’ve made. Since then, the road has been much straighter and the trip much faster, and I’m grateful for the opportunities I’ve had in the (nearly) three years since I’ve been at UCSC.
Before coming to Phenomena, where have you been writing, and what have you been writing about?
My first reporting job was as the astronomy reporter at Science News, based in Washington, D.C. When I moved back to California, I started writing for WIRED, where I report on the life and materials sciences – from giant spiders through marine mammals to materials that change color when they stretch. I’m also working on astronomy features for the news section of the Proceedings of the National Academy of Sciences. While at UCSC, I interned at Nature and wrote about everything from human ancestors to rogue planets, and also spent two quarters interning at Bay Area newspapers, which I loved.
I’m really looking forward to getting back on the astronomy news beat at Phenomena!
What can we expect from the blog?
A thoughtful exploration of the science probing everything that isn’t on planet Earth. I’m aiming for a mix of stories – many about recently published research, but also some excursions into astro history, perhaps some profiles of scientists, some obsession-driven posts. And lots of great photos. Maybe I’ll even open up the Frank Drake archives from time to time. As I get going, I’d be interested in hearing feedback from readers. Which stories or topics are the most satisfying?
Blogging is an excuse for writers to put their obsessions on public display. What obsesses you?
Above all, words. Using language to express ideas that are slippery, to describe something intangible or relay a visceral experience, in a way that leaps off pages or screens – it’s like assembling a jigsaw puzzle. Words are powerful and meant to be used properly.
My other obsessions tend to be fairly transient in duration – I’ll plunge into a subject or idea for a short but utterly immersive period, then slip quietly out and move on to the next. That said, some obsessions do recur. In astronomy? Iapetus, a cranky, bizarre moon of Saturn. Type 1a supernovas – what in the world is going on with those? Ancient observatories, those sites where scientists and philosophers convened to observe the skies. And of course, exoplanets. Also exomoons. For some reason, I really, really love the idea of exomoons.
In life? Ballet. Champagne. I love a good glass of bubbly more than just about anything.
(What are your obsessions, Carl?) [Ed. note: These days, oxygen, for some reason.]
You’ve written about some strange science—what’s the weirdest thing you’ve written about so far?
This question made me laugh. The jungle spiders that build spider-shaped decoys in their webs are definitely bizarre. But using a sky crane to lower a giant robot onto another planet? Totally nuts.
March 6, 2014
The Information Parasites
Parasites can take many forms. Just this week, I’ve written about a giant virus that reproduces inside amoebae (and has survived being frozen 30,000 years in permafrost), along with a wasp that performs brain surgery to zombify hosts for its young. Viruses and wasps are radically different organisms–some would say that viruses don’t even deserve the label of organism. And they make use of their hosts in different ways. The virus sits inside a cell, manipulates its biochemistry to build virus proteins and DNA. The wasp, on the other hand, sips fluids inside a still-living roach, and builds its own proteins and DNA–and then becomes a free-living creature that can climb out of its host and fly away.
So why are they both parasites? The answer lies beyond the details of anatomy and molecules. It’s all about relationships.
Species can have all sorts of influences on each other. They can eat or be eaten, they can pollinate or steal pollen. But there’s one yardstick that scientists can use to measure all the variety in these interactions: the change that one species has on how many offspring the other can have. By that measurement, the differences between giant viruses and brain-surgeon wasps melt away. Each one is a disaster for its partner species. The viruses multiply inside amoebae until they burst. The roach lives until its wasp parasite is ready to depart. In each case, the relationship is good for the parasite (more offspring) and bad for the host (fewer).
When scientists look at life with this definition in mind, they can see a lot of parasites that might not look like parasites. We don’t think of birds as parasites–they’re too beautiful and not in the least bit creepy. But when a cuckoo pushes out the eggs of a reed warbler and puts her own in their place, and when the cuckoo chicks use all sorts of tricks to fool the reed warbler to feed them as if they were its own, we are seeing another parasite at work.
In the journal BMC Evolutionary Biology, a team of scientists in Finland describe another kind of parasite–one that doesn’t steal food or protein synthesis or even parental care. In the words of the scientists, these are “information parasites.”

Top: Great tit. Bottom: Pied flycatcher. Flickr: http://flic.kr/p/7o6KjK http://flic.kr/p/dGnDRc
These information parasites are, once again, birds. Lovely birds, in fact, known as pied flycatchers. And their victims are another species of bird, the great tit (twelve-year-olds at heart are allowed a few moments to get sniggers out of their system).
The pied flycatchers and great tits, both found across much of Europe, have evolved to the point where their existence is quite similar. They eat a lot of the same kinds of food, get killed by the same predators, and even choose the same sites for their nests. This similarity leads to a fair amount of competition, sometimes quite violent. If a bird from one species flies into a crevice to check out a potential nest spot, only to find the other species there, the two birds will fight–sometimes to the death.
The two species aren’t identical, though, and there a couple differences that are particularly intriguing.The great tits build their nests earlier in the year, and the pied flycatchers have a habit of paying visits to great tit nests before building their own.
In recent years, the Finnish researchers have found a likely reason for these visits. The pied flycatchers are gathering intel. They inspect the nests of great tits to help them decide where they will make their own nests. One piece of information they’re interested in is the number of eggs are in a great tit’s nest. If a nest is loaded with eggs, it’s probably a good place for a pied flycatcher to make its own nearby.
The great tit suffers for letting the pied flycatcher get this information. Now a rival bird sets up house on the same territory and starts to compete for the same food. The researchers have found that great tits that attract these neighbors end up with fewer nestlings as a result. The pied flycatchers, on the other hand, have more success in reproducing because they build their nests on good real estate. One species benefits, and one suffers. But the benefit doesn’t come from cockroach innards or cell proteins. The pied flycatcher is stealing information.
Once parasites evolve a strategy for taking advantage of a host, the host generally evolves defenses. Immune systems recognize pathogens and destroy them. The hosts of some wasps will fly away or fight off their attacker. If pied flycatchers really were information parasites, then great tits might evolve defenses to safeguard their information.
When great tits are laying eggs, they search for sheep hair and other materials to keep the eggs covered. It’s not clear why they bother. You could imagine that the covering is a blanket to keep the eggs warm. But the birds don’t bother to keep the eggs covered once they’re all laid and the embryos start to develop. So it’s possible that they’re doing something else with the hair.
One thing that the hair does is hide the eggs. The Finnish scientists wondered if the great tits use hair to hide information from flycatchers. To find out, they ran an experiment.
They put a decoy of a pied flycatcher five meters from great tit nests and played a recording of a pied flycatcher singing for five minutes. The next day, they collected the hair in the nests. The scientists then ran the same experiment, but with decoys of cedar waxwings–birds that live alongside great tits but don’t compete with them.
The great tits responded to pied flycatchers by adding over 40% more hair on top of their eggs than they would otherwise. The scientists concluded that the birds hide the eggs when pied flycatchers show up so that the pied flycatchers won’t see just how well the great tits are doing. Seeing what looks like a meager nest, the pied flycatchers will be more likely to move on.
When hosts evolve defenses against parasites, parasites sometimes evolve counter-defenses. When flu viruses infect a cell, for example, the cell can respond by making an anti-viral protein called interferon. The interferon guides the cell to chop up the invading virus genes. But flu viruses have proteins that block interferon.
Do information parasites have their own counter-defenses? The scientists don’t offer any solid scientific evidence in their new report, but they do mention that they’ve seen something odd. They’ve seen pied flycatchers sneak into great nests and pull hair from the eggs. That may seem like a pointless exercise, since pied flycatchers don’t use hair on their own nests. It’s possible that they’re just trying to steal some reliable information.
When I first started writing my book on the triumph of parasites, I burrowed into the science and was stunned at how many ways there were to be a parasite. Eventually, the bottom just fell out. This is the first time that I’ve become aware of the concept of “information parasites,” but I suspect it won’t be the last.
[Update: Correction--reed warblers are hosts of cuckoos, not cowbirds]
March 3, 2014
Catching Up: Resurrected Viruses, Sex-Driven Smarts, And Some Upcoming Talks
My late winter is revving up into a state of rolling semi-controlled chaos, and so I’ve let a few items slip here at the Loom. Consider this a catch-up post.
1. On Thursday, I wrote my “Matter” column for the New York Times about an intriguing experiment on the evolution of learning. As I’ve written before, animals pay a price to become better learners, and so scientists have been investigating what the benefits are for different species. It turns out that competition for sex can drive the evolution of better learning, at least in flies. Randomly pairing flies into monogamous couples for a hundred generations leads to worse learning.
2. This week my “Matter” column is appearing today, to coincide with the publication of an especially riveting paper: scientists have revived a virus from 30,000-year-old Siberian permafrost. Aside from the dark twist on de-extinction, this story is compelling for another reason: the virus in question is a so-called “giant virus”--the biggest virus ever found, in fact. (I dedicate a chapter of my book A Planet of Viruses to the discovery of giant viruses–one of the most remarkable hiding-in-plain-sight stories around.) And for more on today’s news, check out fellow Phenomena-ster, Ed Yong, reporting for Nature.
3. Talks talks talks! After a quiet few months, I’m on the road. I was in Washington a couple weeks ago to talk about my cover story for National Geographic (video will go online soon, and I will post it here). Then I headed to Auburn last week to talk about genetically modified foods. But I’m just getting started. My future travels include:
–March 20: Rochester NY. Rochester Arts & Lectures. I’ll be talking about the mapping of the brain.
–March 24: Harvard. This talk is entitled, “Darwin in the City: How Modern Civilization Drives Evolution.”
–March 28: Charlotte NC. North Carolina Science Festival. I’ll be doing two talks in one day. One is a panel discussion about the genome. The other will be a public lecture about the microbiome.
–April 25: New York. American Society of Journalists and Authors. I’ll be talking about the craft of science writing.
–April 26: Washington. USA Science & Engineering Festival. I’ll be leading a panel discussion about personalized medicine. Panelists include Francis Collins, the director of the National Institutes of Health.
I also expect a couple more additions to my spring schedule–details to come.
February 27, 2014
Crawling Through The Brain Without Getting Lost

Ampulex compressa. Photo by K. Seltmann, via Creative Commons. Link: http://www.morphbank.net/?id=102143
If you’ve never met the emerald jewel wasp, let me introduce you to my little friend.
The wasp (Ampulex compressa) lives the first stage of its life as a parasite, growing inside the body of a living cockroach. That’s absorbingly horrific on its own, but how it gets into the cockroach in the first place is an especially gruesome delight. Its mother has to play neurosurgeon.
A female wasp seeks out a cockroach host and ambushes it. She inserts her stinger into its abdomen and delivers a paralyzing shot of venom that immobilizes the insect for a few minutes. She pulls her stinger out and then delivers a second injection. This one goes into the cockroach’s head, delivering more chemicals to two sites in the brain of the host.

Image copyright Quade Paul
The result is a cockroach zombie. The neurosurgically altered victim recovers from its paralysis but now lacks the will to flee or fight. The wasp pulls on an antenna and leads the roach, like a dog on a leash, into a burrow. There she glues an egg to the underside of the roach. She leaves the burrow and seals it shut. In the darkness, the roach stands motionless as the wasp larva hatches from its egg and chews a hole into its side. The wasp feeds through the hole for a while, and then slithers inside. Later, it pops out as a full-grown adult.

Image by Ram Gal
At Ben-Gurion University, Frederic Libersat and his colleagues have been studying the emerald jewel wasp for over 20 years, and they continue to learn new things about it. In the journal PLOS One, they’ve now published previously unknown details about the creepiest part of the wasp’s attack: its injection of zombie drugs into the cockroach brain.
To appreciate just how tricky this can be, consider what it takes for doctors to deliver drugs to a human brain. They scan the patient’s brain to map its anatomy in three dimensions. Then they put their patient’s head in a cage, drill a hole in the skull, and then slowly push a tube into the brain. A wasp does much the same thing in about a minute, without ever glancing at a brain scan of its victim.
Some days I wish I was a jewel wasp. Photo source: http://neurosurgerycns.wordpress.com
They pull off this feat with their extraordinary stinger. It measures 2 millimeters long, enabling the wasp to insert it into the roach’s neck and snake it up to the brain. The tip of the stinger has two sets of valves. One set hold the equipment for laying eggs, and the other set hold the equipment for delivering venom. The valves interlock in a tongue-and-groove arrangement so that they can slide over each other, allowing the wasp to lay an egg or deliver a sting with the same organ.
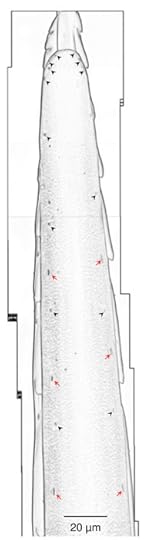
Portion of a wasp stinger. Red arrows mark touch-senstive bell-shaped bumps. Black arrows mark touch- and chemical-sensitive dome-shaped bumps. Gal et al PLOS One.
The stinger, the scientists also found, are studded with little bumps–some shaped like bells, others like domes. Each bell-shaped bump has a touch-sensitive nerve ending inside it, while the dome-shaped bumps have a touch-sensitive nerve ending along with four or five chemical-sensing ones.
To see what those bumps are doing, the scientists put electrodes inside the nervous system of wasps and then pushed the stinger against rubbery lumps meant to simulate a roach’s brain. The wasps’s nerves crackled with activity when the bumps on the stinger tip pushed along a lump. This response suggests that the wasp uses its stinger to feel its way through the roach’s brain.
To see if this was true, the scientists stripped the stinger bumps off of wasps and then let them attack a cockroach. The average time the wasps spent probing the cockroach brain shot up from just over a minute to nearly 20 minutes. That’s what you’d expect if the wasps suddenly were unable to find their way inside a cockroach brain.
The scientists then ran another type of test, presenting healthy wasps with roaches that they had altered in various ways. They took the brains out of some roaches and left them with hollow heads. In other cases, they swapped the brain with a rubbery lump (some lumps were hard and others soft). In still other cases, they injected a toxin into the brains of the cockroaches that silenced their neurons. And in still other cases, they insert scissors into the roaches’ heads and snipped up the brains into a homogenous mush.
The scientists found that some–but not all–of these altered roaches posed a challenge to the wasps. If a wasp stung a roach without a brain, she spent over ten minutes probing its head. A soft rubbery lump also stretched out the time the wasps stung their hosts. And after that long struggle, the wasps withdrew their stinger in defeat, without delivering their zombie venom.
But when the wasps encountered a hard rubbery lump–a lump with the same texture as a brain–they spent just a minute poking the cockroach, after which the scientists found venom in the victim’s head.
Nor did the scientists find any change when the wasps were presented with roaches whose brains had been silenced–suggesting that the wasps don’t sense electrical activity to guide their stinger. On the other hand, a shredded brain left the wasps groping. That result suggests that the wasps need to do more than just feel the roach brain–they need to feel different parts of the brain in order to get where they need to go.
Taken together, these results offer a picture of an exquisitely evolved sensory organ–one adapted not for some all-purpose perception, but solely to navigate the interior of a cockroach’s brain by sense of touch. The full magnificence of this sensory organ may yet to be revealed, however. In their new study, Libersat and his colleagues didn’t determine what the chemical-sensing dome-shaped bumps are for.
Do the wasps taste their way through a cockroach brain? It’s possible–but perhaps the dome bumps provide it with other kinds of information. The scientists speculate that the dome bumps may taste the wasp’s own venom as it’s released into the roach’s brain, so that the parasite can carefully control how much she delivers to her victim (this is neuropharmacology, after all). Or perhaps the wasp can taste the flavor of larva of other species of parasites–in which case she may abandon the already-infected cockroach for a fresh host.
If someone answers that question, I guarantee to let you know. In the meantime, here is a video of a talk I gave for TED-Ed in 2012 about the jewel wasp. And if that’s just not enough for you, check out my book Parasite Rex.
February 24, 2014
Searching For the Oldest Pieces of Earth
About 4.567 billion years ago, a quivering bead of magma 93 million miles from the Sun cooled down until it grew a skin of rock. Eventually, it would be named Earth. We don’t know a lot about what the planet was like back then, because that primordial crust is almost entirely recycled–eroded away, pushed back down into the molten depths of the planet, or smashed to bits by the huge impacts that blasted Earth for its first few hundred million years.
Geologists have wandered the planet to find scraps of the infant Earth. One mineral that is particularly precious to them is known as a zircon. Tiny zircon crystals can withstand billions of years of abuse–getting ripped out of their original rock, incorporated into new rocks, heated up, and squeezed at tremendous pressures–and yet still retain their original chemistry. Zircons have the added attraction of holding onto radioactive isotopes such as uranium. Over billions of years, the uranium decays at a steady rate into lead. By measuring the atoms of uranium and lead in a zircon, scientists can get a tight estimate of the zircon’s age.

Zircon from Jack Hills, Australia, dated to 4.4 billion years old. Photo copyright John Valley, University of Wisconsin
In 2001, scientists digging at a site in the Australian outback called Jack Hills found a zircon dating back about 4.4 billion years. It broke all records for the oldest zircon, and no one has broken its record since.
Estimating the age of zircons from over four billion years ago pushes science’s powers of detection to their limits. And so it’s no surprise that the age of the Jack Hills zircon has been the subject of debate.
Some critics have questioned whether the geological clock in the Jack Hills zircon has been running steadily this whole time. After the zircon got incorporated into younger rocks, it was heated up. Under such conditions, the uranium and lead inside a zircon may migrate around inside the mineral grain. To measure the age of a zircon, scientists only shave off a small portion of the zircon. If there’s been a lot of migration inside the mineral, that portion may be loaded with extra uranium and lead, or it may have lost some of atoms. In either case, the geological clock will be thrown off.
Geologists are constantly raising these challenges and then meeting them. Yesterday in the journal Nature Geoscience, John Valley of the University of Wisconsin and his colleagues published a new study of the Jack Hills zircons. They performed a kind of geological X-ray, mapping the uranium-derived lead atoms from across a zircon grain to figure out how much they’ve moved around.
The lead has indeed moved, Valley and his colleagues have found, but only a little. It has gathered into clumps that are too small and too closely packed to throw off the geological clock. The 4.4 billion-year age stands.
In other words, the Jack Hill zircon existed in rocks that formed about 200 million years after the formation of the planet. That’s a long time for us mere humans, but it’s about four percent of the lifetime of the Earth. Looking inside the Jack Hills zircon, geologists have found hints that they formed in a cool crust below a liquid ocean. If that’s true, then it also means the Earth was hospitable for life by then.
But there’s only so much insight you can get from a zircon that’s twice the thickness of a human hair. What geologists would love to find is an honest-to-goodness rock from 4.4 billion years ago–something you could hold in your hand. The oldest rocks with an uncontested age date back 4 billion years ago–400 million years younger than the Jack Hills zircon. If geologists could find rocks from 4.4 billion years ago, filled with a variety of minerals, they could learn much more about the early Earth.
In the March issue of Scientific American, I have a story about rocks that may indeed be 4.4 billion years old–the oldest rocks on Earth, in other words. But these rocks, along the coast of Hudson Bay, have inspired an intense debate among geologists, some of whom argue that they’re actually much younger. The science is fascinating, and the stakes are big. You’ll need to buy a copy of the issue or access the story through a subscription, but I hope you’ll find it worth the effort.
February 20, 2014
The Ultimate Cold Case
Around 252 million years ago, as many as 96 percent of all species on Earth became extinct. For my new “Matter” column in the New York Times, I write about the scientists who are trying to solve this great murder mystery, and what their work may tell us about how the planet may respond to our own disruptions. Check it out.
February 19, 2014
Scientists On the Loose! My AAAS Talk
On Thursday I participated in an interesting day of talks at the annual meeting of the American Association for the Advancement of Science in Chicago. The theme was “Communicating Science.” I was on a panel in the morning made up of four journalists, who shared our experiences with the changes roiling the field. You can watch it (and the other panels of the day) here. I speak from 10:55 to 18:00. After 48:33, the panel and the audience had a long conversation that I thought was pretty interesting.
I thought I’d put my prepared remarks here, with links, in case anyone wanted to chase down the things I was talking about…
–Good morning. We are going to collectively spare you the hassle of Powerpoint. And so, instead of looking at a slide, I’d like to start this morning’s discussion by having you look at a mental picture. The picture is of Stephen Hawking. You can picture the physicist thanks to us–the media—thanks to the magazine covers, newspaper portraits, web site photos, TV documentaries, episodes of the Simpsons, and dust jackets where he has appeared. For over twenty years, Hawking has been at the media’s frontier, helping to define how scientists present themselves to the public and are represented by others. And just three weeks ago, at age 72, Hawking once again did something new. He posted a two-page document online.
This is actually a much bigger deal than it may sound at first. Hawking recently gave a talk about a new idea he has about black holes. This is interesting, since Hawking has been so important in our current understanding of these strange things. Some recent developments in cosmology and quantum physics have caused him to rethink black holes in a serious way. Hawking once thought that when things fell into black holes, there was no way for us to get any information about them ever again. But now he is suggesting that when things get pulled into a black hole, some information can leak out, in a jumbled form. Black holes are not black holes as we knew them, in other words.
Hawking did what scientists usually do: he wrote up this idea in a paper. But he didn’t proceed to keep it secret until it appeared in a peer-reviewed journal. Instead, on January 22, he uploaded the paper, “Information Preservation and Weather Forecasting for Black Holes” to the physics pre-print site known as arXiv. Two days later, Nature had a detailed article about Hawking paper. New Scientist published an explainer piece the same day. These stories swiftly got a lot of attention on sites like Digg and Facebook, driving hordes of readers their way.
Today, three weeks later, the paper is still only available on arXiv, where anyone can download it for free. The arXiv page conveniently links to some of the blog posts that people have written about the paper—including posts by his fellow scientists. I highly recommend this new piece on Slate by Matthew Francis. Reasonably web savvy teenagers can gather all this information in a few minutes, to digest later at their leisure.
To me this episode epitomizes the huge changes in our field. I’m not saying the individual elements of this story are new. Physicists have given talks for centuries. Arxiv has been around for 23 years. By the early 2000s, people were blogging regularly about science. On February 4 this month, we marked the tenth anniversary of the day a Harvard computer science major launched a site called thefacebook.com.
But recently these elements have crossed two thresholds–of scale and connection. And the result is a drastically new way for scientists to reach the public.
If Hawking had this idea ten years ago, things would have worked differently. To get a wide audience for his new idea, Hawking might have submitted his paper to a prominent journal. The journal would then send it to anonymous reviewers. If the reviewers judged it good science, it would go into press. But it would only be available to people with thousands of dollars to spend on a subscription to the journal.
The journal might promote the paper with press releases. They’d let us journalists look at a preprint—but only if we respected an embargo and stayed quiet till then. Or maybe journalists would get wind of the paper through a press release from Cambridge University.
At this point, the outside world would have known nothing about the paper. Only when the major print outlets unveiled their stories would they find out. Only in the comments the reporters offered from other scientists would people get a hint of what the scientific community thought. And ten or twenty years ago, this process made a few scientists into celebrities—like Steven Hawking.
Every step of this process has changed—or, rather, there is now a set of parallel steps. Arxiv has become a required stop on the road to publication for physicists. Biologists are following their lead now, too. Some of the most provocative biology papers I know of—on topics like exactly when Neanderthals and modern humans interbred—were first posted online on preprint servers like bioRxiv. The curtain-raising ritual at high-impact journals is losing a bit of its magic. It becomes not an unveiling, so much as a stage of maturation in the life of a research project.
I have no idea when or where Hawking will ultimately publish his new paper. It’s possible that the journal he chooses will offer the final paper as freely as arXiv did. Open access publishing is steadily growing. Just yesterday afternoon, AAAS, the host of this meeting, announced they were launching their first open-access online journal, called Science Advances.
Peer review is also becoming more open. Scientists are increasingly reviewing papers in public, after they are published or even when they are on a preprint server–as happens on Haldane’s Sieve. If you visited the early post-peer review forums at places like the Public Library of Science, you heard crickets. Now that’s changing. There are more comments on papers, and more forums. Even the prime portal to biomedical research, Pubmed, is now starting to post comments on papers, which appear right below your search results.
It’s common for scientists to debate new research as soon as it’s published, on blogs, Twitter, or Facebook. New companies are launching in order to measure this response, and to create an alternative to the traditional ways of measuring the impact of a paper. Instead of looking at the number of times it shows up in the footnotes of other papers, maybe the number of Tweets matters, too.
What do these changes mean for people like the four of us on the panel–the journalists? A lot. It makes science journalism more fun. You don’t have to sit dutifully by your computer, waiting for some journal to deign to let you know about a new paper. You can go hunting. You can turn up a new paper that’s just sitting quietly in a preprint archive, and share it with the world.
And you can get a more realistic understanding of how scientists toss around ideas. If research simply appears in an august scientific journal, it can be hard to figure out how it actually fits into the current scientific debates. The last thing a journalist wants to do is present research as if it’s the discovery of extraterrestrial life, when, in fact, it’s arsenic life.
The new ways that scientists share their ideas and opinions helps us. We can take the pulse on Twitter. We can follow comment threads. We can throw questions into these debates in real time if we so wish.
But it also presents new risks that we journalists should be mindful of. The scientist who tweets the most may not be the wisest expert on a particular topic. If you come across a preprint, you have to ask, “Does its mere existence constitute news?” Or is that preprint just a flakey idea that will make it into a serious journal? Should journalists wait for the journals to give these papers their seal of approval? Is that what journals are for now—to designate important science? Or are they simply seizing that role for themselves from the scientific community as a whole?
I honestly don’t have answers to those questions. But Stephen Hawking has made it clear to me that I need to find some.
February 13, 2014
The Phantom Piano
When the brain goes awry, it can reveal to us some clues to how it works in all of us. In my latest “Matter” column for the New York Times, I look at a rare but fascinating disorder that causes people to hallucinate music. How someone could imagine that a piano was playing nearby–or a marching band or church choir–may tell us something about how our brains make sense of the world by making predictions about what comes next. Check it out.
February 10, 2014
Planet Calabash
Many plants have worked their way into our lives, but few have done so with as much flair as the calabash. For over ten thousand years, people have used the calabash (known also as the bottle gourd and formally as Lagenaria siceraria) in all sorts of ways. They’ve eaten it as food. They’ve used it as fishing floats, as pontoons for river rafts, as goblets, as pipe stems. And around the world, people make music with it.
Here’s a whirlwind calabash concert tour around the planet:
Ravi Shankar jams on his sitar, the Indian classical guitar carved from a calabash, at the 1967 Monterey Pop festival:
Naná Vasconcelos plays the berimbau, the Brazilian instrument with a calabash as a resonator, in Rome in 1983:
From Mali, Toumani Diabate plays the kora:
And in China, here’s Crescent Dai performing (and talking about) the hulusi, also known as the bottle gourd flute
The calabash is a domesticated plant that depends on us for its survival, like wheat or almonds. Its closest wild relatives grow in Africa. When people domesticated the gourd, they bred a rugged, light-weight shell that could hold up for years. But the course that the calabash took from wild plant to domesticated tool has been hard to uncover.
Archaeologists have found pieces of calabash dating back 11,000 in East Asia. In the New World, people were using calabashes at least 10,000 years ago.
In 2005, a team of scientists isolated some DNA from ancient calabashes in North and South America and found that they resembled calabashes from Asia more than African ones. Since the people of the New World originally came from Asia, traveling over the Bering land bridge, the scientists proposed that they took calabashes with them.
There’s a problem with this idea, though. If Asians carried calabashes into Alaska, they had to grow them along the way. But the short growing season in the Arctic would have made it impossible to rear the tropical plant. What’s more, the people who have settled in Siberia and Alaska don’t use any plants as containers. They use materials from animals, such as hides.
It was possible that the 2005 study didn’t have the precision to get the true picture of calabash history. The scientists only looked at a few genetic markers–tiny stretches of DNA at certain locations in the genes of different plants. It was possible that these markers had changed too rapidly over thousands of years, so that distantly related calabashes evolved similar sequences.
A group of researchers led by Logan Kistler at Pennsylvania State University decided to take another crack at the calabash. They gathered much more data this time around, gathering living gourds from every continent as well as the Pacific islands, and analyzing nine sets of ancient remains. And instead of looking for a handful of genetic markers, they sequenced 86,000 base pairs from the DNA in the chloroplast, the light-generating structures passed down from female plants to their offspring. By comparing the DNA from the plants, they were able to trace the branches of their family tree.
This is the tree. The x-axis is a time scale, going from 250,000 years ago to today. The numbers at the nodes are statistical measurements of how likely the trees are to be accurate. Branches with a 1 have the highest possible statistical support. The red names refer to gourds from archaeological sites in the New World.

Kistler et al PNAS 2014
As you can see, a wild African gourd belongs to the deepest branch. The tree then splits into two main lineages: one that includes African domesticated gourds, and the other that includes Asian ones. Both the ancient calabashes and the living ones from the New World belong to the African branch of the tree. If the calabashes had taken an Arctic journey, you’d expect a very different tree, with New World plants showing a kinship with Asian ones. This tree makes that journey hard to accept.
Making matters even worse is the great age of the New World lineages. Kistler and his colleagues tallied up the mutations along each branch to estimate how long ago they split from a common ancestor. The New World calabashes share a common ancestor with African calabashes 60,000 to 103,000 years ago. That’s long before people made their way to the New World about 15,000 years ago. However the calabashes originally got to the New World, it wasn’t on anyone’s shoulders.
Kistler and his colleagues argue instead for a different journey–or, rather, different journeys for people and plants. Back about 80,000 years ago, wild calabashes grew only in Africa. Sometimes their seeds were washed out to sea. Computer models of the Atlantic currents indicate that it might have taken just a few months for the wild calabash seeds to travel to the New World. The ocean-faring seeds sprouted in their new home. While Alaska would have been a poor place for the gourds to grow, places like Florida, Mexico, and Brazil would have been ideal.
Africa was likely the first place where people domesticated gourds. Different groups of people may have come across different wild populations of the plants, and selecting the gourds with the toughest shells to use as containers. When this happened is hard to say–the oldest archaeological evidence for gourds in Africa is less than 5,000 years old, but there could be other calabash fragments waiting to be discovered.
It’s tantalizing to look at the asiatica branch of the calabash tree. All the Asian, European, and Pacific gourds share a common ancestor with a domesticated Ethiopian gourd that lived 60,000 years ago. That’s right around the time that modern humans expanded from East Africa into the rest of the Old World. We know that by then they were using ostrich eggs as containers. They didn’t take ostriches with them out of Africa. Did they also take calabashes? That’s possible, but it’s also possible that a wild relative of the Ethiopian calabash spread across the water to coastlines in the Near East or South Asia, where people started to domesticate them.
What is clear is that people carried calabashes a long way, putting them in their canoes as they sailed to the islands of the Pacific. But there’s no evidence that the people who traveled across the Bering Land bridge had calabashes with them. Instead, the evidence suggests, their descendants eventually stumbled across wild calabashes growing along the east coast of North and South America, descendants of seeds that had drifted across the Atlantic while the ancestors of the people of the New World were living in Africa over 40,000 years before. And just as people in other parts of the world had done when they discovered wild calabashes, the people of the New World started using these plants.
Wild calabashes are practically impossible to find any more, either in the New World or in Africa. Humans may be the reason why. Originally, the plants probably developed big gourds to protect their seeds from small predators like birds and rodents. Only big mammals like ground sloths may have been able to feed on them. Like a number of other plants, calabashes may have had seeds adapted to survive a trip through the gut of a big mammal. The mammals could then spread their seeds in their droppings, maintaining the plants across a wide range.
Many of those giant beasts are now extinct. While the end of the Ice Age may have played a role in their demise, it’s also possible that human hunters may have helped push them towards oblivion. Having lost their animal partners, wild calabash would have started to dwindle. Meanwhile, though, humans were caring for a few strains of calabashes that had the traits they valued. Thus evolution pushed the calabashes to their remarkably durable state.
Scientists have long been impressed by the triumph of the calabash. Among domesticated species, only the dog has spread further. But the global journey of the calabash was actually two great trips, one taken by humans over land and another taken by plants, over the sea.