Carl Zimmer's Blog, page 11
June 30, 2014
A Free Digital Biology Textbook Is Now Fully Hatched
Harvard biologist E.O. Wilson has been leading an ambitious project over the past few years to create a free high school biology textbook custom-built for the digital age. It’s called Life on Earth.
In 2012 the team released a sample chapter, and they’ve been releasing more since then. Reviewing the project early on for Download the Universe, anthropologist John Hawks had mixed feelings. He praised its beauty and the pleasure derived from toying with its fancy features, while also questioning how well students will learn from the format.
Now the whole project is complete. You can download the entire book for free here. You’ll need an iPad for the full effect, although I’m currently thumbing through it on iBooks on my laptop. In addition, they’ve put some extra materials together for teachers in iTunes U, which you can access from the book link. I’m curious to know what people think.
June 27, 2014
Catching Up: Brains, Writing, and Hot Hands
The past couple weeks have been a scramble for me (the end of the school year tends to crash like a rogue wave into the lives of parents). During that time, I’ve written a couple new “Matter” columns for the New York Times, both of which concerning how our brains work:
–Last week, I took a look at the first attempts to scan the brain of creative writers. While the research is ambitious, some skeptical scientists don’t think we can dissect the anatomy of creativity.
–This week, I look at our penchant to see order where there’s only randomness. Psychologists call this the “hot hand phenomenon,” named for the conviction people have that basketball players can get on streaks. An experiment on monkeys adds to the evidence suggesting that this phenomenon is an ancient bias in the workings of our brains, which emerges from the way our ancestors hunted for food.
June 25, 2014
The Zoo In the Mouth
There’s a philosophical quandary breeding in your mouth.

Fantail. Photo by Jim Gifford via Flickr/Creative Commons http://flic.kr/p/4pkQSb
Ever since Aristotle, philosophers and scientists have searched for the right way to classify living things. We call living things with feathers “birds,” but we can also divide birds up into smaller groups, like pigeons and storks. We can drill down even further, to different species of pigeons. But it doesn’t feel right to classify birds all the way down to every individual feathered creature on Earth. The fundamental unit of life’s biodiversity has long been the species. Charles Darwin named his book The Origin of Species for a reason.
Darwin threw older notions of species into doubt, challenging the idea that they were fixed since creation or only able to change slightly over time. adjusted over time. In fact, old species give rise to new ones like shoots from a tree. Darwin concluded that where we choose to draw a line to mark the boundary of a species is a matter of convenience.
While Darwin created the modern foundation for biology with his theory, biologists didn’t abandon the word species. It’s still a helpful term for describing how life evolves–even if it’s left scientists arguing about which definition can hold up best against the churning complexity of evolution.
In the 1940s, the biologist Ernst Mayr declared that “species are groups of interbreeding natural populations that are reproductively isolated from other such groups.” In other words, it’s all about sex.
Other scientists didn’t like Mayr’s definition, because reproductive isolation is a squishy term. Stick a squirrel on a remote island, and it can’t reproduce with its fellow squirrels on the mainland. Have you made a new species? Biologists now know that populations gradually become more and more reproductively isolated over thousands or millions of years. Do we have to wait till they’re totally isolated before declaring the two populations new species?
Some scientists suggested that we think of a species in terms as a branch of the tree of life–as the smallest group of organisms that all descend from a common ancestor, and which we can distinguish from other groups. (I wrote a feature a few years ago about these debates over species in Scientific American, which you can read here.)
These definitions work tolerably well for animals and plants. But they’ve turned out to be pretty lousy for microbes. And that’s left scientists in a quandary, because it’s now clear that the vast majority of the genetic diversity on Earth belongs to bacteria, viruses, and other life forms invisible to the naked eye.

Jane Hurd/National Geographic
When microbiologists started to study microbes in the nineteenth century, they went about their business like zoologists or botanists. They’d describe a microbe based on its appearance, what it fed on, and other features they could study in their laboratory. If it seemed different from other microbes that had been previously described, they’d give it an official species name, like Escherichia coli. When later microbiologists would come across a microbe–say, in the blood of a sick patient–they’d identify its species by systematically inspecting its traits, in much the same way a bird-watcher would look at a bird’s plumage and listen to its song.
In the late 1900s, microbiologists were abandoning this method in favor of a new one: identifying species by their DNA. Zoologists and botanists were making the shift, too, but microbiologists were in for a particularly big shock. The diversity of microbes turned out to be mind-bogglingly exuberant. Microbes that had been considered almost identical before the age of DNA turned out to be more genetically different from each other than maple trees and penguins.
As I describe in my book Microcosm, a single species–E. coli–turned out to contain multitudes, from beneficial bacteria that can heal a baby’s dysfunctional gut to all sorts of pathogens that make us sick in frightfully different ways, from making our cells spill their contents to slithering into them like tapeworms.
Making matters even more confusing was the discovery that microbes don’t simply pass down their genes to their offspring the way we do. They are constantly swapping genes with each other, with little respect for any so-called species barrier. Scientists sometimes try to represent this gene traffic by drawing a web of life instead of a tree of life.
Trying to fit microbes into a definition of species based on animals and plants is a bit like trying to herd a flock of flamingos into a school bus. Mayr’s definition is not much help, since microbes don’t have sex like animals do. The branch-of-the-tree-of-life definition is not much help, either. Theoretically, any microbe that picks up a mutation is distinct from other microbes and should be considered its own species. And if it passes that particular mutation to a distantly related microbe, the whole tree-based approach collapses.
The situation is such a mess that some microbiologists have given up on species altogether. In 2012, W. Ford Doolittle wrote an awesomely titled commentary called, “How Bacterial Species Form and Why They Don’t Exist.”
This confusion can make life hard for microbiologists when reporters call. Like many other journalists, I’ve been reporting a lot on the remarkable explorations scientists have been making of the human microbiome–the collection of germs that call us home.
I’ve repeatedly asked, “So, how many species are in us?”
And each time, the microbiologists I talked to would squirm out of a real answer.
As deeply uncomfortable as microbiologists may be with the whole idea of species, they still need some way to measure the diversity of, say, the microbiome. Instead of species, they typically end up using something species-ish.
When microbiologists were shifting to using DNA, they chose one gene as a way to distinguish different kinds of microbes from each other. The gene, found in all living things, is called 16S rRNA. Microbiologists compared the variations in 16S rRNA genes from individual microbes that belonged to a single species–that is, a species as microbiologists would traditionally identify it.
They found that the gene varied by up to three percent. So they decided that if two microbes were 97% identical in this one gene, they belonged to the same group. Instead of a species, they called this group an operational taxonomic unit.
But it eventually turned out that this 97% cut-off was wrong. A good example of how it can lead scientists astray is the case of two species of bacteria that live on our bodies, Streptococcus pneumoniae and Streptococcus mitis. Back in the barbaric pre-sequencing days, microbiologists decided they were two separate species, deserving of two separate names. When you look at how these two microbes make a living, that decision makes ample sense. S. pneumoniae causes pneumonia, while S. mitis can live harmlessly on teeth.
Yet the 16S rRNA sequences of these two “species” are over 97% identical. In fact, they’re 99% similar. While their 16S rRNA genes have remained nearly identical, some of their other genes have veered off in different directions.
In the face of all this delicious confusion, microbiologists need a more powerful way to sort microbes into groups. But there’s been a limit to how fine they can draw their distinctions. Even the best DNA sequencers are not perfect. Here and there, they will make an error in reading a microbe’s genes. When microbiologists are distinguishing different kinds of bacteria with 97% thresholds, those little errors don’t matter. But if microbiologists want to distinguish microbes by tiny differences, making those errors would be akin to mistaking a wolf for a chihuahua.
A. Murat Eren, a microbial ecologist at Marine Biological Laboratory in Woods Hole, Massachusetts, and his colleagues have come up with a powerful new way to chart the diversity of microbes. They call it oligotyping. They line up the 16S rRNA genes from a group of microbes, and then they look for spots in the gene that have the most differences from one microbe to another. The scientists narrow down their search to the fewest spots that can distinguish the largest number of groups, which they call oligotypes. This approach allows them to avoid depending on tiny, error-prone differences that might only be present on a single microbe’s gene.

Photo by Darwin Bell, via Creative Commons https://flic.kr/p/eHmGJ
Recently, Eren and his colleagues tested out oligotyping on the microbiome. They searched the DNA sequences collected by the Human Microbiome Project, a massive survey of microbial genes from over 200 people. Eren and his colleagues limited their search to just the microbes from people’s mouths. That still left them with over 10 million sequences to sift through.
All told, they found at least 362 oligoytpes of bacteria.* Most of their oligotypes belonged to conventional species, but often the scientists found that a single so-called species contained several distinct oligotypes.
These oligotypes are not just minor variations on a theme. They lead different lives. Our mouths are like jungles, with lots of ecological niches. The environment on a tooth is very different from that on the tongue, which is different in turn from the gums. Eren and his colleagues found that each oligotype dwelled in just one or a few parts of the mouth–and did so consistently from one person to the next. Some oligotypes only live on teeth, for example, while others live mostly on the tongue. The evidence for all this diversity already existed in the Human Microbiome Project’s databases, but until now it was hiding in plain sight.
There are plenty of solid, practical reasons for inventing a better way to measure the diversity of microbes–even just in our mouths. Some bacteria that live there can potentially make us sick–not just by causing cavities, but by causing diseases in other parts of the body, such as heart disease. Old-fashioned definitions of species can lead scientists to overlook these pathogens, lumping them into the same group as harmless microbes.
But this research is also important for a more basic reason–because it helps us to wrap our minds around the staggering diversity of life on Earth. One way to start appreciating how vast that diversity is to just open our mouths.
*The paper will be posted at some point this week by the journal. The reference is: Eren et al., “Oligotyping analysis of the human oral microbiome,” PNAS. www.pnas.org/cgi/doi/10.1073/pnas.140...
June 16, 2014
The Feathered River
In the early 1800s, a naturalist named Alexander Wilson was traveling in Kentucky when the sky suddenly became dark. Wilson believed, he later wrote, that it was “a tornado, about to overwhelm the house and everything round in destruction.”
When Wilson got his wits back, he realized the sun had been blotted out by passenger pigeons.
The journals of many early explorers contain similar passages. The passenger pigeon would sweep across the eastern United States in vast flocks, feeding on chestnuts and acorns as they traveled. As Wilson gazed at his passenger pigeon flock, he tried to figure out how many birds it contained. From one side to the other, it was a mile wide. It streamed overhead like a feathered river for more than four hours. Based on that information, Wilson guessed that it contained over 2.2 billion birds–”an almost inconceivable multitude,” he wrote, “and yet probably far below the actual amount.”
In 1914, the passenger pigeon became extinct, likely thanks to industrial-scale hunting. In his book Nature’s Ghosts, Mark Barrow notes that our eradication of such a populous species came as a tremendous shock–one that helped the world appreciate nature’s true fragility.
Because the passenger pigeon disappeared before modern ecology came of age, scientists don’t know much about its natural history. They’ve had to rely mostly on the reports of witnesses like Wilson–reports that encourage us to leap to a fairly simple story: vast flocks of passenger pigeons, followed by a few stuffed corpses preserved in museum.
But in recent decades, some researchers have argued that the history of passenger pigeons was more complicated. In 1985, for example, the archaeologist William Neumann pointed out that Native American archaeological sites don’t contain many passenger pigeon bones. If the birds were blot-out-the-sun abundant for thousands of years, you’d expect Native Americans to have feasted on them. Neumann argued that the nineteenth-century swarms did not reflect the long-term reality of passenger pigeons.
Now a new generation of scientists are tackling this question with a new line of evidence: DNA. It turns out that some stuffed pigeons stored in museum collections still retain some decent-sized chunks of genetic material.
In this week’s Proceedings of the National Academy of Sciences, Chih-Ming Hung of National Taiwan Normal University and colleagues report how they got DNA from the toe pads of three passenger pigeons–two caught in Minnesota and one in Pennsylvania. They managed to get anywhere from 57 to 75% of the entire genome of each bird.

Painting by Louis Agassi Fuertes/National Geographic
When scientists can study that much DNA from an animal, they can learn a lot of things from it. They can learn, for example, how big the population of its ancestors was in the past.
Scientists can manage this feat thanks to the variation in each animal’s genes. If you compare a collagen gene in your DNA to someone else’s, the two copies may be a little different. That’s because mutations arose in the gene as it was passed down through the generations.
In a big population, there will be a lot of these variants, which will get passed down to future generations. A small population, on the other hand, doesn’t have the necessary size to contain many variants. The individuals in that population will pass down their meager genetic variation to their offspring.
The genetic variation in a population today can thus serve as a clue to its size in the past. Even if it later swells up to huge proportions, it may still only have a small amount of variation, because its origins were so modest.
Strictly speaking, scientists can’t measure the true size of an ancient population this way. Only a fraction of individuals in each generation end up reproducing, and so we can only look at the variation of their genes. Scientists refer to this smaller group of reproducers as the “effective population size.” It’s useful to know the effective population size, however, because it can serve as a rough guide to a true population. (Typically, the effective population size is about a tenth of the true population.)
When scientists first began to measure effective population sizes, they would do so by comparing a particular gene in a lot of individuals. In recent years, they invented a neat reverse trick: they can now estimate the effective population size by looking at a lot of genes from just a few individuals. If many of those genes are different from one individual to another, their difference tells scientists that those animals came from a big population in the past. If the genes are very similar, the population was small.
What’s especially remarkable about this method is that it lets scientists track the size of a population through time. That’s because scientists can compare how long ago the variants of each gene arose from a common ancestor. If a lot of gene variants can be traced back to one particular time, that suggests the population was small then. In 2011, Heng Li and Richard Durbin of the Wellcome Trust Sanger Institute used this method to reconstruct human history from just six genomes. They found that human populations shrank drastically between about 50,000 and 20,000 years ago, and then expanded tremendously.
Now Hung and colleagues have used the same method to track passenger pigeon population. Surprisingly, they found that the long-term average effective population size was only 330,000 birds. Scientists have estimated that the population of passenger pigeons in the nineteenth century was between 3 and 5 billion birds. In other words, the effective population size is a thousand times lower than you’d expect.
This mismatch hints that passenger pigeons had a much smaller population at some point in the past. And when Hung and colleagues reconstructed the history of the passenger pigeon population, they found that the birds have indeed gone through some huge fluctuations. About 120,000 years ago, the pigeons were abundant, but they shrank to a small population 21,000 years ago. They then rebounded after the last Ice Age, reaching another peak about 9000 years ago. Since then, they have been declining slowly.
This pattern fits the ecological history of the Ice Ages pretty well. About 120,000 years ago, the climate was warm, glaciers were at their minimum, and forests were widespread across North America. By 21,000 years ago, a lot of passenger pigeon territory was covered by ice, and much of the remaining habitat couldn’t support the oak and chestnut forests that the birds depended on for their food. Once the glaciers retreated, the forests returned, along with the birds.
But Hung and colleagues argue that these long-term climate changes are only part of the explanation for the small effective population size of passenger pigeons. They propose that the birds were like locusts, swiftly expanding when food supplies were good, but collapsing when times got bad. (Real locusts can reach 100 billion in an outbreak, but their effective population size is only half a million.)
In their new study, Hung and colleagues come to a similar conclusion to Neumann’s. The huge flocks that naturalists like Wilson saw in the nineteenth century were not an immutable fixture of the pre-industrial American landscape. Instead, Wilson may have simply been witness to an outbreak of birds.
It’s even possible that settlers from Europe may have been responsible for that outbreak. Native Americans may have kept pigeon populations low by hunting them and collecting acorns and chestnuts. By pushing Native Americans off their land, the Europeans may have allowed the birds to increase their numbers–and helped them even more by planting crops that the birds could feed on.
As researchers assemble passenger pigeon DNA, they also move closer to potentially bringing the bird back from extinction. As I described last year in National Geographic, they could theoretically use the genome of a closely related species, the band-tailed pigeon, as a scaffolding to engineer DNA with passenger-pigeon-specific mutations. Ben Novak of the University of California at Santa Cruz explained in this TEDx video how it might then be possible to breed birds with this DNA until they looked–and perhaps even behaved–like passenger pigeons of old.
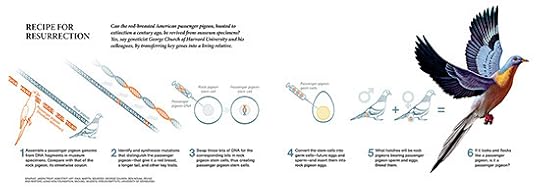
Click to enlarge
Hung’s new study (and similar research being carried out by Novak and his colleagues) suggests that scientists might be able to get passenger pigeons back on their feet without creating the billion-strong flocks that Wilson saw. Even though they sometimes fell to far smaller numbers, passenger pigeons survived for hundreds of thousands of years before people started firing guns at them.
On the other hand, if we were to bring the passenger pigeon back from extinction, it may not stay at some steady population level. If the conditions were right, it might explode to higher numbers. And if we make life difficult for the birds–through climate change, for example–it’s possible that we could drive their numbers down again. The passenger pigeon may be able to do a good impression of a tornado, but it’s still delicately dependent on its ecosystem.
[Apologies for the typos in the original version of this post. I hope I've caught them all now.]
June 12, 2014
Young Animals and Old, Old Plants
Last Friday, I was a guest on the National Public Radio show Science Friday with photographer Rachel Sussman. We talked about her new book, The Oldest Living Things in the World, for which I wrote the introduction. You can listen here. (And you can read my whole introduction to Sussman’s book here.)
I talked on the show about some of the ideas that scientists are exploring about why some species live for a long time and some don’t. What’s intriguing me a lot right now is how old some plants can get. There are a few animal species can reach a very ripe age, such as clams that live for five hundred years. But Sussman’s book is dominated by plants–by ancient trees and shrubs and such–that can live for many thousands of years.
Scientists can’t offer a simple, straightforward answer to why plants can get so much older than animals. But they have gathered a lot of intriguing evidence that may lead them to one. For one thing, the biology of aging is different in some important respects in animals and plants, as Howard Thomas of Aberswyth Aberystwyth University in Scotland Wales explained last year in the journal New Phytologist.
As we animals get older, things go wrong. For example, as our cells divide, their DNA sometimes mutates. This can cause the cells to malfunction or even turn cancerous. This burden of mutations only gets greater the older we get. We can try to fix this damage–repairing DNA, killing off defective cells, and so on–but that takes a lot of energy, energy that animals could otherwise use for other purposes, like reproducing.
Plants don’t seem to have to deal with these challenges. Trees that are 4700 years old don’t have more mutations in their cells than much younger plants. It’s possible that they lack those mutations because a kind of evolutionary struggle taking in the tissues of old plants. If some cells suffer mutations, other cells that are in better shape will take over and continue to grow healthy tissue.
Thomas also suggests that plants aren’t trapped in a trade-off between repairing their cells and growing like animals are. That’s because animals have to eat their food, while plants manufacture theirs from the sun and the air. They’ve got a lot more energy to work with.
The very bodies of plants may also give them an opportunity to grow very old, while ours do not. An animal is made up of two kinds of cells: somatic cells that make up most of the bodies, and a small collection germ cells (sperm or eggs) that can give rise to a new animal. That division gets established early in the development of an animal embryo and never changes. Plants don’t have such a stark division between somatic and germ cells. As they grow, they add new modules, each of which may produce germ cells (hence, a cherry tree is covered in blossoms, rather than just one blossom). Some of those modules may get stressed and even die, but the other modules can survive and continue to grow.
Recently a team of scientists from Ghent University in Belgium pointed out in Trends in Cell Biology that plants are different from animals in another respect: their stem cells.
Stem cells, which grow in both animals and plants, have the potential to grow into new tissue. In animals, they can maintain a healthy, young body. If they stop rejuvenating muscle, skin, and other tissue, an animal becomes old. (See my post this week for more details).
Plants have stem cells, too, which are concentrated where the plants are putting on new growth, such as their stems and root tips. But they also have what you might call stem cells for stem cells. Known as quiescent cells, they form a tiny patch in the middle of a cluster of stem cells. They grow very slowly, and each time a quiescent cell splits in two, one of the new cells becomes a true stem cell. That new stem cell divides rapidly into still more stem cells, which in turn can develop int a root or a leaf or some other part of a plant. But the other cell from that original division is yet another quiescent cell, which remains behind in reserve.
Quiescent cells appear to be vital to plants. If scientists remove all the quiescent cells from a root, for example, some of the stem cells in the root will turn into new quiescent cells. It’s possible, the Belgian scientists write, that they are also crucial to the ability of plants to keep rejuvenating for a long time. They can create a supply of stem cells for millennia.
After thousands of years, in other words, a bristlecone pine may still be young at heart.

Llareta, an ancient shrub. From the Oldest Living Things in the World, by Rachel Sussman
June 11, 2014
Hello, Great-Great-Great-Aunt!
I love writing about evolution’s great transitions–from water to land, from ground to air, and so on. For our species, one of the biggest of those transitions happened when our invertebrate ancestors became vertebrates–complete with our distinctive backbone, muscles, mouths, noses, and eyes. For fifteen years, I’ve been writing about this transition, and it’s been exciting to see more fossils come to light that help us understand how our inner fish got its start. For my new “Matter” column in the New York Times, I take a look at one of the most interesting of these fossils–what one scientist has dubbed a benchmark for our understanding of the first vertebrates. It’s called Metaspriggina, and here’s a video of an animated reconstruction. Get the rest of the story here.
June 10, 2014
The Secret Ingredient in Young Blood: Oxytocin?
Last month, I wrote in the New York Times about a creepy yet potent way to reverse aging. All you have to do is join an old mouse to a young mouse. As the young mouse’s blood flows through the old mouse’s body, it rejuvenates the heart, skeletal muscle, and even the brain.
When scientists saw just how dramatic this reversal could be, they started investigating how it happens. They suspected that it wasn’t blood as a whole that was responsible for the transformation. Blood is a finely blended consommé of cells and free-floating molecules. It was possible that only certain compounds in young blood are required to counter aging. That would be excellent if true, since it would put a damper on any vampire-like strategies for applying this discovery to people. All old people would need to do was take a pill containing the compounds that bring about the change.
As I wrote in my article last month, scientists identified one protein, called GDF11, may help reverse aging in the young blood experiments. But they suspected that more than one molecule would be involved. And today, a team of scientists at the University of California at Berkeley are publishing evidence in favor of a new molecule. What makes this result especially surprising is that this molecule is already fairly famous for other effects on the body. It’s oxytocin.
You may have heard of oxytocin as a love drug, or as a moral molecule. It is certainly true that this hormone, which is produced in the brain, plays some important roles in the social life of mammals. Monogamous voles, for example, appear to depend on oxytocin to strengthen their bonds with their mates. When scientists prevent their cells from taking up oxytocin, the voles become more promiscuous. Likewise, oxytocin plays a part in the mother-child bond. Its concentration rises in women during pregnancy and nursing. If ewes are blocked from taking up oxytocin, they neglect their newborn lambs.
But, as fellow Phenom Ed Yong explained in this 2012 Slate piece, at this point oxytocin should really be called the “hype hormone.” People are way too eager to leap from the existing evidence about oxytocin’s effects to calls for its use as a therapy for children with depression or autism. And as Ed wrote in April in The Scientist, oxytocin also seems to be involved in negative emotions and even lying. Reckless use of oxytocin could have some unwanted effects.
It may seem strange for one little molecule to influence us in so many ways. But that’s true for many hormones. They are signals, but the message they deliver depends on their context. In that respect, hormones are like the words we use to relay messages to each other. Think of the wildly different messages, depending on the context, that just five words can have: “What are you doing here?”
In fact, the effects of oxytocin range far beyond emotions and behaviors. In recent years, for example, scientists have found evidence that oxytocin can reduce osteoporosis and obesity. Oxytocin can relay important signals to cells throughout the body, not just in the brain.
Recently Irina Conboy and her colleagues became intrigued by a few of these experiments. When scientists remove the ovaries from female mice, for example, their levels of oxytocin drop dramatically. The mice also start to age rapidly. Could there be a cause and effect there?
Another clue came from the receptors on the surfaces of cells that can grab oxytocin. The cells with these receptors include stem cells that can produce new muscle. Was it possible that oxytocin sent these cells a signal to develop and renew old muscle?
Clues like these fostered a hunch in the minds of the scientists. Maybe oxytocin was one of the molecules in young blood that could rejuvenate old animals.
As the scientists report in Natural Communications, they ran a series of experiments that strongly suggest that this is indeed the case. They wondered, for example, if naturally aging mice lost oxytocin, in the same way as mice that have their ovaries removed. They found that as mice get old, their oxytocin level drops to a third of the level in young mice. They also found muscle stem cells produce fewer receptors for oxytocin as mice get older.
The scientists then gave oxytocin to old mice. They found that the mice were able to regenerate more new muscle fibers. And when they blocked oxytocin in young mice, the mice couldn’t renew their muscles. In this respect, they became old.
Conboy and her colleagues got a similar result when they engineered mice that could not produce oxytocin. The mice developed normal muscles, but as adults they lost muscle mass much faster than normal mice.
To get a closer look at what oxytocin was doing, the scientists reared muscle stem cells in a dish and added oxytocin to them. Once the cells grabbed onto the hormone, they multiplied quickly. In other words, oxytocin appears to be directly altering the behavior of stem cells, just as the scientists had suspected.
The new study provides a new hypothesis for how we get old. When people are young, they produce lots of oxytocin. On top of whatever psychological effects it may have, that extra oxytocin also tells stem cells to turn into muscle cells, keeping people strong. Young people might also produce GDF11 and other molecules at high levels, and in combination, they may keep all the organs young. And once those signals start to fade in old age, the body starts to fall apart.
Theoretically, giving old people compounds like oxytocin or GDF11 may cause their cells to act young again. The compounds could be the basis for an all-purpose treatment for the diseases of old age, from osteoporosis to heart disease to Alzheimer’s.
Theoretically.
It’s worth bearing in mind that all the studies I’m writing about have only been carried out in mice or rats. We can’t say for sure that the effects would carry over into human trials. We don’t even know if oxytocin is high in children and low in old people–not to mention what the “right” level of oxytocin would be to reverse aging.
It’s also worth bearing in mind that there may very well be a good reason that youth-generating signals fade as we get older. If the signals don’t deliver exactly the right message, in exactly the right context, our cells might misinterpret them in a disastrous way. Instead of just multiplying to restore weakened muscles, for example, stem cells might grow uncontrollably, leading to cancer.
But Conboy and her colleagues respond to that concern by pointing out that we already know a lot about oxytocin as a drug in people. Because its other effects have gained so much attention, it’s already been extensively tested. In its synthetic form, pitocin, it’s approved for use in pregnant women who are past term, in order to speed up their labor. Human clinical trials are already underway to try out oxytocin as treatment for psychological disorders. While it’s not free of side-effects, oxytocin has never been linked to cancer in all the testing that it’s undergone.
Of the many messages oxytocin delivers around our bodies, it’s possible that the message to stay youthful is relatively clear. On the other hand, if there’s one thing oxytocin has taught us so far, it’s that hype can’t replace real research.
June 8, 2014
Life Magnified
If you travel through Dulles Airport in the near future, you may see some lovely scientific images on the walls. It’s an exhibit called “Life: Magnified,” organized by the National Institute of General Medical Sciences, the American Society for Cell Biology and the Metropolitan Washington Airports Authority’s Arts Program. If you aren’t passing through Dulles, you can see the images on the web.
Here are a few of my favorites. You can see high-resolution versions on the web site, plus many others.
First, the mouthparts of a Lone Star tick (an awesome beast):

Igor Siwanowicz, Janelia Farm Research Campus, Howard Hughes Medical Institute, Ashburn, Va.
Neurons in the cerebellum, a region of the brain:

Thomas Deerinck, National Center for Microscopy and Imaging Research, University of California, San Diego
HIV (yellow) attacks an immune cell (blue):

Seth Pincus, Elizabeth Fischer and Austin Athman, National Institute of Allergy and Infectious Diseases, National Institutes of Health
New yeast emerge after two yeast cells have sex:

Juergen Berger, Max Planck Institute for Developmental Biology, and Maria Langegger, Friedrich Miescher Laboratory of the Max Planck Society, Germany
Hairs on a gecko lizard’s toes, allowing them to stick to walls:

Dennis Kunkel, Dennis Kunkel Microscopy, Inc.
An ovary from an anglerfish:
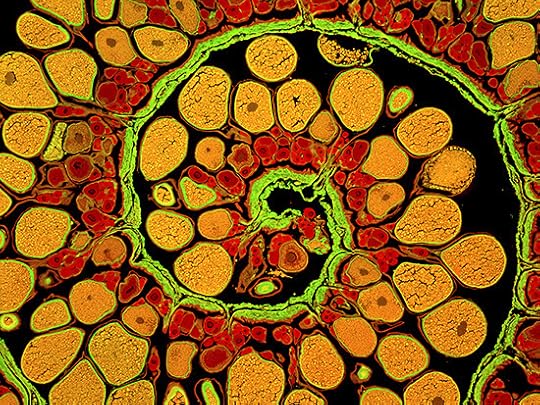
James E. Hayden, The Wistar Institute, Philadelphia, Pa.
June 5, 2014
The Value of Nature–to the Dollar?
The debates raging over how to deal with climate change often swirl around costs. Some warn that doing anything to stop our planet from warming will cost us dearly in jobs and revenue. Others warn that the cost of letting Earth get warmer is far more steep. It could flood cities, worsen droughts, and make it harder to grow food in many places.
Left out of these debates is the effect that climate change will have on nature–and the services that we depend on nature for. We take those services for granted, but if we damage the ecosystems that provide them, we’ll miss them. In my new “Matter” column for the New York Times, I take a look at how some scientists are trying to put a price tag on the global services of ecosystems, including protection against floods and erosion. If they’re right, the value is colossal–about twice the world’s gross product. Check it out.
June 4, 2014
Diagnosis: One Test to Rule Them All?
In the New York Times, I tell the story of a boy named Joshua Osborn who almost died because no one could figure out what made him sick. As House has taught us, diagnosis is an important yet difficult art. But scientists are developing a new way to search for the causes of diseases–by simply looking at millions of pieces of DNA from the patient. In Joshua’s case, a little of the DNA belonged to the culprit–an obscure kind of bacteria called Leptospira–and the discovery pointed to a treatment that quickly wiped it out. This kind of testing is still a long way from regular use, but Joshua’s very existence offers the compelling case that it’s worth trying to develop. Check it out.




