Carl Zimmer's Blog, page 5
May 15, 2015
Bamboo Mathematicians
In the late 1960s, a species of bamboo called Phyllostachys bambusoides–commonly known as the Chinese Mainland Bamboo or Japanese Timber Bamboo–burst into flower. The species originated in China, was introduced to Japan, and later into the United States and other countries. And when I say it flowered, I mean it flowered everywhere. Forests of the plant burst into bloom in synchrony, despite being separated by thousands of miles. If, like me, you missed it, you will probably not live to see it happen again. The flowers released pollen into the wind, and the fertilized plants then produced seeds that fell to the ground. The magnificent bamboo plants, which can grow 72 feet tall, then all promptly died. Their seeds later sprouted and sent up new plants. The new generation is now close to fifty years old and has yet to grow a single flower. They won’t flower till about 2090.
We can say this with certainty this because Chinese scholars have kept such careful records for such a long time. In 999 A.D. they recorded a flowering of Chinese Mainland Bamboo. It was probably an astonishing sight, since no one alive at the time had ever seen the species flower before. The bamboo plants died, their seeds sprouted, and the forests did not flower again till 1114. After the species was imported to Japan, the Japanese recorded flowers in the early 1700s, and then again in 1844 to 1847. The flowering in the late 1960s was just the next burst of a 120-year cycle.

An 1885 illustration of Chusquea abietifolia, with a 32-year flowering cycle. Gray Herbarium Library, Harvard University Herbaria
This remarkable cycle would be fascinating enough on its own. But it turns out a number of other species of bamboo grow flowers on cycles lasting decades, too. A species called Bambusa bambos flowers every 32 years, for example. Phyllostachys nigra f. henonis takes 60 years.
Three biologists at Harvard got puzzled by these cycles and recently set out to find an explanation for how they evolved. In the journal Ecology Letters, they offer up a tantalizing hypothesis: bamboo cycles have reached their remarkable lengths through some simple arithmetic.
Like all scientists, these biologists (Carl Veller, Martin Nowak, and Charles Davis) stand on the shoulders of giants. Or one giant in particular–the ecologist Daniel Janzen, who over the years has cast off a huge number of creative, influential ideas with unsettling ease.
In the mid-1970s Janzen came up with an explanation for why bamboo plants would flower in synchrony. He noted that rats, birds, pigs, and other animals devour colossal numbers of bamboo seeds. Each gobbled-up seed represents the loss of a potential offspring. If there are enough seed-predators, and they are hungry enough, they can wipe out a bamboo plant’s entire set of seeds.
Bamboo plants might fare better, Janzen argued, if they flowered at the same time. They would overwhelm their enemies with food. Even if they gorged themselves to bursting, they would still leave some seeds untouched. Those surviving seeds would then have enough time to grow into plants that could defend themselves with tough fibers and bitter chemicals.
Once bamboos fell into flowering lockstep, it would be hard for them to slide out. If a few bamboo plants flowered a few years too early, animals would feast on their seeds, and their out-of-sync genes would fail to make it into future generations.
Other scientists have found support for Janzen’s idea. Swamping enemies with seeds really does lower the overall harm that seed-eaters cause to each individual plant. But Veller and his colleagues still had questions. How did the bamboo plants get into those beneficial flower cycles to begin with? And how did various species end up with such long–and such different–flowering rhythms?
The scientists developed a mathematical model based on what’s known about bamboo biology. They started out with a bamboo forest in which almost all the plants flower annually, as some bamboo species do.
But the population also contained some mutants. They had mutations in their flower-timing genes, so that they needed two years to flower instead of one. Some of the two-year mutants flowered in even years, while others flowered in odd years. Spending two years between flowering instead of one could have some advantages for bamboo plants. The plants could have more time to gather more energy from sunlight, which they could use to make more seeds, or give their seeds more defenses against predators.
As more of the forest becomes two-year plants, there are fewer plants releasing their seeds every year. Eventually, Veller and his colleagues found, a year arrives when the annual bamboo plants can’t produce enough seeds to survive the onslaught of animals. In one fell swoop, they’re wiped out. If it’s an odd year, then the odd-year two-year plants can get wiped out, too. If it’s an even year, the even-yeared plants take the fall. Either way, the whole forest gets abruptly synchronized into flowering every two years.
It’s also possible that the forest wouldn’t just have two-year mutants, but mutants that took three years or more to flower. Veller and his colleagues found that in their mathematical model, bamboo plants with longer flowering cycles could also take over. Exactly which cycle won out was partly a matter of chance, because how many seeds bamboo plants successfully produce in a given year can fluctuate due to the weather and other unpredictable conditions. Whichever cycle emerges as the dominant one, the whole forest then evolves to stay synchronized. Any outliers flowering out of sync get wiped out, just as Janzen had proposed.
There is one exception, though: a mutant bamboo plant can evolve a new cycle that’s a multiple of the original one. Imagine that a two-year bamboo turns into a four-year one. Every year it flowers, it’s protected by the two-year plants flowering at the same time. And it’s got an advantage over them: it can spend the extra time making more seeds.
Even though the four-year flowers need twice as long to produce their seeds, the scientists found, under some conditions they can still become increasingly common over a few centuries. Eventually, the whole forest will lock onto the four-year cycle.
But bamboo can’t evolve the other way, the scientists found. If a four-year forest produces a two-year mutant, it will flower half the time in years when it has no protection from predators. The only direction it can go is towards longer cycles. If a four-year forest produces an eight-year mutant, it can have the same advantage that the four-year plants originally had: well-protected time.
Veller and his colleagues realized that they could test this model. Over millions of years, they reasoned, species should have multiplied their flowering cycles. It’s likely that they could only multiply the cycles by a small number rather than a big one. Shifting from a two-year cycle to a two-thousand-year cycle would require some drastic changes to a bamboo plant’s biology. Therefore, the years in a bamboo’s cycle should be the product of small numbers multiplied together.
The mathematics of bamboo offers some promising support. Phyllostachys bambusoides has a cycle of 120 years, for example, which equals 5 x 3 x 2 x 2 x 2. Phyllostachys nigra f. henonis takes 60 years, which is 5 x 3 x 2 x 2. And the 32 year cycle of Bambusa bambos equals 2 x 2 x 2 x 2 x 2.
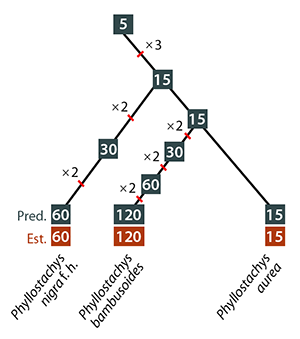
Veller et al 2015 Ecology Letters
The scientists found more support when they looked at how bamboo species have evolved. Here’s an evolutionary tree of Phyllostachys bambusoides and its close relatives. It’s possible that their common ancestor had a five-year cycle, and then the cycle multiplied by small factors along each branch of the tree.
But could this just be a kind of meaningless bamboo numerology? Is it just a coincidence that these species display such elegant multiplications? Veller and his colleagues carried out a statistical test on bamboo species with well-documented flowering cycles. They found that the cycles are tightly clustered around numbers that can be factored into small prime numbers. It’s a pattern that you would not expect from chance. In fact, they argue, this test offers very strong evidence for multiplication (for stat junkies: p=0.0041).
There is plenty of opportunity to put this model to the test. A lot of species of bamboo have long flowering cycles that no one has measured very carefully. Scientists could see how newly studied cycles fit into Veller’s model. If scientists find a new species of Phyllostachys that’s got a 23 year cycle, for example, it would be mathematically impossible for it to have evolved from a five-year ancestor. One thing’s for sure, though. If this model requires scientists to sit around watching bamboo, waiting for it to flower, this is going to take a few generations of scientists to settle.
May 12, 2015
Can Scientists Turn Birds Back Into Dinosaur Ancestors?
We know they evolved from dinosaurs about 150 million years ago, but it remains to be discovered precisely how the DNA of ground-running dinosaurs changed–a transformation that turned arms into wings, produced aerodynamic feathers, and created a beak. It’s possible that some clues to those genetic changes can be found in living birds themselves. By blocking some of the recently evolved steps in the development of bird embryos, we might be able to get birds to grow some dinosaur anatomy.
A team of researchers recently used this approach to understand how dinosaur snouts turned into bird beaks. Beaks are really just insanely extravagant versions of little bones called premaxillae. (You’ve got a pair just behind your front teeth.) The researchers blocked some proteins produced on the face of chicken embryos and found that the chickens failed to make beaks. Instead, their premaxillae became an unfused pair of bones–a lot like you might find in living beakless relatives of birds, such as alligators. Here, a normal chicken skull is on the left, an altered one is in the middle, and an alligator is on the right.
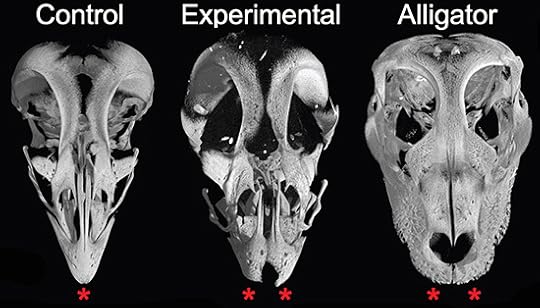
Bhullar et al, Evolution 2015
As I write in my column today in the New York Times, some researchers remain skeptical that these chickens are really developing the beakless heads of their ancestors–that they’ve run evolution in reverse, in effect. More precise experiments on chicken DNA could confirm that this is what indeed happening.
Some people may find this exciting because it could presage the coming of dino-chickens. But no one has any idea of how long it would take to figure out how to reverse the rest of a bird’s body. A chicken with nothing more than a snout, by this measure, is profoundly underwhelming.
But for those who are interested in how evolution actually happened, it’s already very thought-provoking. For example, the scientists picked out two proteins to block specifically to turn beaks into snouts. To their surprise, this procedure simultaneously changed other bones in the skulls of the birds, turning them back to dinosaur-like shapes.
When birds evolved beaks, other parts of their head was also undergoing some evolutionary changes. The palate bones in the roof of their mouth became very thin, serving mainly to transmit forces from muscles at the back of the head to the beak. When the scientists blocked proteins in chicken embryo faces, they changed the palate bones as well as the beak. This figure, which looks up at the palate from underneath, shows what happened:
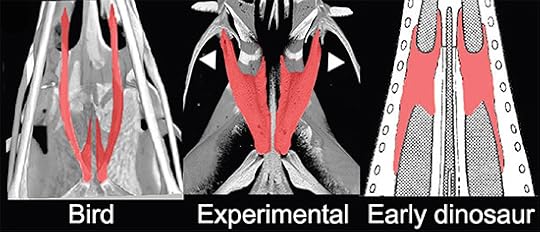
Bhullar et al 2015 Evolution
Scientists have long known that a single gene can have several effects on an animal. This multi-tasking is called pleiotropy. The new experiment hints that the bird beak didn’t evolve simply through a series of little steps, each having a single effect on bird heads. Instead, birds might have taken some bigger evolutionary leaps.
April 28, 2015
Talking About Editing Human Embryos on the Radio
This morning I went on the NPR show “On Point” to talk about using CRISPR to edit embryos. I’ve embedded it below, and you can also listen to it at this link.
It was fascinating to listen to my fellow guests. Nobel-prize winner Craig Mello basically said that if we can make it safe, then let’s go for it. Marcy Danovsky of the Center for Genetics and Society argued that the world needed to put rules in place to ban human germline engineering.
Towards the end of the show, I went on a bit of an anti-GATTACA rant. GATTACA, in case you haven’t seen it, is a movie that puts a biotech twist on Brave New World. Danovsky and other critics frequently warn that embryo editing could lead to a world like the movie–in other words, rich people would create a genetically altered population of super-smart, super-healthy people, leaving the have-nots in the dust.
If we’re going to talk about international bans, I’d like an international ban on invoking GATTACA in these discussions. It’s like saying, “We shouldn’t genetically engineer people because we will end up with an army of flying monkeys who will enslave the rest of us.” I mean, we can imagine an army of flying monkey overlords, and we can all agree that an army of flying monkey overlords would be a bad thing. But is that the most useful way to talk about the real social and medical impacts of a new technology?
Here are a few reasons for my view:
1. Good luck genetically engineering intelligence. We could clearly cure hemophilia with CRISPR, because it’s caused by a single mutation to a single gene. But the genetic basis of intelligence involves hundreds of genes, as far as scientists can tell, and their effects are very dependent on the environment in which a child grows up.
2. Genes get around. The only way to keep the GATTACA flying monkeys as a distinct population would be to stop them from having children with unengineered people. That would require social engineering that would make the genetic engineering look like a grade school science fair project.
3. You don’t need CRISPR to create health inequity. We already live in a world with big inequities in well-being. We didn’t have to wait for genetic engineering to make that happen. And the fact that CRISPR could create inherited changes is also not so special. Socially based inequities get passed down through the generations too. This kind of argument would disappear if the world agreed to provide free CRISPR engineering to all prospective parents if they wanted it.
So let’s have a debate without the flying monkeys, shall we?
April 22, 2015
Editing Human Embryos: So This Happened
Earlier this week, Chinese researchers reported that they edited the genes of human embryos using a new technique called CRISPR. While these embryos will not being growing up into genetically modified people, I suspect this week will go down as a pivotal moment in the history of medicine. David Cyranoski and Sara Reardon broke the news today at Nature News. Here I’ve put together a quick guide to the history behind this research, what the Chinese scientists did, and what it may signify.
There are thousands of genetic disorders that can occur if a mutation happens to strike an important piece of DNA. Hemophilia, sickle cell anemia, cystic fibrosis– the list goes on and on. As I wrote in the Atlantic in 2013, a particularly cruel genetic disorder, fibrodysplasia ossificans progressiva causes people to grow a second skeleton. It’s caused by a mutation that changes a single “letter” of a single gene, called ACVR1. The protein encoded by the gene doesn’t work properly, triggering a wave of changes in people’s bodies, with the result that when they heal from a bruise, they replace entire chunks of muscle with new bone.
In some cases, people can offset many of the symptoms of genetic disorders with simple changes, like watching what they eat. In other cases, like hemophilia, they have to take regular doses of drugs to remain healthy. In other cases, like fibrodysplasia ossificans progressiva, there’s no effective treatment yet.
For decades, scientists have tried to develop a new way to treat genetic disorders like these: to heal the patient, heal the gene.
This approach came to be known as gene therapy. As I wrote in Wired, gene therapy soared to heady heights of hype in the 1990s. Researchers developed viruses that they could load with working versions of people’s defective genes. They injected the viruses into their subjects, and the viruses delivered the genes to some cells–enough cells, in theory, to start doing the work required to make the people healthy again.
Gene therapy research crashed around 2000 when one volunteer died during a study due to an overwhelming immune response to the viruses he received. Since then, gene therapy researchers have found safer, more efficient viruses, and now gene therapy is starting to emerge in the clinic.
But the revival of gene therapy doesn’t necessarily mean that viruses are the best of all possible tools to fix broken genes. What if, for example, you could just remove the mutant DNA in a gene and replace it with the right sequence?
For a long time, that question was best left to late nights at bars and episodes of Star Trek. Nobody knew how to manipulate DNA with that kind of precision. But in just the past couple years, scientists have created exactly this kind of gene-editing tool, which is known as CRISPR.
As I wrote recently in Quanta, CRISPR didn’t spring fully formed from someone’s mind. It’s actually a collection of molecules that bacteria use to fight viruses. They can create molecules that can latch precisely to certain stretches of DNA and cut them apart. Shortly after scientists figured out how bacteria use CRISPR, they began to wonder if they could use it, too.
It soon became clear they could. They could easily synthesize “probe” molecules that would grab onto a specific stretch of DNA in just about any cell. Enzymes could then chop out that stretch. If the scientists supplied a different version of that stretch of DNA, the cell would incorporate it where the original stretch once was.
Delivering CRISPR into the bodies of people with genetic disorders could conceivably repair their genes. Of course, the success of this kind of treatment would depend on how efficiently the molecules could get inside the cells that needed repairing, and how accurately they cut the DNA. Still, some early experiments on animals suggest that the approach may someday work on people.
But what if you didn’t have to wait until so late in the game to repair a broken gene? If a fertilized egg ended up with a defective gene, you could conceivably use CRISPR to fix the mistake. That single cell could then give rise to an entire healthy human with trillions of cells that all had the correct version of the gene.
Last month, a team of leading scientists–including pioneers in both gene therapy and CRISPR–declared that this would be a bad idea. “At present, the potential safety and efficacy issues arising from the use of this technology must be thoroughly investigated and understood before any attempts at human engineering are sanctioned, if ever, for clinical testing,” they declared in a piece they published in Science.
But meanwhile, a team of researchers led by Junju Huang at Sun Yat-sen University were testing out CRISPR on human embryos. Huang told Nature that both Nature and Science rejected the paper based on ethical objections. So they ended up publishing the results in the journal Protein Cell (open access, by the way).
The scientists tested out CRISPR as a form of embryonic gene therapy. Imagine an embryo had a mutation in a gene called beta-globin involved in making hemoglobin. It would develop into a person with the blood disorder beta-thalassemia. Would it be possible to cure the embryo by rewriting the gene?
The researchers set out to do the study on human embryos–but they didn’t want to use embryos that might ever actually be able to develop into a fully-formed human being. When fertility doctors fertilize eggs with in vitro fertilization, they sometimes end up with two sperm delivering their DNA into a single egg. These “tripronuclear zygotes” can start dividing as normal embryos do, but their abnormal collection of genes causes them stop developing when they’re still just tiny clumps of cells. The researchers argue that this failure makes tripronuclear embryos “an ideal model system” for studying CRISPR therapy. (Bioethicists, start your engines!)
All told, the researchers injected 86 embryos, 71 of which survived long enough for them to study. CRISPR only managed to cut DNA in a fraction of the embryos, and in only a fraction of those embryos did cells manage to take up the new version of the target gene (called beta-globin).
Two big problems stick out from the results.
One is the fact that CRISPR sometimes missed its target and inserted DNA in the wrong places. Such a misfire wouldn’t just fail to fix a disease like beta-thalassemia. It could create a disease of its own.
The other big problem is that the embryos that did get edited correctly were actually a mix of edited cells and unedited cells–what’s known as a mosaic. Mosaics can give doctors a lot of headaches, as I’ve written in the New York Times. If fertility doctors used CRISPR to create healthy, hemophilia-free embryos, they’d need to make sure the embryos were repaired by picking off a cell and examining it up close. A cell from a mosaic embryo could give doctors the wrong picture of the embryo as a whole.
The authors conclude their paper by warning that these failures need to be “investigated thoroughly before any clinical application.”
Just because this experiment came out poorly doesn’t mean that future experiments will. There’s nothing in this study that’s a conceptual deal-breaker for CRISPR. It’s worth recalling the early days of cloning research. Cloned embryos often failed to develop, and animals that were born successfully often ended up with serious health problems. Cloning is much better now, and it’s even getting to be a business in the world of livestock and pets. We still don’t clone people, though–not because we can’t, but because we choose not to. We may need to make the same choice about editing embryos before too long.
[Update: fixed the details on the beta-globin gene]
April 21, 2015
When Darwin Met Another Ape
On March 28, 1838, Charles Darwin paid a visit to the London Zoo. At age 29, he was far from the scientific celebrity he would eventually become. It had only been two years since his return from his round-the-world voyage on the Beagle, and he was still methodically working his way through the heap of fossils and living specimens he had accumulated along the way. It would take more than two decades before he would present the world with his theory of evolution. In 1838, the theory was still in a primordial form in his mind. Darwin was struggling to find an explanation for how living things–humans included–got to be the way they are. And so, on that chilly spring day, Darwin went to the zoo and stepped into a cage with an orangutan.
 It’s the sort of moment that was made for Hollywood. Indeed, when Hollywood turned its attention to Darwin, with the 2009 movie Creation, the actor Paul Bettany recreated the scene by climbing into another cage with another orangutan. And that image ended up on the posters for the movie, a powerful allegory for our reckoning with the fact that we are cousins to other apes.
It’s the sort of moment that was made for Hollywood. Indeed, when Hollywood turned its attention to Darwin, with the 2009 movie Creation, the actor Paul Bettany recreated the scene by climbing into another cage with another orangutan. And that image ended up on the posters for the movie, a powerful allegory for our reckoning with the fact that we are cousins to other apes.
Hollywood certainly gets lots of stuff wrong about science on a regular basis. But, in this case, Hollywood deserves some credit. This scene really did happen, and it really did have a profound impact on Darwin–and, by extension, on science.
Darwin’s biographers have retold the scene several times. They’ve relied on a few key pieces of evidence to reconstruct it, such as a letter Darwin wrote to his sister Susan after his first visit to the zoo, along with mentions in his books The Descent of Man and The Expression of Emotion in Man and Animals. Yet even today, 177 years later, there’s still much to learn about Darwin’s fateful encounter. In the journal Studies in History and Philosophy of Biological and Biomedical Sciences, John van Wyhe, a historian at the National University of Singapore, and Peter C. Kjaergaard of the Natural History Museum of Denmark present previously unpublished notes that Darwin scribbled down about the London Zoo’s orangutans.
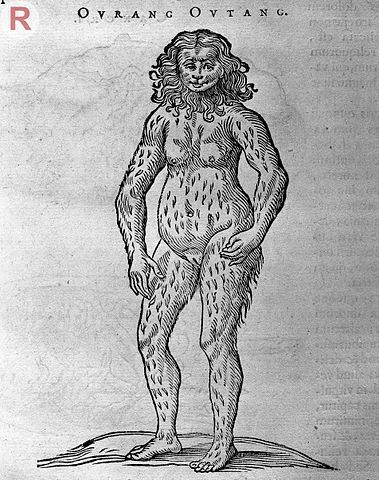
Wellcome Institute
In 1838, orangutans were still frighteningly unfamiliar to Europeans. In fact, all the great apes were a mystery because they lived thousands of miles away, deep inside African and Asian jungles. Early European explorers would report encounters with fierce, human-like creatures, usually told second-hand. In the early 1600s, a Dutch physician named Jacobus Bontius who lived on the island of Java wrote of wild apes there called “Ourang Outang,” meaning “man of the forest.” The picture that accompanied his description looks like a woman with a lion’s mane.
Bontius’s name for these creatures stuck. In fact, seventeenth century naturalists came to use “Orang-Outang” to refer to any ape, even a chimpanzee. Over the next two centuries, some dead orang-outangs made their way to the anatomical labs of Europe, and a few live ones made it to zoos. They were juveniles, often taken from their mothers, and they didn’t last long in captivity before dying of diseases. Baby orangutans, chimpanzees, and gorillas all looked a lot more alike than mature individuals, and so they all continued to be called orang-outangs.
Not only were they similar to each other, but they were disconcertingly similar to humans. Some naturalists responded to this realization by placing apes just below humans on the Great Chain of Being, a kind of divine ladder that separated species on different rungs from lower to higher forms. Linnaeus, on the other hand, put orangutans and other apes in the same genus as humans. Rousseau even wondered if they were “a race of genuine wild men.” Even more scandalously, Lamarck proposed that orangutans were the ancestors of humans.
Upstanding Victorian scientists in Britain were scandalized by such revolutionary talk. The geologist Charles Lyell mocked Lamarck for the idea that an orangutan could evolve to the point that it attained “the dignity of man.”
Darwin, on the other hand, warmed to the idea of evolution after his return to England in 1837. And he wasn’t about to declare humans beyond its influence. To understand the link, if any, between humans and orangutans, he decided to investigate it for himself. Hence he climbed into a cage with a young female orangutan named Jenny.
In his letter to his sister, Darwin described his first encounter:
the keeper showed her an apple, but would not give it her, whereupon she threw herself on her back, kicked & cried, precisely like a naughty child.— She then looked very sulky & after two or three fits of pashion, the keeper said, “Jenny if you will stop bawling & be a good girl, I will give you the apple.— She certainly understood every word of this, &, though like a child, she had great work to stop whining, she at last succeeded, & then got the apple, with which she jumped into an arm chair & began eating it, with the most contented countenance imaginable.
Van Wyhe and Kjaergaard have now found two folded sheets of cream-colored paper in the collection of Darwin’s documents archived at Cambridge University. Each is marked “Man,” which was how Darwin labeled his notes on human evolution. (You can see the original sheets here, although deciphering Darwin’s chicken scratch is best left to experts like Van Wyhe.)
Darwin wrote these particular notes in September 1838, when he returned to the zoo to spend more time with Jenny and another orangutan named Tommy. It’s fascinating to look at what details jumped out at Darwin, and to try to infer why they matter so much to him at that point in his scientific thinking.
Sex was on his mind, for one thing. He jotted down that zookeepers told him that monkeys could “know women perfectly.” Orangutans, he wrote, “seem to have trusted sight & not smell for knowing sexual difference.” Jenny, he added, was “particularly fond of watching boys bathe.”
To these observations he added, “How wonderful. early men have seen women naked, must then smell & afterwards association by sight–this is most curious as proof of origin of mankind.”
Darwin also looked for emotional displays in Jenny’s face, judging her to be “decidedly jealous. She would make her jealousy known by showing her teeth and “making peevish noise.” If she saw others getting attention, he wrote, she “shook the cage & knocked her head against door because she could not get out. –Jealous of attention to other.”
To Darwin, this was not anthropomorphism–imposing human experiences on non-humans. Instead, he was convinced that orangutans and humans had similar emotions because they shared a common ancestor.
Humans are also notable for how we make and use tools. And so Darwin paid close attention to how Jenny played with objects (the strike-throughs are Darwin’s):
I saw = make swing of straw in whisp =
In play she arrange straw in row, stuffing it through cage, like silly listless child — Played with two sticks, carrying them climbing up with them & trying to reach them — has is very fond of playing with anything soft, covered itself herself up with two pocket handkerchiefs just like girl with shawl spread them out — considered them as her property would not give them up to me. but the keeper brought them & gave them. followed me & bit me for having taken it away & tried to pick my pocket. –
She is fond of breaking sticks & in overturning things to do this (& she is quite strong) she places tries the lever placing stick in hole & going to end as I saw. — She will take the whip & strike the giraffes, & take a stick & beat the men. — When a dog comes in she will take hold of anything, the keepers say, decidedly from knowing she will be able to hurt more with these than with paw.
Humans are also self-aware, and in recent decades scientists have tested other animals for self-awareness by having them look in mirrors. Darwin had much the same idea in 1838:
Both were astonished beyond measure at looking glass, looked at it every way, sideways, & with most steady surprise.– after some time stuck out lips, like kissing, to glass, & then the two did when they were first put together. — at last put hand behind glass at various distances, looked over it, rubbed front of glass, made faces at it — examined whole glass — put face quite close & pressed it — at last half refused to look at it — startled & seemed almost frightened, & evidently became cross because it could not understand puzzle. — Put body in all kinds of positions when approaching glass to examine it.
We see in these newly revealed notes that the iconic image of Darwin in the orangutan cage is just the tip of a scientific iceberg. Darwin didn’t just jump in a cage, high-five an orangutan, and head back home for afternoon tea. For months, he became something approaching a primatologist. Not only did he observe emotions and behaviors; he also ran experiments, giving orangutans mirrors, tickling them, offering them food. The whole experience left Darwin firmly persuaded that the differences between humans and apes were of degree, not of kind. Humans and orangutans clearly shared a common ancestor, and the evidence endures today in our bodies and behaviors. Such a notion might scandalize Lyell, one of the greatest scientists of the day, but the young Darwin didn’t care.
For all his attacks on Lamarck, Lyell never totally ruled out the possibility of evolution. And after Darwin published The Origin of Species in 1859, Lyell, now in his mid-sixties, came around and accepted it. In 1863, he wrote to Darwin,
When I came to the conclusion that after all Lamarck was going to be shown to be right, and that we must go the whole orang I re-read his book, and remembering when it was written, I felt I had done him injustice.
Van Wyhe and Kjaergaard borrowed Lyell’s phrase for the title of their new paper, “Going the Whole Orang.” It’s open access, so you can read the whole thing for yourself here. It’s worth your time.

Photo by Nadia Drake
April 7, 2015
Why Do We Get Allergies?
The more you think about sickness and health, the trickier it gets to draw a clean line between them. We tend to think of ourselves as being prepared by nature for a good life. If we can just keep bacteria and viruses from killing us, and avoid walking into open elevator shafts, we’ll live a long, healthy life.
But we are actually the products of evolution, and evolution can’t give us perfect health. It has endowed us with powerful immune systems, thank you very much. And it has endowed us with quick reflexes that can, in some cases, keep us out of open elevator shafts. But evolution doesn’t automatically march to perfection. It stops short, leaving us with grave imperfections.
We have lots of defenses against cancer, for example, but they weaken as we get old. That’s a recipe for heartbreak in millions of families. But in the game of evolution, that’s a winning formula. Natural selection strongly favors defenses against cancer that threaten our ability to survive to adulthood and have kids. But if we die of cancer at age sixty, our kids are well on their way, carrying out genes down to the next generation.
This evolutionary perspective could change the way we think about our health in many ways. Take allergies. They affect millions of people, causing everything from hay fever to anaphylactic shock. One of the world’s leading immunologists, Ruslan Medzhitov, is convinced that allergies are actually adaptations we use to defend ourselves from noxious chemicals. As awful as allergies can get, we wouldn’t want to live without them.
I’ve written a profile of Medzhitov. It appeared today originally in Mosaic, but it’s now propagating through the Internet. You can also find it on Ars Technica, Discover, Gizmodo, Digg, and elsewhere. Check it out at the outlet of your choice. And good luck this pollen season!
April 2, 2015
Please Welcome Maryn McKenna to Phenomena!
 Today, Phenomena gains a phenomenal new member: Maryn McKenna. If you’ve read her books such as Superbug or kept up with her blog of the same name, you know that nobody does a better job of analyzing the threats we face from infectious diseases. To celebrate the launch of “Germination,” her blog here at Phenomena, I asked Maryn some questions about how she got here, and where she’s headed.
Today, Phenomena gains a phenomenal new member: Maryn McKenna. If you’ve read her books such as Superbug or kept up with her blog of the same name, you know that nobody does a better job of analyzing the threats we face from infectious diseases. To celebrate the launch of “Germination,” her blog here at Phenomena, I asked Maryn some questions about how she got here, and where she’s headed.
You gained the nickname “Scary Disease Girl” from fellow journalists. What was the path that led you there?
A complicated one–the kind that makes sense in retrospect but seemed random at the time. I started as a newspaper reporter, with a just-out-of-grad-school specialty of conducting investigations by digging through documents. The first investigation I did, though, was not science but finance, about bad savings and loan organizations in the Midwest. That attracted the attention of editors at larger papers, and I moved through a couple of other jobs doing investigative work.
Oddly, all the investigations after that first one were about public health — cancer clusters, drug abuse, Gulf War Syndrome — so after a few, I had turned into a public-health reporter. On the strength of them, I was asked to apply for what was at the time the best public-health reporting job in the country, covering the Centers for Disease Control and Prevention, the CDC, from its home base in Atlanta. The instruction when I was hired was, “Just get inside there and tell us who these people are,” and so I spent 10 years talking my way into every investigation I could. That’s really where the nickname originates, because the stories I ended up telling were the big outbreaks of those years: the anthrax attacks, the arrival of West Nile virus, the international epidemics of SARS and H5N1 flu.
That experience led to my embedding with the CDC’s disease-detective corps, the Epidemic Intelligence Service, for a year, and writing a book about them, Beating Back the Devil (2004); and one of the investigations in that book provided the germ of my next book, Superbug (2010), which is about the global rise of antibiotic resistance.
Before joining us at Phenomena, what have you been writing about diseases, and where?
I left newspapers in 2006 to freelance, so, like most science journalists, I write for a variety of places: Wired, Scientific American, Slate, Atlantic — a range. But for the past five years, the core of my scary-disease identity has been a blog at Wired, also called Superbug. At that site, which was one of the launch entries at Wired’s “all-star science blog” platform, I’ve explored emerging infections, the recurrence of problems we thought were beaten, the difficulty of getting rid of infections we thought we could eradicate, and the mistaken health and social policies, and personal mistakes, that allow disease organisms to flourish.
What’s the scariest disease you’ve ever written about?
I suspect people expect me to say something like, “Ebola!” — but while Ebola is a dreadful disease in its worst manifestations, and devastating to West Africa, I personally find it less frightening as a public health threat than more widespread, less-noticed infections. There was a point where I was seriously unsettled by the possibility of a pandemic resulting from the global spread of avian flu; I remember calling up a friend, another disease reporter, and confessing that for the first time ever, what I was learning was frightening me. And I find it stunning that the insect-borne disease Chagas is now so endemic in the southwestern United States that it is a risk in blood transfusions and organ transplants.
But the problem that most worries me now (and was the subject of my recent TED Talk) is the unchecked spread of antibiotic resistance. The Review on Antimicrobial Resistance, a project in England, estimates that if we can’t get things under control, resistance will kill 10 million people per year by 2050, and cost $100 trillion. That’s definitionally scary to me.
A year ago, you began writing for “The Plate,” a group blog at National Geographic about food. How did you get interested in food as a subject to write about?
I came to writing about food through writing about food problems: foodborne illness, and also antibiotic overuse in food production, and the drug resistance that results. That opened a door to writing about things that are not strictly related to disease: the evolutionary history of wheat, for instance, and how the names of foods . I’ve always been fascinated by food; as a young business reporter I talked my way into places doing small batch chocolates and cheeses and beer. And in the years since, it’s become a topic that essential to our culture. But my goal in writing about food is always to link it back to larger issues: sustainability, economic pressure, social justice — as well as disease.
 You’ve written for a number of newspapers and magazines. Is there anything different about blogs for you?
You’ve written for a number of newspapers and magazines. Is there anything different about blogs for you?
The pleasure and terror of blogs is how much they require speaking in a personal voice and engaging with the audience in an unfiltered way. We take this for granted now, but for a traditionally trained journalist, taught to stay out of the story, it was challenging to learn. But in compensation, I appreciate the opportunity to be incremental in bringing a story to my audience. I don’t always have to have the full chronology, and all the arguments, in one post; I can present it to readers piece by piece and be confident they will stay with me for the unfolding. That’s particularly welcome since all of us here are telling stories about science, which changes from study result to study result, so we can contextualize and reframe post by post.
What plans do you have for Germination?
I’m excited to bring the various pieces of my blogging life to one site (though my food-related writing will still be flagged at The Plate as well). I hope to be covering the full slate of public health and infectious disease topics: scary diseases, little-noticed infections, cool microbiology; the complexity of big campaigns such as polio eradication, and the challenge of our widely distributed food-production system. Readers should expect coverage of new research results, interviews with little-known scientists, glimpses of the underbelly of the food system, and some whimsy.
March 24, 2015
Lemon-Scented Malaria
Parasites are life’s great success story, abundant in both species and sheer numbers. One secret to their success is the ability that many parasites have to manipulate their hosts. By pulling strings like a puppet master, they use their hosts to advance towards their own goal of planetary conquest. Creepy is the best word to describe most of their strategies. They turn some hosts suicidal. They castrate others. They turn still others into zombie bodyguards. But a new study published today suggests that the parasite that causes malaria may use a more pleasant strategy. It lures mosquitoes to infected hosts with a lemony scent.
Malaria is caused by single-celled parasites called Plasmodium. A female mosquito carries them in its gut as it flies around in search of a victim to bite. After the parasites mature, they push through the insect’s gut wall, eventually making their way into its salivary glands. When the mosquito lands on a person and drills into the skin, it pushes some of its Plasmodium-laden saliva into the wound.
The parasites now begin their long journey through the human body. They get pushed by the surges of the bloodstream to the liver, where they invade cells and multiply inside them. The infected liver cells erupt with the next stage of Plasmodium’s life cycle, called merozoites. The merozoites end up back into the bloodstream, where they now invade red blood cells. They multiply yet again, rupturing the blood cells and invading new ones. Eventually the parasites achieve the next stage in their life cycle, when they’re ready at last to get sucked up by a hungry mosquito in a meal of blood.
If Plasmodium can’t get into a mosquito, all of this multiplication is for naught. So anything that the parasite can do to increase the odds of a successful exit can potentially be favored by natural selection. Last year, for example, a team of researchers found that mosquitoes were attracted to mice infected with Plasmodium parasites–but only when they were ready to leave their rodent host. The scientists found evidence that the parasites engineer this attraction by changing the odor of the mice. Infected mice give off odor molecules that draw mosquitoes to them.
Those scientists speculated that perhaps the parasite alters its hosts chemistry to make new odors. But recently Audrey Odom of Washington University and her colleagues raised another possibility: maybe the parasites themselves produce mosquito-attracting chemicals.
Other scientists had explored this possibility before without much to show for their efforts. But Odom and her colleagues suspected that previous researchers hadn’t looked hard enough. So the Washington University team added Plasmodium to much larger volumes of blood than before–400 milliliters–and then snagged odors rising off the blood with more sensitive traps. The efforts paid off: Odom and her colleagues found that when Plasmodium infected red blood cells, it produced chemicals called pinene and limonene.
You have probably smelled these chemicals before. Pinene is part of the blend of odors that make up the scent of pine trees. Limonene gets its name from lemons, which produce it in their rinds.
If you’re confused at this point about a single-celled blood parasite producing a fragrant odor, you have every right to be. To make sense Odom’s weird discovery, we have to take a sharp detour through more than a billion years of evolution.
It’s 1.3 billion years ago. The planet is ruled by bacteria and protozoans. Animals and plants won’t evolve for many hundreds of millions of years. On the surface of the ocean, some bacteria are capturing sunlight with photosynthesis, while protozoans are preying on them. Somehow, this story goes off-script, and some protozoans end up with photosynthetic bacteria trapped inside them. Instead of becoming food, the bacteria supply the food, powering the protozoans with photosynthesis. Over many generations, the bacteria become an inseparable part of their host. The combination of these two kinds of life become a new kind, which we call algae.
This primordial algae had many descendants. Some of them evolved into green algae, and eventually gave rise to plants on land. Another lineage of algae were swallowed up by yet another protozoan, and became another form of algae found on Earth today, known as red algae. Some red algae live now as free-floating photosynthesizers in the ocean. Others took up inside corals, providing coral animals with sustenance from the sun. And still other red algae became parasites of animals. Some of these parasites eventually became Plasmodium.
Plasmodium’s ancestors lost the ability to photosynthesize a long time ago. But they still hold onto some of the ancestral enzymes from the bacteria that their forebears swallowed 1.3 billion years ago. As a result, Plasmodium is weirdly similar to flowers and trees. Some scientists have even taken advantage of this evolutionary kinship by looking at weed-killers as potential drugs for malaria.
This ancient heritage also explains why Plasmodium can smell like lemons. Odom and her colleagues found that the parasite make pinene and limonene using enzymes that are related to the ones that plants use to make these chemicals.
There are reasons to think that the parasite are using these chemicals to lure mosquitoes. While we’re painfully aware of the appetite mosquitoes have for blood, the fact is that mosquitoes also feed on flower nectar. They depend on the nectar for sugar they need to fuel their flights. Many insects are keenly sensitive to certain colors and odors that flowers produce, which guide them reliably to their next meal of nectar. Odom and her colleagues found that the antenna of malaria-carrying species of mosquitoes are exquisitely sensitive to pinene and limonene. If you want to attract mosquitoes, it makes sense to make those chemicals.
While this research is tantalizing, it is only the first step in testing the hypothesis that Plasmodium makes a fragrant odor to lure its next host. So far, Odom and her colleagues have only demonstrated that the parasites are making these chemicals inside red blood cells. It’s certainly conceivable that in a living host, these odors could escape into the lungs and leave the body with exhaled air. It remains to be seen if they really do get out, and if they make a difference to the success of Plasmodium. Or perhaps this parasite perfume has some other function, and it remains bottled up inside sick hosts.
(For more examples of parasite manipulation, see my cover story in the November 2014 issue of National Geographic or my book Parasite Rex.)
Reference: Kelly M, Su C-Y, Schaber C, Crowley JR, Hsu F-F, Carlson JR, Odom AR. 2015. Malaria parasites produce volatile mosquito attractants. mBio 6(2):e00235-15. doi:10.1128/ mBio.00235-15.
[Update: Link to paper fixed]
March 10, 2015
Whales on the Wrong Side of the World
In May 2010, a whale showed up on the wrong side of the world.
A team of marine biologists was conducting a survey off the coast of Israel when they spotted it. At first they thought it was a sperm whale. But each time the animal surfaced, the more clearly they could see that it had the wrong anatomy. When they got back on land, they looked closely at the photographs they had taken and realized, to their shock, that it was a gray whale. This species is a common sight off the coast of California, but biologists had never seen one outside of the Pacific before.
Aviad Scheinin, one of the marine biologists on the survey, posted the news on the web. “Nice Photoshopping,” someone replied.
Three weeks later, Scheinin got one more bit of news about the whale. It was photographed off the coast of Spain, having traveled 1864 miles. Then it disappeared.
After three years, a second gray whale appeared off the coast of Namibia in 2013. Comparing photographs, scientists could see that it was a different animal than the one that visited Israel. After lingering along the coast of Namibia for a month, the whale vanished.
These two sightings have left whale experts startled. In an interview with the Orange County Register, one scientists compared the feeling to walking down a street in California and seeing a giraffe.
But according to a new study, these two whales may be a hint of the new normal. Gray whales may be poised to move into the Atlantic, because we’re opening a path for them through the Arctic. But it’s not an unprecedented invasion. To some extent, it’s a case of history repeating itself.

A feeding gray whale. Davis Melzer/National Geographic
California’s gray whales give birth each winter in the lagoons of the Baja Peninsula. Then they migrate up the west coast to the Arctic for the summer. They power these tremendous migrations–the longest of any mammal–by ramming their mouths into the sea floor and filtering out tiny crustaceans from the sediment. When they rise back up to the ocean’s surface, they bring with them wide muddy plumes.
Aside from the California population, the only other known population of gray whales is a small group of animals on the western side of the Pacific. But scientists have had hints for a long time that gray whales might once have lived in the Atlantic as well.
In the eighteenth century, whaling ships off the coast of New England chased what naturalists at the time referred to as “scrag whales.” Their descriptions of scrag whales are a match for gray whales. In the 1800s, fossil-collectors picked up whale vertebrae on the coast of England. Many years later, paleontologists found that the bones belonged to gray whales.
These findings suggested that gray whales once lived in both the Atlantic and Pacific. That’s the case today for other filter-feeding whales (known as baleen whales). Species such as humpback whales and fin whales split into Atlantic and Pacific populations a couple million years ago and have remained distinct ever since.
Scientists suspected that gray whales spread across both oceans millions of years ago. Later the planet has cooled, creating an icy Arctic that formed a barrier between the two populations. The gray whales of the eastern Pacific would migrate as far north as they could manage before reaching the ice, and then head back south. Presumably the Atlantic gray whales had a similar migration. Isolated for millions of years, the gray whales of the two oceans might well have evolved into different species. If that were true, then whalers must have driven the Atlantic gray whale species to extinction, while sparing the Pacific one.

Engraving of gray whale by Charles Scammon, 1872
To explore the mystery of these whales further, a team of researchers has taken a fresh look at the fossils of Atlantic gray whales. Instead of just observing the anatomy of the bones, the scientists probed them for ancient DNA. They also measured the amounts of carbon isotopes in the bones to determine their age. The fossils ranged in age from just a few hundred years old to over 50,000 years old.
The scientists were able to use all this information to draw a family tree of gray whales, showing how Atlantic and Pacific gray whales were related to each other. They could also estimate how long ago the branches split apart.
The gray whale’s tree turned out to be different from those of other baleen whales. The Atlantic and Pacific populations of gray whales are not a pair of ancient, distantly related lineages. Instead, the Atlantic gray whales are actually made up of at least four different lineages. And each of the Atlantic branches is most closely related to a different branch of Pacific gray whales.
In other words, Pacific gray whales have periodically swum across the Arctic Ocean and into the Atlantic and established populations that survived for millennia. The scientists can identify several waves of immigration. One took place about 79,000 years ago, and then three others happened more recently, between about 10,000 and 5,000 years ago.
The timing of these colonizations is telling: the whales appear to have moved into the Atlantic whenever it was warm enough for them to get through. Between 135,000 and 70,000 years ago, the climate was so warm that the Bering Strait was open year-round, giving gray whales access to the Arctic Ocean. Once these gray whales got to the Atlantic, they then endured until at least 5,000 years ago.
Then a new ice age began. Glaciers grew, sea levels dropped, and gray whales could no longer get across the Arctic. Sixty thousand years passed before the ice age ended with a sudden burst of warmth. And that’s when new waves of gray whales came into the Atlantic. The Arctic then cooled somewhat, closing the door once more.
Now we are warming the Arctic again by releasing greenhouse gases into the atmosphere. If history is any guide, global warming in decades to come may open up the Arctic for Pacific gray whales, some of whom may wander off their regular migrations and end up in the Atlantic.
These gray whales will encounter an ocean far different from the ocean their cousins arrived in thousands of years ago. They will have to deal with busy shipping lanes where they may get killed in collisions, along with oil drilling and industrial fishing operations. On the other hand, the authors of the new study predict that the gray whales will have lots of good habitat to live in. As sea levels rise, there will be more shallow shelves where the whales can scoop up mud to find food. Today, a gray whale outside the Pacific seems like a case of Photoshopping. Soon, however, we may be photoshopping a whole ocean of whales.
March 6, 2015
Junk and Jewels in the Genome
In this Sunday’s issue of the New York Times Magazine, I have a feature about clashing visions of the genome. Is it overwhelmingly made up of “junk”–pieces of DNA that provide us with no useful function–or is it rife with functional pieces that we have yet to understand? Or is the reality of the genome a confusing mixture of the two?
To research this story, I shed some blood so that I could compare my genome to that of an onion. This print-out, annotated by T. Ryan Gregory, shows that an onion has five times more DNA in its genome than mine. I also spent time in the lab of John Rinn at Harvard, where scientists are discovering hints that our genome encodes exotic molecules that may be essential for our well-being. And I talked to a range of scientists about the challenges of understanding what any given piece of DNA is “for,” and what sort of assumptions one should bring to the challenge. Finally, I dug deep into the history of this question, which has roots reaching all the way back to Darwin. Check it out.
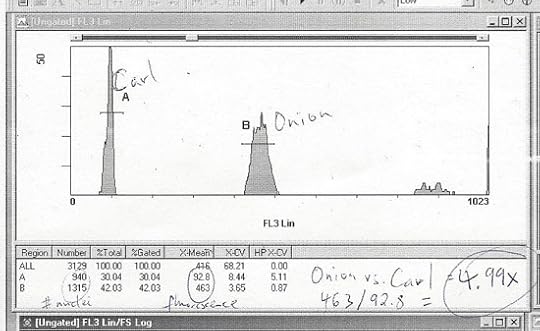
Courtesy of T. Ryan Gregory
P.S. This is an incredibly rich topic, and I welcome readers to discuss it (especially the stuff I didn’t have room to get to in my feature) in the comment thread below. I’ll also post some interesting papers here, too.
The Genomic Challenge to Adaptationism, by Sahotra Sarkar. British Journal for the Philosophy of Science, 2014. [subscription required]
Junk or Functional DNA? Germain et al., Biology and Philosophy, 2014. pdf
An Evolutionary Classification of Genomic Function, by Dan Graur et al. Genome Biology and Evolution 2015.
Discovery and Annotation of Long Noncoding RNAs. Mattick and Rinn. Nature Structural & Molecular Biology 2015. [subscription required]