Carl Zimmer's Blog, page 7
January 9, 2015
The common–and fairly awesome–cold virus
Like me, you may be snuffling with a cold today. You’re infected–typically in your nose–with a virus. The dominant cold-causing virusers are known as rhinoviruses, and they’re quite lovely. Here’s I’ve embedded a video of one, which lets you orbit the virus like you’re visiting an alien moon:
(The video is based on this 2014 study of the structure of the rhinovirus shell.)
How is it that rhinoviruses manage to infect so many of us? For my “Matter” column this week in the New York Times, I look at a new study that offers a clue: they may escape the reach of our immune system by lurking in the cool refuge of our noses. Check it out.
January 6, 2015
A Very Different Kind of Selfie
Human sexuality is obviously complicated. But it’s a mistake to think that, if you could somehow strip away human culture, sex would get simple. Even if you could find the simplest animal out there with a sex life, you wouldn’t find that imaginary simplicity.
This week I’ve written an essay on just such an animal, the worm Caenorhabditis elegans. With only a thousand cells in its entire body, the worm is unquestionably simple But it’s also arguably the best-studied animal on the planet. And yet its sex life–featuring self-fertilizing hermaphrodites with some males on the side–remains bizarrely mysterious.
I’ve written an essay for the online magazine Evolution: This View of Life on C. elegans, its strangely complicated sex life, and how that sex life–like other things in biology–is only starting to make sense in the light of evolution. Check it out.
January 1, 2015
On Genes and Time
You’d be forgiven for calling FTO the “fat gene.” There are two variants of the gene, and in study after study, one of those variants, known as rs993609, is associated with more weight, as well as a much higher risk of obesity. The comparison holds up in different countries, and in different ethnic groups. The link is so clear that it might seem like saying FTO can make you fat is as true as saying two plus two equals four.
Now try to imagine discovering that before World War II two plus two equaled zero.
That’s the gist of a new study on FTO’s effects over time. As I explain in my new “Matter” column for the New York Times, scientists have looked at the link between FTO and body-mass index in a long-term study of thousands of people over many decades. They found that people born before 1942 show no link. None. The link is strong in people born afterwards, and gets stronger with time.
I find this study interesting because it provides a twist on the old debate about nature versus nurture. Take two people with identical genes and put them in different environments, and some of their genes may respond in different ways. That’s long been a good counterargument against genetic hyper-determinism. Here’s a case where the entire environment–the world in which we live, work, and eat–has changed. As a result, a gene that would have gone overlooked if scientists looked for it a couple generations ago now leaps out as a clear factor in one of the world’s most pressing medical problems.
It’s possible that other genes will show patterns of their own over time. Putting a time axis on how genes affect our health may make studying the matter far more challenging–as well as using that information to guide medicine. What proves true now may not be true a few years from now.
PS– There’s a fascinating side-story about FTO that I didn’t have space to get into, which University of Chicago Vincent Lynch raised on Twitter. Ever since FTO’s effect was discovered in 2007, scientists have been puzzling over how that effect comes about. They’ve discovered that the FTO protein does all sorts of interesting things, including affecting the brain biochemistry involved in appetite. But a study that came out in March suggests the risk variant may have nothing to do with the FTO protein at all.
“Wha….?” you say? Well, here’s the thing. A gene like FTO is made up of two different kinds of pieces of DNA, called introns and exons. Cells make FTO proteins by only reading the exons of the FTO gene, essentially ignoring the introns. Packing genes with unread DNA may not sound smart, but evolution doesn’t build life the way you might.
It turns out that the rs993609 mutation sits in an intron in the FTO gene. So it doesn’t appear to change the FTO protein. Instead, scientists suspect that the rs993609 mutation sits in a little stretch of DNA in that intron, called an enhancer. They speculate that this enhancer can somehow coax our cells to make copies of another gene that lies elsewhere in our DNA. The rs993609 mutation changes how the enhancer acts on that other gene.
So rs993609 is “in” the FTO gene–in a geographical sense–and yet the FTO gene itself may not make post-war folks fat.
This molecular mystery doesn’t affect the patterns in the new study I wrote about in my column. But it will be important to understanding the biology of obesity. And it serves as a good lesson in how tricky it can be to talk about genes.
December 26, 2014
2014: A Storyful Year
Thanks to everyone for sharing a year of science with me over the course of 2014. It was a year of frantic writing, as I tried (and failed) to keep up with all of the new research that expanded my appreciation of the natural world. In addition to blogging here, I wrote my weekly “Matter” columns for the New York Times, published a few longer pieces, and spent time in Second Edition World, revising a couple of my books. (Details to come in a few months.)
Looking over the year, I put together a list of the pieces I was most fond of (plus some radio work). If you’re looking for some reading (or listening) to fill the languorous spaces between gift-opening and holiday-meal-snarfing, check these out…
From the Loom:
What Slipped Disks Tell Us About 700 Million Years of Evolution
Parasite Cuisine: Eating the Eaters
The Mystery of the Sea Unicorn
The Erotic Endurance of Whale Hips
How A Dog Has Lived For Eleven Thousand Years–In Other Dogs
From the New York Times:
White? Black? A Murky Distinction Grows Still Murkier
The Strange Tale of a New Species of Lizard
Scientists Rein In Fears of Ebola, a Virus Whose Mysteries Tend to Invite Speculation
How Caffeine Evolved to Help Plants Survive and Help People Wake Up
Our Microbiome May Be Looking Out for Itself
Putting a Price Tag on Nature’s Defenses
In a First, Test of DNA Finds Root of Illness
Young Blood May Hold Key to Reversing Aging
The Continuing Evolution of Genes (See also this TED-Ed video for which I wrote the script, based on some the research in this article.)
Seeing X Chromosomes in a New Light
From around the longform universe:
Secrets of the Brain (National Geographic, February)
Why Do We Have Blood Types? (Mosaic, July 15)
The New Science of Evolutionary Forecasting (Quanta, July 14)
How Lives Became Long (the introduction to Rachel Sussman’s photography book, The Oldest Things in the World)
The spoken word:
Worth (Radiolab–I talked about putting a price tag on nature)
The Black Box (Radiolab–I talked about anesthesia and the mysteries of consciousness)
Translation (Radiolab–I talked about how we translate the messages in our genes into our biology)
Safety Carl Versus Gamera (Story Collider)
Darwin in the City (Harvard lecture)
My Guide to the Giant Sandworms of Dune (Studio 360)
Ebola and a Planet of Viruses (Radio Times on WHYY)
December 23, 2014
Antisocial Medicine
One of the biggest surprises to come out of microbiology in recent years is that bacteria have a social life. Rather than existing as lonely, autonomous creatures, bacteria live in communities, in which they cooperate, compete, and communicate. In the January issue of Scientific American, I have a feature about how some scientists are trying to translate their growing understanding of the social life of bacteria into a new kind of medicine. By preventing microbes from cooperating, we may be able to bring infections to a halt. Better yet, this kind of antisocial medicine may even be able to avoid–or at least slow down–the evolution of drug resistance in bacteria.
Here’s the introduction to my piece:
At the University of Zurich, Rolf Kümmerli investigates new drugs to stop deadly infections. He spends his days in a laboratory stocked with petri dishes and flasks of bacteria—exactly the place where you would expect him to do that sort of work. But Kümmerli took an odd path to get to that lab. As a graduate student, he spent years hiking through the Swiss Alps to study the social life of ants. Only after he earned a Ph.D. in evolutionary biology did he turn his attention to microbes.
The path from ants to antibiotics is not as roundabout as it may seem. For decades scientists have studied how cooperative behavior evolves in animal societies such as ant colonies, in which sterile female workers raise the eggs of their queen. A new branch of science—sometimes called “sociomicrobiology”—is revealing that some of the same principles that govern ants can explain the emergence of bacterial societies. Like ants, microbes live in complex communities, where they communicate with one another to cooperate for the greater good. This insight of social evolution suggests a new strategy for stopping infections: instead of attacking individual bacteria, as traditional antibiotics do, scientists are exploring the notion of attacking entire bacterial societies.
New strategies are exactly what we need right now. Bacteria have evolved widespread resistance to antibiotics, leaving doctors in a crisis. The Centers for Disease Control estimates that in the U.S. alone, 23,000 people die of antibiotic-resistant infections. Strains of tuberculosis and other pathogens are emerging that are resistant to just about every antibiotic in medicine’s arsenal. “It already is a substantial problem,” says Anthony S. Fauci, director of the National Institute of Allergy and Infectious Disease. “And there’s every reason to believe it’s going to get even worse.”
The standard response to this crisis has been to slow the evolution of resistance and find new drugs to replace old ones as they grow weak. But this is only a treadmill solution. Bacteria are relentlessly evolving resistance and will continue to do so unless we find a different way to fight them. “Every time we develop a new drug, it fails,” says John Pepper, a theoretical biologist at the National Cancer Institute. “So the solution is, ‘Quick! Make another antibiotic!’ That helps for a few months. But that’s just not good enough any more.”
You can read the rest here. (Subscription to Scientific American required.)
December 16, 2014
Wi-Fi Brain Implants For Robot Arms
For many paralyzed people, their problem is a communication gap. They can generate the signals in their brain require to control their muscles–to walk, to wash dishes, to weed a garden. But damage to their nervous system prevents those signals from reaching their destination.
Last year, in a feature I wrote for National Geographic about the brain, I recounted the work of scientists and engineers who are trying to bridge that gap. Their dream is to create a technology that reads signals from people’s brains and uses them to control machines. The machines might be robot arms that people could use to feed themselves, or computers to compose emails, or perhaps even exoskeletons that could enable people to walk.
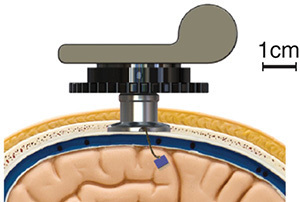
Scientists have been investigating these brain-machine interfaces for decades, and in recent years they’ve made some impressive advances–some of which I described in my story. But it would be wrong to giddily declare that scientists have reached their goal. You need only look at this picture below to get a sense of how far we are from science-fiction dreams.

UPMC
This woman, Jan Scheuermann, is at the forefront of brain-machine interface research. She volunteered to have an electrodes injected into the surface of her brain. Researchers at the University of Pittsburgh connected the electrodes to pedestals on top of her scalp. Cables can be attached to the pedestals; they connect to a computer and a power source.
Scheuermann and the scientists worked together to train the computer to recognize signals from her brain and use them to control a robot arm. In December 2012, Scheuermann made news by controlling the robot arm so well she could feed herself a bar of chocolate.
But this system was hardly ready for prime time. The electrode apparatus has to pass through a hole in a patient’s skull, creating the risk of infection. The cables tether the patient to bulky machines, which would make the whole system cumbersome rather than liberating.
In addition, the robot arm had plenty of room for improvement. It had seven degrees of freedom. Scheuermann could control its shoulder, elbow, and wrist joints. The hand, however, could only open and close. So Scheuermann had the same kind of dexterity as if she wore a mitten.

A 1958 pacemaker–wired to a cart of machines. Source: http://www.ncbi.nlm.nih.gov/pmc/artic...
None of this was any reason to dismiss brain-machine interfaces as having reached a dead end. The history of pacemakers started out in much the same place. Today, people can walk around with pacemakers implanted in their chests without anyone around them having the slightest awareness that a device is regulating their heartbeat. Sixty years ago, however, the first pacemakers were enormous, cumbersome affairs. Implanted electrodes were tethered to wires that ran to big machines. Patients either had to lay next to the machines or trundle them around on a cart. The pacemakers were also relatively simple, delivering fixed patterns of electricity to the heart. Too often, they failed to keep the heart working.
In the 1960s, pacemakers became portable and battery-powered. They still needed external wires, but the wires now ran to a small box that a patient could carry on a belt. Finally, pacemakers disappeared into the body completely. In 2009, doctors began implanting pacemakers that not only had their own power supply but could also communicate medical information to doctors with a Wi-Fi connection to the Internet. Pacemakers also deliver more sophisticated signals to the heart, using algorithms to adjust their rhythms. If someone looked at the ungainly state of pacemakers in 1960 and declared them hopeless, they would have been profoundly wrong.
Two new studies are pushing brain-machine interfaces forward in the same way.
Yin et al Neuron 2014 http://dx.doi.org/10.1016/j.neuron.20... 
The first study advances the electrode end of the interface. A team of scientists led by Arto Nurmikko of Brown University developed an implant that requires no wires. The implant can pick up signals from 100 different electrodes. It contains microelectronics that can turn these signals into a Wi-Fi transmission broadcast at a rate of 200 Mb per second. The researchers implanted the device in monkeys and found that they could pick up signals from five yards away with a quality on par with signals delivered by cables. The monkeys went about their business freely, and the scientists could pick out signals they used to walk on a treadmill. When the monkeys fell asleep, the scientists could detect shifts in their brain waves. The whole apparatus runs for over two day straight on a double AA battery.
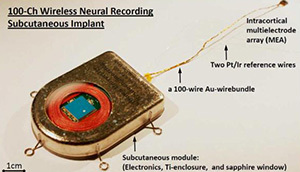
Yin et al IEEE Trans Biomed Circuits Syst. Apr 2013; 7(2): 115–128. doi: 10.1109/TBCAS.2013.2255874
For now, this device will probably be most useful to researchers who study the behavior of animals. But Nurmikko and his colleagues are also learning lessons for the next generation of brain-machine interfaces for people. In another promising line of research, they have designed a prototype of a fully implantable device. The electrodes go in the brain, while the power source and transmitter sit atop the skull, below the scalp. In the future, scientists may be able to make new devices that take advantage of both studies–implants that can be sealed in the head, transmit a lot of data wirelessly, run efficiently on a long-lasting battery, and not heat up the way electronics sometimes do.
Meanwhile, at the other end of the interface, Scheuermann has been testing out a new and improved robot arm. The Pittsburgh team programmed four different positions that the hand could take, such as pinching the index and thumb together. The researchers had no idea if all those extra degrees of freedom would be too much for their interface to handle. Could it pick out signals in Scheuermann’s brain that were meaningful enough to make full use of the arm’s range of motion?
To train Scheuermann, the scientists had her start her practice on a virtual robot arm, which she used to grab virtual objects on a computer screen. The computer system learned how to recognize certain patterns of neuron signals as commands to change the shape of the robot hand. At the same time, Scheuermann’s own brain became more adept at controlling the robot arm, producing stronger signals. Finally, the scientists had Scheuermann try to pick up a number of different objects. Here’s a sampling of her successes:
Scheuermann, as the scientists had hoped, learned how to manage her new arm. It wasn’t a perfect education. Scheuermann sometimes failed to grab objects, and the scientists never managed to record a success on certain tasks, such as pouring water from one glass into another.
Still, the results were encouraging–and sometimes intriguing. The scientists found some groups of neurons that would fire together in distinctive patterns as Scheuermann moved the arm through all ten dimensions. In other words, these neurons weren’t limited to just bending the elbow or pinching a thumb. In the future, it may be possible to harness these flexible signals to make the arms even more proficient, and to fill the communication gap even more.
Wifi Brain Implants For Robot Arms
For many paralyzed people, their problem is a communication gap. They can generate the signals in their brain required to control their muscles–to walk, to wash dishes, to weed a garden. But damage to their nervous system prevents those signals from reaching their destination.
Last year, in a feature I wrote for National Geographic about the brain, I recounted the work of scientists and engineers who are trying to bridge that gap. Their dream is to create a technology that reads signals from people’s brains and uses them to control machines. The machines might be robot arms that people could use to feed themselves, or computers to compose emails, or perhaps even exoskeletons that could enable people to walk.
Scientists have been investigating these brain-machine interfaces for decades, and in recent years they’ve made some impressive advances–some of which I described in my story. But it would be wrong to giddily declare that scientists have reached their goal. You need only look at this picture below to get a sense of how far we are from science-fiction dreams.

UPMC
This woman, Jan Scheuermann, is at the forefront of brain-machine interface research. She volunteered to have an electrodes injected into the surface of her brain. Researchers at the University of Pittsburgh connected the electrodes to pedestals on top of her scalp. Cables can be attached to the pedestals; they connect to a computer and a power source.
Scheuermann and the scientists worked together to train the computer to recognize signals from her brain and use them to control a robot arm. In December 2012, Scheuermann made news by controlling the robot arm so well she could feed herself a bar of chocolate.
But this system was hardly ready for prime time. The electrode apparatus has to pass through a hole in a patient’s skull, creating the risk of infection. The cables tether the patient to bulky machines, which would make the whole system cumbersome rather than liberating.
In addition, the robot arm had plenty of room for improvement. It had seven degrees of freedom. Scheuermann could control its shoulder, elbow, and wrist joints. The hand, however, could only open and close. So Scheuermann had the same kind of dexterity as if she wore a mitten.

A 1958 pacemaker–wired to a cart of machines. Source: http://www.ncbi.nlm.nih.gov/pmc/artic...
None of this was any reason to dismiss brain-machine interfaces as having reached a dead end. The history of pacemakers started out in much the same place. Today, people can walk around with pacemakers implanted in their chests without anyone around them having the slightest awareness that a device is regulating their heartbeat. Sixty years ago, however, the first pacemakers were enormous, cumbersome affairs. Implanted electrodes were tethered to wires that ran to big machines. Patients either had to lay next to the machines or trundle them around on a cart. The pacemakers were also relatively simple, delivering simple patterns of electricity to the heart. Too often, they failed to keep the heart working.
In the 1960s, pacemakers became portable and battery-powered. They still needed external wires, but the wires now ran to a small box that a patient could carry on a belt. Finally, pacemakers disappeared into the body completely. In 2009, doctors began implanting pacemakers that not only had their own power supply but could also communicate medical information to doctors with a WiFi connection to the Internet. Pacemakers also deliver more sophisticated signals to the heart, using algorithms to adjust their rhythms. If someone looked at the ungainly state of pacemakers in 1960 and declared them hopeless, they would have been profoundly wrong.
Two new studies are pushing brain-machine interfaces forward in the same way.

Yin et al Neuron 2014 http://dx.doi.org/10.1016/j.neuron.20... 
The first study advances the electrode end of the interface. A team of scientists led by Arto Nurmikko of Brown University developed an implant that requires no wires. The implant can pick up signals from 100 different electrodes. It contains microelectronics that can turn these signals into a WiFi transmission broadcast at a rate of 200 Mb per second. The researchers implanted the device in monkeys and found that they could pick up signals from five yards away with a quality on par with signals delivered by cables. The monkeys went about their business freely, and the scientists could pick out signals they used to walk on a treadmill. When the monkeys fell asleep, the scientists could detect shifts in their brain waves. The whole apparatus runs for over two day straight on a double AA battery.

Yin et al IEEE Trans Biomed Circuits Syst. Apr 2013; 7(2): 115–128. doi: 10.1109/TBCAS.2013.2255874
For now, this device will probably be most useful to researchers who study the behavior of animals. But Nurmikko and his colleagues are also learning lessons for the next generation of brain-machine interfaces for people. In another promising line of research, they have designed a prototype of a fully implantable device. The electrodes go in the brain, while the power source and transmitter sit atop the skull, below the scalp. In the future, they may be able to make new devices that take advantage of both studies–implants that can be sealed in the head, transmit a lot of data wirelessly, run efficiently on a long-lasting battery, and not heat up the way electronics sometimes do.
Meanwhile, at the other end of the interface, Scheuermann has been testing out a new and improved robot arm. The Pittsburgh team programmed four different positions that the hand could take, such as pinching the index and thumb together. The researchers had no idea if all those extra degrees of freedom would be too much for their interface to handle. Could it pick out signals in Scheuermann’s brain that were meaningful enough to make full use of the arm’s range of motion?
To train Scheuermann, the scientists had her start her practice on a virtual robot arm, which she used to grab virtual objects on a computer screen. The computer system learned how to recognize certain patterns of neuron signals as commands to change the shape of the robot hand. At the same time, Scheuermann’s own brain became more adept at controlling the robot arm, producing stronger signals. Finally, the scientists had Scheuermann try to pick up a number of different objects. Here’s a sampling of her successes:
Scheuermann, as the scientists had hoped, learned how to manage her new arm. It wasn’t a perfect education. Scheuermann sometimes failed to grab objects, and the scientists never managed to record a success on certain tasks, such as pouring water from one glass into another.
Still, the results were encouraging–and sometimes intriguing. The scientists found some groups of neurons that would fire together in distinctive patterns as Scheuermann moved the arm through all ten dimensions. In other words, these neurons weren’t limited to just bending the elbow or pinching a thumb. In the future, it may be possible to harness these flexible signals to make the arms even more proficient, and to fill the communication gap even more.
December 10, 2014
The Caterpillar Defense
Let’s say you’re a baby bird. In particular, you’re a chick belonging to the species Laniocera hypopyrra, which also goes by the elegant common name of the cinereous mourner. You hatch out of your egg and find yourself in a nest up in tree in a rain forest in Peru. You can’t fly. You can only wait for your parents to bring you food. You are, in other words, easy pickings.
So what might you do to avoid getting snatched up by a predator? Perhaps you might hold very still so as not to attract attention. Perhaps you might also grow dull, bark-colored feathers to help you blend into you background.
If you’re a cinereous mourner, however, this is not what you do. As you can see in this video, you grow brilliant orange plumage. You make yourself absurdly easy to see.
In 2012, a team of scientists first pointed out how odd it was that cinereous mourner chicks were so bright. Weirder still, the chicks become drab as they grow up. The researchers based their study solely on museum specimens of the bird, so they couldn’t learn all that much about what it’s like to be a bright orange chick in a predator-rife jungle. But they proposed that the chicks were actually ensuring their safety by growing bright feathers. They might be poisonous, for example, with toxic chemicals in their feathers. Their bright colors were thus a warning. Or perhaps they were harmless, but they were mimicking some unknown animal that was harmful.
Now Gustavo A. Londoño of the University of California at Riverside and his colleagues have observed cinereous mourner chicks in the wild. They’re the ones who filmed the footage above. They were struck by the way that the chicks creep around their nests, like a caterpillar. Not just any caterpillar mind you. Like this one:
This bizarre caterpillar, which lives near cinereous mourner nests is enormous. It’s 12 centimeters long, or about the size of a cinereous mourner chick. And its hairs are tipped with an irritating toxin. Londoño and his colleagues propose that the chicks both behave and look like this caterpillar (which belongs to a yet-to-be-described species).

Londono et al 2014 American Naturalist
The resemblance goes beyond just color, Londoño observes. The chicks grow unusual feathers with long white tips–which resemble the hairs of the caterpillars, as shown in this figure.
Londoño argues that cinereous mourner chicks practice something called Batesian mimicry. It’s a strategy that scientists have found in many species, especially butterflies. But this is the first time anyone’s found a possible case of Batesian mimicry in a bird. It’s possible that the cinereous mourner chicks were driven to evolve this unusual strategy because they’re especially vulnerable. Londoño and his colleagues have found that predators kill off chicks in at least eighty percent of nests, possibly because the parents don’t guard them enough. Under such extreme risk, the chicks have evolved an extreme solution.
December 5, 2014
Masters of Electricity (The Video Version)
This week I wrote my New York Times column about one of those remarkable studies that makes you realize how little we understand about the natural world. Ken Catania, a biologist at Vanderbilt University, performed some elegantly simple experiments that revealed that electric eels use electricity as a taser and as a remote control for their prey.
To read mostly straight-text accounts of the research, you can read my column, as well as fellow Phenom Ed Yong’s blog post. But the video that Catania filmed as part of his research is so interesting I thought offer a moving-pictures recap here.
Here’s what it’s like when an eel goes after a fish. The eel’s pulses of electricity are converted to sound. Basically, there’s an electric blast, a lunge, and it’s all over for the little fish.
But when Catania slowed down his video (he films it at 1000 frames a second), he could see that right after the eel starts sending out pulses, the fish just froze. Here are a couple examples. The red frames show when the eel is discharging electricity. The blast contracts all the fish’s muscles at once, causing the animal to freeze. It’s a natural taser.
Finally, here’s a movie that shows how the eel can flush out hidden prey. The fish here has its brain removed, and it’s tucked away in a walled-off recess in the eel’s tank. The barrier is made of agar, which allows electricity and waves to pass through.
The eel comes up, giving off short paired pulses of electricity. These pulses are like probes. Instead of immobilizing the fish, the pulses make the fish twitch. The waves set off by the twitch reveal its hiding place, and the eel comes in for a taser attack.
The full paper is here.
November 20, 2014
Your Inner Feather
Feathers are like eyes or or hands. They’re so complex, so impressive in their adaptations, so good at getting a job done, that it can be hard at first to believe they evolved. Feathers today are only found on birds, which use them to do things like fly, control their body temperature, and show off for potential mates. The closest living relatives of birds–alligators and crocodiles–are not exactly known for their plumage. At least among living things, the glory of feathers is an all-or-nothing affair.
But the more we get to know feathers, the more we can appreciate how they evolved. The general rule is that complex things–be they feathers, hands, or eyes–take a very long time to evolve. As I wrote in National Geographic in 2011, the fossil record has gone a very long way in helping us to understand how feathers took on the form we see today. Birds evolved from dinosaur ancestors, and those ancestors already had feathers. Feathers started out as simple filaments, turning to fuzz, and then diversifying into a lot of different forms–including the ones that eventually let birds take to the air.
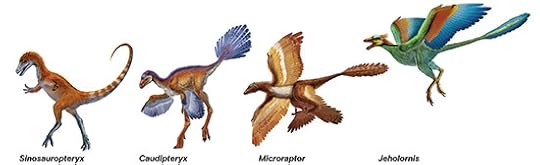
A sampling of feathered dinosaurs and early birds. Xing Lida/National Geographic
Now a new study in the journal Molecular Biology and Evolution offers an even deeper look into the history of feathers. Instead of looking at fossils, the scientists look at the genetic recipe for feathers written in the DNA of birds. It turns out that a lot of that recipe already existed hundreds of millions of years before anything vaguely resembling a feather existed on Earth. In fact, you, my fine unfeathered friend, have most of the genetic information required for making feathers, too.
Scott Edwards, a Harvard ornithologist, and his colleagues couldn’t have carried out this study even a few years ago, because scientists have only recently figured out a lot of the details of how feathers develop. Bird embryos starts out featherless. But in their skin, they develop lots of tiny blobs of cells known as placodes in which cells are switching on genes in a distinctive pattern. The reason that certain genes switch on in the placodes and others don’t is that genes have little on-off switches near them. If a particular combination of proteins lands on a gene’s switch, the gene will start making a protein of its own.
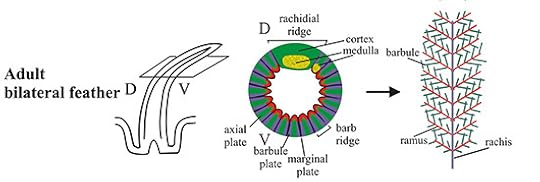
The development of a feather. The middle figure is a cross-section of the primordial feather shown on the left. Source: Ng et al 2012. PLoS Genet 8(7): e1002748
At first, the cells in the placodes multiply quickly. Then they start grow into shafts, which then split open to form feathers. Depending on the bird, and on the spot on the bird’s body where it grows, the feather may split into a downy plume, or into a paddle-shaped flight feather, or into an ornamental tail feather. Along the way, the cells differentiate, producing different combinations of proteins. The cells that make up the central shaft of the feather are stiffened with certain types of keratin, for example, while cells that are in the more delicate regions of the feather produce more flexible forms of the protein from different genes. Clusters of cells produce pigment molecules to give the feather colors and patterns. Every cell has an entire genome, which means it has all the genes for making any part of the feather. But its switches ensure that it only uses a certain combination of those genes.

Frizzled chicken. Photo by Alisha Vargas via Creative Commons https://flic.kr/p/7XmQqu
Edwards and his colleagues combed the scientific literature for genes that are important for feather development. Scientists have studied frizzled chickens, for example, identified the mutation for the breed’s frizzles, and thereby identified a gene essential for developing feathers. All told, Edwards and his colleagues found 193 feather genes this way. Their list included 67 that encode variations of keratin, and 126 that help establish the pattern of feathers.
Next, the scientists searched for the switches that control those genes. This isn’t so easy. The switches are short stretches of DNA, often nestled deep inside much longer stretches of DNA that are just gibberish. What’s more, genes have distinctive segments that let you know you’re looking at a gene. The switches are much harder to distinguish from the gibberish.
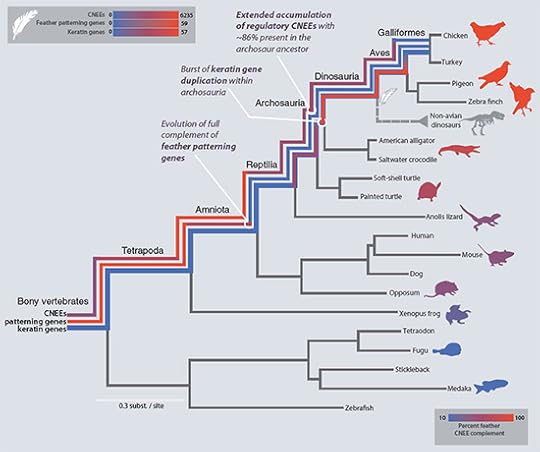
The scientists used several strategies to zero in on the switches. They took advantage of the fact that most switches for a gene are close to the gene itself. So they only searched in the neighborhood of the 167 feather genes. They also took advantage of the fact that switches evolve relatively little, because most mutations will be harmful to them. So the scientists compared the DNA around feather genes in several different species and sought out the stretches that were noticeably similar from one species to the next. Using these two strategies, the scientists discovered a staggering 13,307 feather gene switches (technically known as conserved nonexonic elements, or CNEEs for short).
Next, the scientists asked when each part of this feather cookbook evolved. If they found a gene or a switch only in the DNA of birds, then they could be confident that it had evolved after the ancestors of birds split off from the ancestors of alligators and crocodiles. But if they found a gene or a switch in birds and alligators and crocodiles, then it must have evolved earlier, in their common ancestor.
(You may be asking, how did these new genes evolve? The short answer is that they can evolve through the duplication of old genes, or the transformation of genetic gibberish–a k a noncoding DNA. If you want to get more details, watch this TED-Ed video I wrote the script for.)
To see how far back the evolution of feather genes went, the scientists compared birds to a wide range of vertebrates, including humans, turtles, and pufferfish. They found that the instructions for making feathers got their start a long, long time before feathers themselves (see the tree at the bottom of the post or embiggen it here). The genes that establish the basic pattern of placodes already existed in the common ancestor of living fish and birds (and us)–in other words, about half a billion years ago. Even more feather genes evolved as our common ancestors climbed ashore and walked around on land 350 million years ago. Many switches for feather genes also emerged during this period, too.
About 300 million years ago, our ancestors began to lay hard-shelled eggs. Those early animals would give rise to mammals, reptiles, and birds (collectively known as amniotes, named for the amniotic egg). Edwards and his colleagues found that the first amniotes already had the entire complement of feather patterning genes. That means you, as an amniote, have them too.
Later, the early amniotes split away into their major lineages. The lineage that includes alligators, birds, and extinct dinosaurs–called archosaurs–originated about 250 million years ago. Edwards and his colleagues detected many new keratin genes evolving during the origin of archosaurs, along with 86 percent of their 13,000 or so feather gene switches.
It would not be another 100 million years or so before the oldest known birds flew. And yet just about everything you need to make a feather, genetically speaking, was already in place.
It may seem strange to consider the fact that you, as a mammal, have all the known genes required to pattern a feather, and yet you do not look like Big Bird. The reason for this discrepancy is that genes can do different jobs. Depending on where and when they make their proteins, they can build different kinds of anatomy. But it didn’t take much rewiring of genetic switches to turn the scaly skin of early reptiles into feathers. Indeed, the deep history of feathers could explain why paleontologists are finding so much evidence of simple feather-like filaments not just in dinosaurs, but in their close relatives like pterosaurs. Evolution was tinkering with the same toolkit.
Edwards and his colleagues noticed something else intriguing in the genomes of birds. They found a lot of switches that were not near feather genes, but were unique to birds. When the scientists looked for the nearest genes, they noticed that many of the genes help birds grow. They control the size of bird bodies, for example, or the size of their limbs.
This is an intriguing finding, because the fossil record reveals that as dinosaurs evolved into birds, their bodies shrank, while their arms got long for their size. The shift made it possible for birds to generate a lot of lift with big wings, which only had to keep a small body aloft.
Edwards and his colleagues may have found the molecular signature of that change. If they’re right, the cookbook for feathers is very old, but it took the evolution of a new kind of body for birds to use their feathers to fly.
From Lowe et al 2014 (Click to embiggen)
With apologies to Neil Shubin for riffing on his title.



