Carl Zimmer's Blog, page 6
March 4, 2015
Genius and the Brain
The 92nd St. Y in New York is presenting “Seven Days of Genius” this week. As part of the festivities, the video site Big Think invited me to film a conversation with neuroscientist Heather Berlin about the nature of genius and the origin of creativity in the brain.
Here’s the video, which we taped at YouTube headquarters:
March 2, 2015
We Are Instant Number Crunchers
If you have ever struggled through a math class, you may not think of numbers as natural. They may seem more like a tool that you have learn how to use, like Excel or a nail gun. And it’s certainly true that numbers pop in the archaeological record just a few thousand years ago, with the abruptness you’d expect from an invention. People then improved the number system after that, with the addition of zero and other upgrades.
But scientists have found that we are actually born with a deep instinct for numbers. And a new study suggests that our number sense operates much faster than previously thought. It might be better called our number reflex.
Some of the most compelling evidence for the number sense comes from studies on babies. In a 2010 study, for example, Elizabeth Brannon of Duke and her colleagues showed 6-month-old babies pictures of dots. As they switched between different pictures, they tracked how long the babies looked at each one. In some cases, the pictures were identical. In others, the dots differed in size or spacing. And in still other cases, Brannon and her colleagues added extra dots to the pictures.
When Brannon and her colleagues looked over their data, they found that the attention of the babies tended to be grabbed when they switched the number of dots. What’s more, the babies looked longer at a picture when the difference in the number was bigger.
The number sense in infants is the raw material for math aptitude later in life, as Brannon documented when she followed up on the infants three years later. Brannon found that their sensitivity to numbers as six-month-olds predicted how well they scored on math tests as three-year-olds. Other scientists have also found that a link between number sense and math skills in fourteen-year-olds.
Having discovered our number sense, Brannon and other researchers have begun probing our brains to see how it works biologically. It’s not easy to tease out the number sense from all the other things our brains do when they take in a visual scene. There’s a huge amount of information to decipher in an instant of vision, and our brains use a complex network of regions to get the job done.
When light hits our eyes, the retina takes the first pass at processing the image and then fires signals down the optic nerve to the back of the head. The visual cortex then teases out some basic features, such as brightness, edges, color, and so on. The regions where this processing takes place then send signals to other parts of the brain, which detect more complex things, like body movements and faces.
Some researchers have proposed that our awareness of numbers only emerges late in this pathway. We may first have to detect other features of a scene, and then analyze them in order to figure out how many objects there are in a group. If we look at three lemons on a counter, for example, we might first have to calculate the total area of yellow in our field of vision, determine how much yellow is in each lemon, and then divide the former by the latter.
To probe where our number sense lies on the path of thought, Brannon and her colleagues placed EEG caps on people’s heads. Then they showed their volunteers pictures of dots. As in Brannon’s earlier experiments, they varied the pictures with extra dots, as well as changing the size or spacing. Each time, the scientists recorded the electricity produced by people’s brains as they processed what they saw.
Analyzing the different responses, the scientists noticed one fascinating spike of electrical activity that emerged from the back of the brain. The strength of the spike varied with the number of dots people saw. The more dots, the bigger the spike.
The size and spacing of the dots, by contrast, had no effect on the spike. If we sensed numbers only by analyzing other features of objects, then you might expect to see an influence. But Brannon and her colleagues could find none. They conclude that this spike represents our direct detection of numbers.
What makes this spike even more intriguing is how fast it occurs: just 75 millisecond after the scientists present a picture. At that stage in visual perception, the visual cortex is just starting to process signals from the eye. Numbers, the new research suggests, are so important that we start sensing them before we’re even aware of what we’re seeing.
(For more on our number sense and other discoveries about the brain, see my ebook anthology, Brain Cuttings.)
February 22, 2015
Is It Worth Imagining Airborne Ebola?
Back in September, when the West African Ebola outbreak was getting worse with every passing week, a lot of people began to worry that the virus could spread by air. And even if it couldn’t spread by air yet, they worried that it might be on the verge of mutating into an airborne form.
When I talked to virus experts, they saw little ground for either concern. The epidemiology of the outbreak, like previous ones, had the sort of pattern you’d expect from a virus that spreads mainly through contact with body fluids. A look at the evolutionary history of viruses indicates that a fluid-adapted virus would be unlikely to switch to going airborne with just a couple mutations. (I wrote in the New York Times about these conversations here and here.)
The anxiety over airborne Ebola has faded. The outbreak itself has dwindled down dramatically, although driving it down to zero may prove hard. But a new “Opinion/Hypothesis” piece published in the journal mBio, called “Transmission of Ebola Viruses: What We Know and Do Not Know,” has breathed some new life into the old worry.
The piece was written by Michael Osterholm of the University of Minnesota and a number of other researchers. Back in September, Osterholm wrote a controversial op-ed in the New York Times, declaring, “If certain mutations occurred, it would mean that just breathing would put one at risk of contracting Ebola.”
In the new mBio piece, Osterholm and his colleagues survey a number of past studies on how Ebola spreads. These studies don’t tell us as much as we’d like. We know less about Ebola than we do about, say, influenza, because it’s a lot rarer and a lot deadlier. Scientists thus have fewer opportunities to study it, and when they do, they have to take enormous precautions. But the evidence we do have offers a pretty clear picture, Osterholm and his colleagues write: “Available data indicate that direct physical contact and exposure to infected body fluids are the primary modes of Ebola virus transmission.”
Those fluids may be the blood of a sick patient, or diarrhea, vomit, or sweat. People can get infected by touching those fluids, but it’s also conceivable that the virus can reach a new victim in a spray of fluid. The droplets in these fluids don’t travel far, so they don’t create airborne transmission in the same sense that a virus like measles is airborne–with tiny aerosols drifting on air currents. Some animal studies have shown that Ebola can spread without direct contact, but they don’t demonstrate clear evidence that aerosols delivered the virus. Still, Osterholm and his colleagues note that when Ebola victims are autopsied, the viruses sometimes turn out to be present in their lungs. A cough or a sneeze could conceivably deliver virus-laden aerosols.
While that’s theoretically possible, Osterholm and his colleagues acknowledge that this route has never been documented in humans. “This could be because such transmission does not occur or because such transmission has not been recognized, since the number of studies that have carefully examined transmission patterns is small,” they write.
There are other factors in Ebola outbreaks that we still don’t understand well. Some evidence suggests that certain people may become “superspreaders,” transmitting Ebola to many more people than usual, but we don’t know what’s responsible for these differences. It’s also possible that different strains of Ebola have genetic differences that cause some to spread faster than others. Some preliminary studies suggest that people who got sick in the West African outbreak build up more viruses in their bodies than people in earlier outbreaks.
After surveying what we do and don’t know about Ebola transmission, the authors offer what they call a hypothesis: Ebola might indeed be able to become airborne. Infected people might cough up virus-laden droplets, which other people might then breathe into their lungs, setting off an infection. Mutations could make this route easier for the virus to take. “We agree this is an improbable (although not impossible) scenario,” Osterholm and his colleagues acknowledge, but they point out that Ebola has sprung many surprises on us in the past. “We should not assume that Ebola viruses are not capable of surprising us again at some point in the future,” they conclude.
I got in touch with some other experts to see what they thought about this new piece. The most positive of them was Pardis Sabeti, a Harvard scientist who has been analyzing the genes of Ebola viruses to track their evolution. “I think that overall it is a really nice and thorough review,” she told me. She agreed it was important to figure out whether different Ebola lineages spread differently. As for airborne Ebola, she considered it unlikely although not impossible. “We should continually monitor its properties as it continues to evolve,” she said.
But other researchers were less enthusiastic. “I don’t see any new data that really sheds any new light on things in terms of the outbreak,” said Thomas Giesbert of the University of Texas. Most of the scientists I reached out to found the hypothesis of airborne Ebola even less impressive. “I guess you can make hypothesis about anything, and a ‘hypothesis’ about ‘potential’ isn’t very strong,” said Edward Holmes of the University of Sydney. “It fails to deliver,” said Vincent Munster of the National Institutes of Health.
Vincent Racaniello of Columbia was even harsher: “It can be viewed as a scare tactic, although to what ends I do not know,” he said.
Racaniello and the other critics note that there’s no evidence of that the Ebola virus has evolved in any significant way during the latest outbreak. And the fact that no one has found compelling evidence of aerosol transmission since the virus was discovered in 1976 suggests that shifting to that route is a major challenge, not an easy evolutionary maneuver.
In fact, viruses in general don’t show the massive evolutionary potential that Osterholm and his colleagues see in Ebola. Smallpox and influenza have infected billions of people by airborne transmission for thousands of years, and there’s no evidence that they have evolved a new route. Poliovirus and norovirus take the oral route, as they always have.
“No human virus has ever changed the way it is transmitted – at least in the 100 years or so we have been studying them,” said Racaniello. “There is no reason to believe that Ebolaviruses will become respiratory pathogens.”
(For more, see this blog post Racaniello published this weekend. For more on viruses generally, see my book A Planet of Viruses.)
February 10, 2015
Parasitic Wasps Infected with Mind-Controlling Viruses
 In November, National Geographic put a ladybug and a wasp on its cover. They made for a sinister pair. The wasp, a species called Dinocampus coccinellae, lays an egg inside the ladybug Coleomegilla maculata. After the egg hatches, the wasp larva develops inside the ladybug, feeding on its internal juices. When the wasp ready to develop into an adult, it crawls out of its still-living host and weaves a pupa around itself.
In November, National Geographic put a ladybug and a wasp on its cover. They made for a sinister pair. The wasp, a species called Dinocampus coccinellae, lays an egg inside the ladybug Coleomegilla maculata. After the egg hatches, the wasp larva develops inside the ladybug, feeding on its internal juices. When the wasp ready to develop into an adult, it crawls out of its still-living host and weaves a pupa around itself.
As I wrote in the article that went with that photograph, the ladybug then does something remarkable: it becomes a bodyguard. It hunches over the wasp and defends it against predators and other species of parasitic wasps that would try to lay their eggs inside the pupa. Only after the adult wasp emerges from its pupa does the bodyguard ladybug move again. It either recovers, or dies from the damage of growing another creature inside of it.
How parasites turn their hosts into zombie slaves is a tough question for scientists to answer. In some cases, researchers have found evidence suggesting that the parasites can release brain-controlling chemicals. But the wasp uses another strategy: there’s a parasite within this parasite.
In the Proceedings of the Royal Society, a team of French and Canadian researchers now lay out the evidence for this strange state of affairs. As they studied this manipulation, they reasoned that the best place to look for clues was inside the heads of parasitized victims. They discovered that the brains of the bugs were loaded with viruses. When the scientists sequenced the genes of the virus, they found it was a new species, which they dubbed D. coccinellae Paralysis Virus, or DcPV for short.
The scientists found DcPV in the wasps as well–but not in their brains. In female adult wasps, the virus grows in the tissues around their eggs. Once a wasp egg hatches inside a lady bug, the virus starts replicating inside it, too. The larva then passes on ladybug develops an infection as well.
DcPV causes no apparent harm to the wasps, but the ladybug is not so lucky. The virus makes its way into the ladybug’s head, where it attacks brain cells and produces new viruses in pockets inside the cells. Many brain cells die off during the infection.
The researchers hypothesize that the virus is responsible for the change in how the ladybug behaves. To get the ladybug to guard the wasp, the virus may partially paralyze its host, so that it becomes frozen over the parasite. Because the paralysis isn’t complete, the ladybug can still lash out against predators. But these may just be wild spasms in response to any stimulus. The bodyguard effect may become stronger as the infection robs the ladybug of the signals from its eyes and antennae.
The fate of a parasitized ladybug–to die or to walk away–may depend on how it handles a DcPV infection. In some cases, the virus may be fatal–possibly by triggering a massive immune response that kills not just the virus but the ladybug itself. In other cases, the ladybug’s immune system may eventually be able to clear the virus out of its system, letting its nervous system heal.
In either case, the bodyguard paralysis lasts long enough to protect the wasp while it develops into an adult. Whether the ladybug lives or dies doesn’t matter to the wasp–or to the virus. The new wasp carries a fresh supply of DcPV. If it’s a female, it will be able to use the virus to infect both its own young, and its ladybug slave.
In recent years, scientists have developed a deepening appreciation for the importance of our microbiome–of the bacteria and viruses that make our bodies their home. While some microbes invade our bodies, others reside inside of us and help keep us healthy. Parasitic animals have microbiomes of their own, and this new study suggests that they can use them for suitably sinister ends.
(For more information on the sinister tricks of parasites, see my book Parasite Rex.)
February 5, 2015
How The Measles Virus Became A Master of Contagion
Here are two recent stories about viruses. They started out alike, and ended up very differently.
In October, a woman in Guinea died of Ebola, leaving behind two sisters, one of them two years old, the other five. A relative named Aminata Gueye Tamboura took the orphaned children back to her home in northwest Mali–a 700-mile journey. Tamboura didn’t know it then, but the girl, named Fanta Conde, was infected with Ebola as well. For three days, they traveled on buses and in taxis as Fanta grew ill, developing a scorching fever and a perpetual nosebleed. Soon after arriving in Mali, she died.
Yet Tamboura never became infected with Ebola. Nor did Fanta’s sister or her uncle, who also made the trip. Nor did anyone else who shared the buses and taxis with Fanta, or who encountered Fanta elsewhere on her doomed journey. After Fanta’s death, the entire country of Mali braced for a devastating outbreak. But the outbreak never came.
The other story began in December. Someone–we don’t know who–paid a visit to Disneyland in California. That person, who we’ll call Patient Zero, was infected with the measles virus. But Patient Zero probably didn’t realize that he or she was incubating it, because the obvious symptoms, such as a rash and a high fever, wouldn’t emerge for several days. Strolling around Disneyland, Patient Zero cast off the measles virus in all directions, infecting dozens of people. Those people later developed measles, and may have spread the virus to others. By the end of January, the Disneyland outbreak had reached 94 cases, and that number is certain to rise higher.
These two stories show just how different viruses can be. For all the fear that Ebola can inspire, it’s a pretty bad transmitter. Measles, on the other hand, is among the most contagious viruses on Earth. There’s no single secret to measles’s power of contagion. Its adaptations for spreading are sprinkled across its whole life cycle.
While the biology of measles has only come into focus in the past few years, physicians have long been aware of its contagiousness. In 1846, a Danish doctor named Peter Panum recorded the first detailed account of a measles outbreak during his stay on the Faroe Islands, located between Scotland and Iceland. The disease leaped from one village to another. Out of the blue, someone would develop a blotchy pink rash that would spread across the entire body. A fever would ignite. “The patients were bathed in perspiration,” Panum wrote, “and, when the bedding was raised or the shin exposed, vapors literally rose from them like clouds.”
Because the Faroe Islands were so remote, Panum had an easy time observing the disease spread from person to person. He developed an eerie power of prediction. If one person developed a rash from measles, Panum knew that everyone else in the patient’s house would get sick two weeks later.
Panum noticed other predictable patterns, too. On average, he estimated, every infected person infected seven to nine other people. Today, the estimate for the average number of infections spread from each sick person is higher–between 12 and 18. By comparison, the figure for Ebola is only about two.
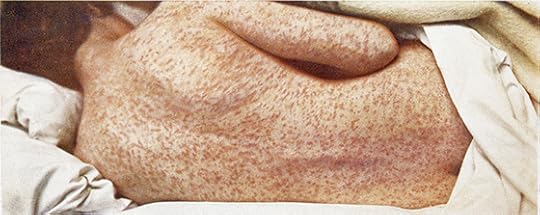
1908 image of measles rash via Wellcome Institute
What made Panum’s observations all the more impressive is that he made them without knowing what was causing the measles outbreak. Scientists would not come to understand the nature of viruses for another five decades. The measles virus itself would not be discovered till in 1954, over a century after Panum’s stay on the Faroe Islands.
For the past five decades, scientists have been studying the measles virus, and yet many details of its life cycle are only now coming to light. As a virus, it has to do three things in order to avoid extinction: it has to invade a new host, make copies of itself, and get those copies to another host. At every step of the way, scientists are finding, the measles virus cranks up its chances of successful spread.
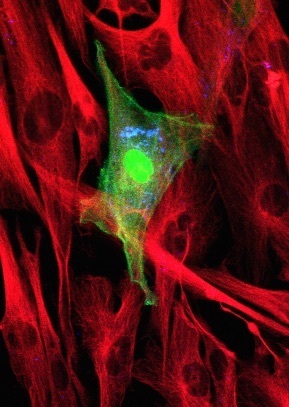
An immune cell infected with measles viruses. The viruses are engineered to produce a green-glowing protein. Photo by Paul Duprex
People get infected with measles viruses by breathing them into their lungs. The lining of the lungs contains immune cells that destroy incoming invaders and kill off infected cells. The measles virus boldly attacks these very sentinels. It uses a molecular key to open a passage into the immune cells. Once inside, it starts making new viruses that infect other immune cells. The virus-laden cells then creep from the windpipe to the lymph nodes, which are crowded with still more immune cells. It’s like a walk in Disneyland, except inside a person’s body. From the lymph nodes, infected immune cells spread the virus throughout the body. If the virus manages to slip into the nervous system, it can cause permanent brain damage.
After several days of multiplying, the virus starts making preparations to leave its host. Some of the infected immune cells creep up into the nose. The interior lining of the nose is made up of sheets of epithelial cells. The immune cells nuzzle up to the epithelial cells. A protein on their surface, made by the viruses, fuses them to the epithelial cells, allowing the virus to cross over. Now the measles virus is another step close to leaving its host and finding a new one.
Each infected epithelial cell start making huge numbers of new measles viruses, which it dumps out into the nasal cavity, where they can get exhaled. Meanwhile, the infection also damages the upper airway, causing infected cells to rip free and get coughed out of the body.
People sick with measles release clouds of virus-laden droplets. The big droplets fall quickly to the ground or other surfaces, where they can stay infectious for hours. The small droplets meanwhile rise into the air, where they are lofted by currents and can deliver measles to people far away.
The sheer number of viruses produced by each sick person, along with the adaptations the viruses have for penetrating deep into the airway, make them tremendously contagious. If someone gets sick with measles, up to ninety percent of people in the same home who aren’t already immune will get sick, too. And because infected people can transmit the virus for days before symptoms emerge, the virus can spread to many homes before anyone realizes an outbreak is underway.
The late-arriving symptoms of measles are the outward sign that people’s immune systems are starting to fight the virus. Much of the battle takes place between uninfected immune cells and infected ones. The fight decimates the immune system. Even after people have conquered a measles infection, it can take weeks for their immune system to get back to full strength.
In this fragile state, people become vulnerable to other diseases such as pneumonia. The danger posed by these infections depends on how much care patients can get. In industrialized countries, only a tenth of 1 percent of people who get measles die. In developing countries, the rate is 5 to 10 percent. In refugee camps, the figure can be as high as 25 percent.
While people cope with these post-infection troubles, the virus has moved on to its next hosts. The contagion of measles is part of a “one-and-done” strategy that the viruses have evolved. After people recover from measles infections, their immune systems will protect them for life. As a result, the virus needs to be highly contagious for its long-term survival.
This strategy also means that measles vaccines can be extremely effective. By teaching people’s immune systems what the measles virus looks like, vaccines provide protection for life.
All these features of the measles virus add up to a startling paradox. Despite being far more contagious than the Ebola virus, the measles virus is a far better candidate for complete eradication from the face of the Earth.
Ebola viruses mainly circulate between animals (scientists suspect bats are their normal host). Every few years, they get into humans and cause an outbreak. Bringing Ebola outbreaks to a halt doesn’t mean that the virus has become extinct. It just means that it has retreated back to its regular host.
Measles, on the other hand, only infects humans. If we could make our species measles-free, that would mean the virus had become extinct, never to return. And the life cycle of measles actually makes it possible to block its transmission from person to person. It’s very rare for infections to last more than a couple weeks, so that there isn’t the risk of people surreptitiously spreading the disease for years. People who do get sick won’t get sick again, taking them out of the pool of potential hosts. And we are fortunate to have a safe, effective way to break measles transmission: a vaccine.
While the eradication of measles is possible, that doesn’t mean it will be easy. It requires long-term commitment from the entire world. If a country immunizes less than 95 percent of its population, the virus can still spread efficiently from person to person.
Despite these challenge, the world has made giant advances against measles in the past few decades. Before the development of measles vaccines in the early 1960s, 7 to 8 million children died around the world every year. In 2014, that figure was down to 145,000 deaths. The World Health Organization estimates that between 2000 and 2013, measles vaccination prevented 15.6 million deaths. In the coming decade, new vaccination campaigns may drive down deaths from measles even more.
But the Disneyland outbreak demonstrates just how quickly the measles virus can undo years of public health efforts. By 2000, the United States had reduced measles to the point that it could no longer circulate on its own inside the country. A few cases cropped up each year, imported by people traveling from other countries where measles is still a problem. But in recent years measles cases have bounced back–helped by a growing number of unvaccinated people.
The rate of measles vaccination is slipping year after year. In Arizona charter schools, 9 percent of kindergarteners have been exempted from vaccination. People who don’t vaccinate their children tend to live close by each other, creating pockets of vulnerability where measles outbreaks can endure, as the virus finds one host after another. The vulnerable also include children who are either too young or too sick with other diseases to get a vaccine.
It’s possible that the Disneyland outbreak will mark a turning point–a recognition that vaccination is a social contract we make to each other, so that we don’t allow the virus to infect our fellow citizens. Perhaps we will someday even eradicate measles from the face of the Earth. That will unquestionably be a boon for humanity. But it’s also possible that it could open the way to a new disease.
That’s because the measles virus has cousins.
Measles belongs to a cluster of viruses called morbilliviruses. They infect a wide range of animals, from whales to wildebeest, from pandas to primates. It appears that morbilliviruses use the same strategy as measles–coming in through immune cells and going out through epithelial cells. And they’re also just as contagious. Some studies suggest that measles started out several thousand years ago as one of these wild morbilliviruses. According to one theory, after we domesticated cattle, a cow morbillivirus jumped into humans. As human populations grew dense, the new measles virus found a comfortable new home.
Scientists have documented virtually no cases of morbilliviruses spreading from animals to humans. Given the staggering contagiousness of morbilliviruses, that’s pretty amazing. It’s possible that our immunity to measles also protects us other morbilliviruses. These animal viruses may sometimes make incursions into our species, but the conditions are so harsh that they never have time to adapt to our biology.
If that’s true, it’s possible that the eradication of measles would open up a new ecological niche that another animal morbillivirus could invade. This possibility doesn’t mean that we shouldn’t stop fighting measles. Instead, we should broaden our efforts. Even as we eradicate measles, we should become better acquainted with related viruses and prepare for the possibility that they may become new threats. With the lessons we learn from eradicating measles, we can be ready to battle the next master of contagion.
For more information about viruses, see my book A Planet of Viruses.
Thanks to Paul Duprex of Boston University for images and fact-checking.
February 1, 2015
Our Inner Viruses: Forty Million Years In the Making
Each year, billions of people get infected with viruses–with common ones like influenza and cold viruses, and rarer ones like polio and Ebola. The viruses don’t stay all that long inside of us. In most cases, our immune systems wipe them out, except for a few refugees that manage to escape to a new host and keep their species alive. In some cases, the viruses kill their unfortunate hosts, and end their own existence as well. But in some exquisitely rare cases, viruses meld with the genome of their hosts and become part of the genetic legacy their hosts pass down to future generations.
Scientists know this melding has happened because viruses have distinctive genes. When scientists scan the human genome, they sometimes come across a stretch of DNA that bears the hallmarks of viruses. The easiest type of virus to recognize are retroviruses, a group that includes HIV. Retroviruses make copies of themselves by infecting cells and then using an enzyme to insert their genes into their host cell’s DNA. The cell then reads the inserted DNA and makes new molecules that assemble into new viruses.
Most of the time, retroviruses behave like other viruses, jumping from host to host. But sometimes a retrovirus will end up in the genome of an egg or sperm. If it then ends up in a new embryo, the embryo will carry a copy of the virus in every single cell–including its own egg or sperm. And on and on, from parents to children to grandchildren.
If the virus DNA remains intact, it still has the capacity to multiply. It may produce new viruses that break out of a cell, and even leap into a new host. But over the generations, the virus DNA may mutate and degrade. It may no longer be able to escape its own cell. But the virus may still have a bit of life left to it: it can make new viruses that insert their genes back into the genome at a new location. Here’s a simplified diagram of how it works…

Dewannieux and Heidmann 2013 dx.doi.org/10.1016/j.coviro.2013.08.005
This process has generated a huge amount of viral DNA in the human genome. We carry about 100,000 pieces of DNA that came from retroviruses–known as endogenous retroviruses. All told, they come to an estimated 5 to 8 percent of the entire human genome. That’s several times more DNA that makes up all 20,000 of our protein-coding genes.
When biologists started sequencing the genomes of other species, they discovered that it’s taken millions of years to pile up all this viral DNA. They found some of the same endogenous retroviruses in the genome of chimpanzees, for example. Since our ancestors split from theirs about seven million years ago, this shared viral DNA must come from our common ancestor.
Gkikas Magiorkinis, a University of Oxford virologist, and his colleagues have now carried out large-scale survey of endogenous retroviruses in humans, apes, and Old World monkeys–a group of species that all descend from a common primate ancestor that lived some 40 million years ago. They catalogued the viruses in each species and compared them to the versions in the other primates. They were able to reconstruct the history of our viral DNA in unprecedented detail, even coming up with estimates for the rate at which the viruses inserted new copies into our genome.
The scientists can trace our viral DNA to 30 to 35 separate invasions. Once each virus established itself in our ancestors’ DNA, it produced copies of itself scattered through the genome. The rate at which new copies were inserted rose and fell over time, and at different rates in different branches of the primate tree. Here’s an overall look at the history of the viruses. (“Loci” here refers to new copies of viruses inserted into the genome in a given interval of time.)
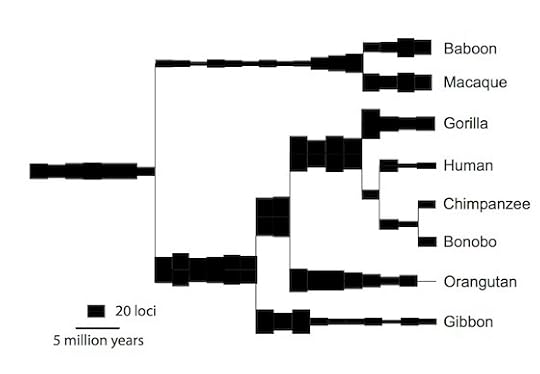
Magiorkinis et al, Retrovirology 2015
Our monkey-like ancestors 40 million years ago acquired new virus copies at a fast clip–much faster than in our own lineage in the past couple million years. One virus in particular, known as HERV-H, was responsible for most of the new copies. It may have evolved adaptations that made it into a superspreader inside the genome.
In the Old World monkeys–represented in the new study by baboons and macaques–the rate of new virus copies pretty much stayed the same over the past 30 million years. But the apes tell a different story. The rate dropped in every ape branch. The same shift occurred in parallel in the ancestors of humans, chimpanzees, gorillas, orangutans, and gibbons.
It’s possible that some of this decline had something to do with the fact that we have been fighting back against our inner viruses for millions of years. A newly inserted virus may disrupt an essential gene, and the result may be that its cell may become cancerous. Scientists have documented this threat by studying mice, which are often the victims of retrovirus-driven cancer. And they’ve also found that mammal cells can minimize this risk in many ways. One way is to coil up virus DNA so that it can’t get copied. Another way is to make special proteins that damage newly made virus genes.
One thing that apes have in common is that they’re big. If you’re a big animal, that means you have more cells, and more cells should mean you have a bigger risk of developing cancer. Yet we don’t. The risk of cancer in a human is no greater than a mouse. It’s possible that an increase in body size drives the evolution of new defenses against cancer. And those defenses may include doing a better job of keeping viruses in check.
But Magiorkinis and his colleagues suspect that getting big can only explain some of the decline. In the human lineage in particular, viruses have slowed down drastically. Here’s a graph from their new study that tracks the frequency of new virus copies in the human genome over time:
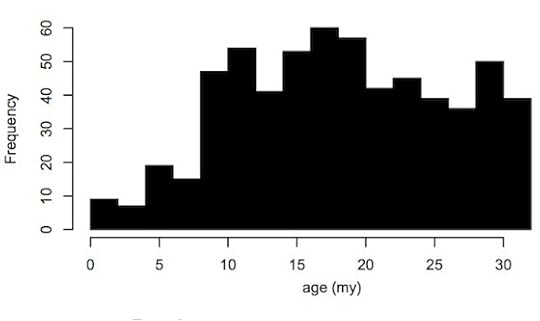
Magiorkinis et al, Retrovirology 2015
In the past million years, only a single virus has continued to multiply–known as HERV-K. Today, you can find some HERV-K copies in some people and not in others. The pattern of these copies suggests that as recently as 250,000 years ago, HERV-K was still making new copies.
It’s possible that HERV-K is completely dead now. There’s no evidence that HERV-K or any other endogenous retrovirus is actively spreading or causing cancer. It’s hard to say at this point why humans have put the brakes on endogenous retroviruses. But Magiorkinis has one suggestion: our ancestors may have reduced their odds of picking up new viruses.
Retroviruses like HIV can be spread by blood. Other primates use their teeth as weapon, either to kill prey or to fight other primates. If their victim has an infection, they can get infected, too. Ancient humans evolved to gather food with tools rather than teeth. Males stopped chomping other males, a shift that is reflected in the shrinking canine teeth of our ancestors. By making ourselves less vulnerable to blood-borne viruses, we put a stop to the influx of retroviruses overrunning our genomes.
There’s no way of telling if we are done with new endogenous retroviruses for good now, or if HIV or some other new retrovirus will manage to work its way into our genes. But the history of our inner viruses is still important to our health. Scientists have found HERV-K proteins made in tumors, suggesting that cancer cells may harness some of the biochemical power in these ancient parasites. Knowing their past can let us understand how they’ll affect us in the future.
(For more information, see my book A Planet of Viruses.)
Reference: Magiorkinis et al, “The decline of human endogenous retroviruses: extinction and survival.” Retrovirology.
January 29, 2015
The Tumor Within A Tumor
Biologists who study cancer have been borrowing a lot of concepts from evolution in recent years. That’s because the changes that occur inside a tumor bear some striking resemblances to what natural selection does to a population of animals, plants, or bacteria. Evolutionary biologists who study societies–from human tribes to ant colonies–have investigated how cooperation can evolve when cheating can let some individuals get ahead. Now scientists are finding evidence of cooperation and cheating among cancer cells. In my column this week for the New York Times, I look at the social life of cancer–and how we might undermine it to fight the disease.
This video, made by the authors of a new study I write about in the column, presents the gist of this idea–of killing a tumor by creating a new tumor inside of it.
January 21, 2015
Frankenstein Can’t Come Out And Play Today
In the standard Frankenstein story, a scientist creates an unnatural monster that breaks out of the lab and runs amok. But why should unnatural make something unstoppable? The contrary is possible, too. Imagine a different story: Frankenstein’s monster escapes, realizes that it can’t survive in the outside world, and retreats back to the lab. This story line may not make for a satisfying movie, but it might be a good goal for real life.
The fear of the unstoppable unnatural has been with us ever since scientists began moving genes between species in the 1970s. In a 1973 experiment, researchers transferred a gene from a frog into Escherichia coli. The gut microbe used the frog gene to make a frog protein.
It wasn’t long before researchers figured out how to use genetic engineering to turn microbes into factories. When scientists inserted the gene for human insulin into E. coli, the bacteria were able to manufacture a drug that had previously been harvested from cow pancreases. E. coli became the workhorse of biotechnology, spewing out drugs, vitamins, and industrial materials. (For more on E. coli’s strange yet significant history, see my book Microcosm.)
At first, the prospect of foreign genes in E. coli was terrifying. Some critics warned that insulin-producing bacteria would escape from fermenting tanks, get into people’s bodies, and cause an epidemic of diabetic comas. That never happened, probably because insulin does E. coli no good at all. The human gene is a burden to the microbe, draining off energy and resources it could use to grow.
Nevertheless, it was conceivable that some other creation might turn out to be dangerous. The scientific community responded by laying down guidelines for working with genetically engineered creatures. Most of the guidelines involved creating physical barriers to keep organisms from escaping factories or labs. But scientists have also created biological barriers, by changing the creatures themselves to make it hard for them to survive outside the lab.
For example, scientists who study the plague engineered a safe strain of the bacteria Yersinia pestis that they could work with in their labs. Y. pestis needs iron to survive, and it uses special molecules to scavenge the element from our bodies. To make a safe strain, scientists shut down some of the iron-scavenging genes in the bacteria. The bacteria could still grow in a flask if they got a rich supply of iron. But inside people, where iron is scarce, they would starve.
At least that was the plan. In 2009, a University of Chicago scientist named Malcolm Casabadan got infected by a lab strain of Y. pestis and died of the plague. Unfortunately, neither he nor anyone else knew that he suffered from a genetic disorder called hemochromatosis, which caused him to accumulate high levels of iron in his blood. Investigators concluded that his body probably served the same role as an iron-rich lab flask. Inside him, the hobbled bacteria could grow.
Casabadan didn’t die because the engineered Y. pestis that infected him was unnatural. The problem was that it wasn’t unnatural enough. That is, it could still find a place in the natural world where it could thrive. Some scientists think a better safeguard would be to create life that is fundamentally unnatural–in other words, that cannot possibly survive without our help, because the natural world is alien to it.
Fortunately, this goal does not require scientists to create an utterly alien form of life, complete with some alternate form of heredity to take the place of DNA. Scientists can take advantage of the fact that all living things on Earth are incredibly similar, chemically speaking.
All living things build proteins from about twenty building blocks, called amino acids. By combining the amino acids in different sequences, life can produce a vast range of proteins. But there are hundreds of other kinds of amino acids in nature, and scientists have created many others that are never found in nature.
In theory, living things should be able to use these amino acids to build their proteins, too. They don’t, however, because all living things share a nearly identical code for translating the information in their genes into proteins.
Genes are made of a different set of building blocks, called bases. To build a protein, a cell reads three bases at a time (a codon) and then selects a corresponding amino acid. If a base called guanine appears three times in a row in a gene, for example, a cell will pick out an amino acid called glycine.
For the most part, all living things rely on the same genetic code. That’s why E. coli that acquires a human insulin gene makes insulin, too, instead of collagen or hemoglobin. It’s also why viruses can invade our bodies and use their own genes and proteins to hijack our cells to make new viruses. We all use the same language, and so our programming can be hacked.
About a decade ago, Farren Isaacs, then a postdoctoral researcher in the lab of George Church at Harvard, started tinkering with the genetic code, trying to change the rules. Last year, he and his colleagues reported that they had reassigned one codon in E. coli to an artificial amino acid. (It’s known as p-acetyl-L-phenylalanine, or pAcF for short.) They sprinkled the new codon across the genome of the bacteria, which then made some of its proteins using pAcF.
The recoding had a remarkable effect on the bacteria: they became immune to a virus that specializes on infecting E. coli. By changing the code, the scientists made the bacteria harder to hack. (I wrote about this work in more detail in 2013 in Nautilus.)
While these bacteria could make unnatural proteins, they didn’t depend on the proteins for survival. Growing on a regular diet of natural compounds, they could still thrive. Isaacs went on to Yale, where he continued his research, as have Church and his colleagues at Harvard. And in Nature, each team has now published the next logical experiment in this line of research. Each group has recoded E. coli so that it now depends on an artificial amino acid. Without it, the bacteria cannot build essential proteins, and they die.
Because these bacteria can’t find these artificial amino acids in the outside world, the scientists reasoned that they couldn’t survive on their own. To test that possibility, they transferred the recoded microbes to dishes where they got an ordinary diet. In all but one trial, the scientists found no evidence of the recoded bacteria surviving without their essential amino acid. And in the one trial where they did survive, they barely clung to life, easily outcompeted by ordinary E. coli.
To further reduce the odds of the bacteria surviving on their own, the researchers are now building in other features. Church’s group, for example, is reassigning other codons to other unnatural amino acids, further reducing the odds even more that mutations can rescue the bacteria. Ultimately, they hope to push these creatures into an alternate biological universe, walled off from our own.
What could we do with such creatures? We could potentially use them not just in protected labs, but in the outside world. They might clean up oil spills, for example, surviving as long as we supplied the artificial amino acids they needed to build proteins. When their job was done, we could shut off the supply and they’d die. It’s conceivable that scientists could recode plants as well, creating crops that could only grow with our help.
When I talked to other experts about this research, they were pretty impressed. “It’s a landmark moment,” Tom Ellis of Imperial College told me. “I think that it will have an immediate positive impact,” said Karmella Haynes of Arizona State University.
But when I talked to bioethicist Paul Wolpe of Emory University, he thought it unlikely that we’re home-free when it comes to risks from genetic engineering. In the past, people have introduced animal and plant species to new places with the best of intentions, only to see them cause unanticipated harm. “While I applaud these first steps, caution should be the guide here,” Wolpe said.
I expect that most of the conversations these odd bugs will inspire will be about practical matters–about making valuable stuff and avoiding risks to our health. But this research speaks to something deeper. When we try to figure out the definition of life, we look around at the life we know and look for the features all living things have in common. But scientists have also wondered if life as we know it may take up a tiny portion of the space of all possible forms that life can take.
These altered bacteria tell us that suspicion is likely true. With a few years’ work, they’ve made creatures that are probably unlike anything that ever lived on Earth. And within their universe–the universe of artificial amino acids that exists in Massachusetts, Connecticut, and a few other places on Earth–they are as alive as we are.
January 19, 2015
Can the Microbiome Mutiny?
It’s an ugly fact of life that getting old means getting infections. Old people get attacked more by pathogens, and the damage that these germs cause can speed up the aging process, leading to even more infections. The standard explanation for this vulnerability is that the immune system falters in old age, opening an opportunity for pathogens to invade. But in the journal Biology Direct, Viktor Muller of Eotvos Unversity and his colleagues propose that something else is also going on in the aging body. Maybe the microbiome senses that its host is in bad shape and rises up in rebellion. The scientists call their idea “the Microbiome Mutiny Hypothesis.”
It may seem like a strange notion, but several lines of evidence suggest it’s worth considering. First of all, a lot of the pathogens that attack the elderly come from within. They grow quietly and harmless for decades inside people’s bodies and then switch over to causing dangerous infections later in life. The answer to why old people get infections must address why harmless bacteria turn bad in old age.
To understand this turn, we have to abandon any strict division between “good” germs and “bad” ones. For the germs themselves, these are just two ends of a seamless spectrum. Depending on how they use their host, microbes may cause no harm, a little, or a lot. And the virulence of a microbe–the amount of harm it causes–can itself evolve over time. Under some conditions, natural selection may favor gentle handling. But in other situations, causing deadly disease may be the winning strategy.
A lot of factors go into determining which strategy will be a winner for a given microbe. For some microbes, the best way to multiply may be ripping open host cells and feasting on their contents. This may kill a lot of their hosts, but that may not matter to the microbes, since they can escape to a new host–say, by causing diarrhea that contaminates a water supply. But in other conditions, killing a host may be a bad long-term strategy–if, for example, the odds are low that a microbe will get from one host to the next.
It’s even possible for organisms to evolve the ability to switch between these strategies, using the best strategy for different environments. In a 2013 experiment, Oxford scientists observed this switch evolve before their eyes.
They studied phages, which are viruses that infect bacteria. A phage invades a bacterium and makes new copies of itself. The bacterium eventually ruptures, spilling out the next generation of phages.
The scientists reared phages under unusual conditions–they mixed together a very high concentration of phages with bacteria. As a result, each microbe tended to get infected by more than one phage. The phages would then make copies of themselves at different rates. When the microbe ruptures, out would come a mixture of phages. The faster breeders dominated over the slower ones.
The scientists let the phages evolve in these conditions for 50 days. When they were done, the phages could now adjust their speed. If they found themselves alone in a host cell, they grew slowly. But if they sensed other phages in the cell, they sped up, so as to outcompete their rivals. As a result, their host died faster.
Muller and his colleagues propose that some of the microbes that live in our bodies can also switch from benign to deadly for similar reasons. While we’re healthy, they growing slowly, causing us no harm. But as we approach the end of our lives, the microbiome shifts to a more aggressive strategy.
“Killing the goose that lays the golden eggs might not be such a bad idea if the goose is going to die soon, anyway,” the scientists write.
There’s good evidence that microbial residents can eavesdrop on our health. A pathogen called Pseudomonas aeruginosa, for example, can sense certain molecules our brains release in response to stress. They respond by unleashing a toxin that help them grow–while also damaging our lungs.
Muller and his colleagues offer some ways to test their hypothesis. If they’re right, then infections in old age aren’t just the result of a slack immune system. Instead, bacteria and viruses sense a changed environment and respond by making new molecules, which they use to grow aggressively and cause harm. If scientists disable these molecules, then the pathogens should become tame again.
It would be interesting to see the Microbiome Mutiny hypothesis put to such a test. Conceivably, scientists could someday turn the test into a treatment. Rather than blasting the elderly with broad-spectrum antibiotics, doctors could just disarm the mutiny.
January 16, 2015
Sitting on a Cliff Vs. Falling Off a Cliff
The Steller’s sea cow is gone. This mega-manatee swam the North Pacific for millions of years, and then in the 1700s humans hunted them to extinction. Today on the front page of the New York Times, I write about a warning from a team of scientists that if we keep on doing what we’re doing now–industrializing the ocean and pouring carbon dioxide into the atmosphere in greater and greater amounts–a lot of other marine animal species will go the way of the Steller’s sea cow.
Yet this story is actually a fairly hopeful one. The scientists compared the pace of extinctions at sea to those on land and found that the oceans are basically where the land was in 1800–with relatively few extinctions yet, on the verge of massive changes to the habitat that could wreak much bigger havoc. The oceans still have a capacity to recover, if we choose to let them.
It’s hard to strike that balance, but it’s important. By coincidence, a group of marine biologists has just published a provocative opinion piece calling for more skepticism about “ocean calamities”–the claim that the oceans are getting hit with some global shock of one sort or another. (You can read the piece in Bioscience for free.) They complain that too often scientists see a small-scale change in one region of the ocean and blow it up to a global catastrophe. The scientists pick apart some of these cases, such as the belief that jellyfish are taking over the planet. The strongest evidence for their rise turned out to be a natural increase of one population of jellyfish that is part of a natural cycle.
That doesn’t mean that thee are no ocean calamities. The scientists see strong evidence for devastation from overfishing, for example. And that doesn’t mean that dangers that don’t seem to have had big impacts yet won’t have them in the future (see ocean acidification). But leaping to the apocalypse based on limited or ambiguous evidence is bad science and bad policy, the scientists argue:
We conclude that a robust audit of ocean calamities, probing into each of them much deeper than the few examples provided here, is imperative to weeding out the equivocal or unsupported calamities, which will confer hope to society that the oceans may not be entirely in a state of near collapse and which will provide confidence that the efforts by managers and policymakers targeting the most pressing issues may still deliver a healthier ocean for the future.
I wanted to check in with the authors of the Bioscience piece about the new study I wrote about for the Times. If they thought this new study was an egregious case of calamity-mongering, I needed to know that, and I would make it clear in my piece.
But that’s not what I found. When I spoke to Robinson Fulweiler, a marine biologist at Boston University, she said, “I was really excited to read their paper, and I actually felt good about their conclusions.” She thought the scientists did a good job of gauging what’s happened to the oceans so far, the risks they face in the future, and–importantly–the steps that we can take, armed with our knowledge of the situation.
When we’re contending with our effects on the planet, it may be tempting to go limp and say we’re all doomed, or to wave it off as some huge delusion. But the reality of the oceans calls for a different response altogether.



