Carl Zimmer's Blog, page 8
November 19, 2014
The Good Viruses?
When I talk about my book A Planet of Viruses, people often ask me if there are any viruses that are actually good for you. In an Ebola-obsessed age, it may be hard to imagine how the answer could be yes. But–yes! Or, at least, possibly yes. In my New York Times column this week, I write about a provocative new study that suggests a virus can play the same nurturing role as the microbiome, encouraging the growth of a healthy gut and a robust immune system. Check it out.
November 14, 2014
The “Natural” Of Family Life
Family life is fascinating–whether the family involved is made up of humans, monkeys, or hippos. Recently I’ve been exploring the complexities of mammal family life, and I’ve been thinking about what this research can and cannot tell us about our own experiences in families.
Last week in the New York Times, I wrote my column about some intriguing research on what happens when monkey mothers nurse their babies. Their milk doesn’t just deliver nutrients. It also has messages–different levels of hormones–that influence how babies develop, both physically and psychologically.
This week I’ve taken a darker turn. My column is about why male mammals sometimes kill unrelated infants.
It may sound strange, but the “why” of infanticide has a lot in common with the “why” of nursing’s effects on babies. In both cases, scientists have found evidence that these are adaptive responses. In the case of nursing, the babies appear to be reading the messages in their milk to judge the condition of their mother. They’re then adjusting their development to make the best use of her resources. In the case of infanticide, males that compete with other males to be with females can get more of an opportunity to have babies of their own by killing unrelated babies first.
Both stories are fascinating, and not just for their immediate results. We can’t help but wonder what scientific research of other mammals may tell us about ourselves. Yet the insights are not straightforward, and so we have to be on our guard not to draw simplistic conclusions.
We don’t yet know how much of an influence hormones in milk have on human development, for example. Monkeys and humans both nurse, but they nurse in very different ways (human mothers don’t keep their babies nursing constantly, for example). And human babies are influenced by many other factors as they develop.
As for mammal infanticide, it may be tempting to see it as an explanation for human child abuse. But you can’t draw a simple parallel between, say, the death of a human child in a chaotic home where there’s been lots of drug abuse and the death of a baby monkey that’s been systematically stalked for days by an adult male.
Nor do these stories give us easy answers about what is right or wrong in human family life. To try to make that leap is to commit a moralistic fallacy. I’ve had a lot of experience watching people make that fallacy. That’s one of the hazards of reporting on evolution.
I first wrote about infanticide 18 years ago. I was working at Discover, and I had taken a trip to Sumatra not long before, where I had spent some time with scientists at a forest reserve. Some of them were following monkeys, observing their family life. They told me how males sometimes killed babies. There were no killings when I was there, which isn’t surprising given that they happen quickly and relatively rarely. But even as I watched the monkeys leap through the high canopy, busy with their own complicated social lives, I kept wondering about why they would turn homicidal.
When I got back home, I spoke to Sarah Hrdy, an anthropologist who developed the argument that infanticide is part of a reproductive strategy in the 1970s. I talked to her critics, and I also talked to scientists who found evidence for infanticide in other species. You can read the feature I ended up putting together here.
Shortly after the article came out, the creationists came after me. Specifically, the president of the Institute of Creation Research at the time, John D. Morris. He published a piece called, “Will Infanticide Follow Abortion As Acceptable Behavior?”
He ended it this way:
The discussion reviewed her [Hrdy's] theories and others who followed, but the most chilling aspect was the possible use of this animal behavior as a model for human behavior. The article’s author, Dr. Carl Zimmer, stopped short of advocating human infanticide, but clearly the issue was raised and not discarded. Those who today practice infanticide were discussed without condemnation. A comparison to the disparity in frequency of child-killing between biological fathers and step-fathers was made. Nowhere was the practice of infanticide condemned, and, of course, no mention was made of it as a sin.
The article did speculate on the various reasons why langurs (and other animals) choose to kill their young, and comparisons were made to human situations. The reader is left to shudder at the thought of the possibilities, but be numbed to its consequences, by greater familiarity with it.
One only has to look at Hitler’s Germany to recognize similarities. Atrocities escalated until unthinkable things were attempted. It is my conviction that the trends in this country are the same. A wholesale turnover of politicians, media elite, and education theorists would help to stem the tide. The groundwork has already been laid, the damage may have already been done. We will soon reap even more awful fruits of evolutionary thinking, unless we go “Back to Genesis.”
Morris was wrong in all sorts of ways (beginning with the fact that I’m not “Dr.” Zimmer). But his screed was valuable as an example of how easily people can–intentionally or not–make a fallacious leap from nature’s “is” to human “oughts.” In order to make our own moral judgments–about breast-feeding, about protecting children from abuse–we need to understand how evolution has shaped us. That’s no simple task, and when it comes to judging the right thing to do for children, it’s just the beginning.
November 6, 2014
Norovirus: The Perfect Pathogen Emerges From the Shadows
As the year comes to a close, people are starting to puke. The notorious stomach bug known as norovirus is starting its annual rampage,, which will last from late fall through winter. A couple years ago, in the midst of another norovirus season, I wrote about the virus’s spectacular biology on the Loom. Noroviruses (unlike the Ebola virus) are extraordinarily rugged, able to waft through the air and survive for days on surfaces where it can cause a new infection. In a scientific review, one CDC scientist went so far as to declare, “noroviruses are perhaps the perfect human pathogen.”
This exquisite potency makes noroviruses a massive burden on our collective health. According to the latest estimates, noroviruses infect about 20 million Americans every year, and many more worldwide. But despite the scale of their threat, the fight against noroviruses has been slow. That’s because no one has been able to rear human noroviruses in the lab. To run experiments to see how noroviruses makes us sick, to develop vaccines, and to test out antiviral drugs, scientists desperately want a recipe for brewing up batches of noroviruses.
The inability to raise noroviruses stems, in turn, from our ignorance about some of the most important aspects of their biology. Scientists know that the virus attacks the gut, but they haven’t known for sure which kind of cell it attacks, or how it does so. They know that different noroviruses are more dangerous to people with different blood types–despite the fact that norovirus does not cause blood disease.
But now a team of scientists led by Stephanie Karst of the University of Florida may have cut through a lot of these mysteries. Karst and her colleagues have figured out how noroviruses get into our cells. And it turns out that some of our harmless gut bacteria are helping the viruses get there.
For years, scientists had assumed that noroviruses infected the cells that make up the inner lining of the intestines. After all, those cells (called epithelial cells) were the first ones that the viruses would encounter when they arrived in the gut.
If noroviruses infected epithelial cells, scientists could also explain the puzzling link to blood types. Our blood types are determined by the type of carbohydrates that festoon our red blood cells. But our gut epithelial cells also put the same carbohydrates on their surface too. Noriviruses can bind to these carbohydrates (known officially as human blood group antigens, or HBGAs).
Add up all the evidence, and you got a pretty straightforward scenario: noroviruses arrive in the gut, latch onto the HBGAs on the epithelial cells, invade those cells, and voila, a weekend of vomiting and diarrhea.
As sensible as that scenario sounded, though, there was one big problem: when scientists would run experiments, the viruses didn’t show any interest in infecting epithelial cells. Nor did the viruses seem to stay around on the surface of the intestines. Karst and her colleagues infected mice with a mouse version of norovirus, they found that it somehow burrowed deep inside the intestinal lining.
Buried deep in the lining of our gut, there are pouches of immune cells that protect us from intestinal infections. As food slides down through the intestines, the epithelial cells pick out suspicious-looking proteins and deliver them into those pouches. Cells known as B cells can then make antibodies that attack dangerous pathogens.
The deep dive that the noroviruses were taking raised the possibility that they were infecting B cells in the gut. Karst and her colleagues got even more interested in B cells when they ran another experiment on mice. The scientists were hoping to better understand how a norovirus infection may protect a mouse for future infections. As part of their experiment, they reared mice that couldn’t make B cells.
You might expect that the mice would become less able to withstand norovirus infections, since they couldn’t make antibodies. But the opposite was true: without B cells, the mice became more resistant.
Pondering all these pieces of evidence, Karst and her colleagues suspected that maybe B cells–not epithelial cells–really were the target of noroviruses.
The scientists tested out the idea on mouse noroviruses. When they mixed mouse noroviruses in a dish with mouse B cells, the viruses could indeed invade the cells, as the scientists predicted. But when they tried to infect epithelial cells, on the other hand, the viruses failed to invade.
The scientists couldn’t be sure that what was true for mice was true for humans. But testing their idea on human noroviruses would be a lot harder, since Karst and her colleagues didn’t have an endless supply of pure noroviruses.
Instead, the scientists had to collect stool samples from sick patients. They diluted the virus-laden stool and mixed it with human B cells. Just as they had hoped, the viruses infected the B cells.
There was, however, a fascinating catch. If the scientists put the stool through very fine filters–fine enough to exclude bacteria–the noroviruses could no longer infect the B cells.
This failure suggested that resident gut bacteria–or at least one species of bacteria–were helping the noroviruses.
It would have been absurd for Karst and her colleagues to test out every species of gut bacteria to see which one was aiding the noroviruses. Our guts contains many hundreds of species. Fortunately, previous research by other scientists allowed Karst and her colleagues to avoid this brute-force approach.
It turns out that blood type cells and epithelial cells are not the only cells to produce blood-type molecules. Certain species of bacteria have HBGAs, too. It’s not clear why they have the same molecules as we do. But whatever the reason, noroviruses can grab onto bacterial HBGAs as well as they can onto our own.
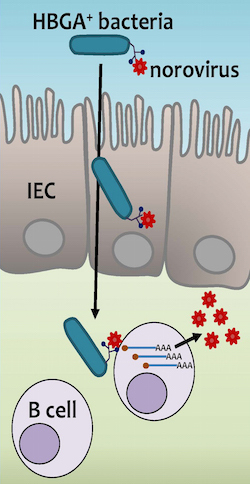
Diagram by Stephanie Karst
Karst and her colleagues picked out one of the species that other scientists had shown could bind noroviruses. It’s a common kind of bacteria called Enterobacter cloacae. The scientists added Enterobacter cloacae to filtered stool samples that contained human noroviruses. And then they combined this mixture with human B cells. Now they could get human noroviruses to infect B cells.
This experiment doesn’t reveal precisely how Enterobacter cloacae help noroviruses get into B cells. It’s possible that the bacteria ferry them into the hidden pouches where B cells lurk. It’s also possible that when the viruses latch onto the bacteria, the connection triggers a change in their surface molecules, making it possible for them to infect the cells. Karst hopes to get some answers with more research.
But this new results may offer an explanation for why people’s blood types make them more or less vulnerable to noroviruses. Let’s say you’re type B. Your immune system learns to recognize type B HBGAs as harmless, because they’re part of your own body. It’s possible that if you’re colonized by bacteria that have type B HBGAs, too, your body will tolerate them as well.
But if you get infected with bacteria that carry type A HBGAs, your immune system may make antibodies and attack them as foreign. That’s the likely reason that getting a tranfusion with the wrong blood type can be so dangerous. If you are Type B, for example, you have lots of antibodies to Type A HBGAs. So your body will attack Type A blood as foreign.
The new study from Karst and her colleagues may also explain why noroviruses seem to care about your blood type. Your blood type may determine the kinds of bacteria that can survive in the gut–and thus the kinds of bacteria that noroviruses can latch onto and use to get into B cells.
It would be great to say that this discovery immediately points to a sure-fire cure for the noroviruses blues. It doesn’t, alas. Karst and her colleagues were able to block norovirus infection in mice by using antibiotics to wipe out their gut bacteria. Without bacteria to help, the virus couldn’t get into B cells. But that’s the sort of cure that’s worse than the disease. The microbiome performs lots of important tasks, including helping with digestion and creating a kind of ecological barrier that prevents nasty pathogens from invading. Take it away, and you could get very sick–much sicker than you’d be with a norovirus infection.
Nevertheless, this discovery is still important, because it explains why previous attempts to raise noroviruses have failed. The viruses were provided with the wrong target and didn’t get the help they needed to hit it. Now, Karst hopes, she and her colleagues can finally develop a recipe to brew up lots and lots of human noroviruses for research on vaccines and antivirals.
And that’s the only sense in which the phrase “lots of lots of noroviruses” can make us happy.
October 30, 2014
For Your Halloween Viewing Pleasure: Two Mindsucker Movies
Last night at the National Geographic Society in Washington, I gave a talk with photographer Anand Varma about how parasites manipulate their hosts–the subject of my cover story in the November issue of National Geographic and Varma’s aesthetic obsession for the past couple years. Along with his gorgeous photos, Varma also showed off some lovely/creepy videos. I thought I’d share a couple of them with you. Pop them into full screen for full appreciation.
First off: Ophiocordyceps, a fungus that takes control of an ant. The fungus spores invade an ant’s body and then fill much its interior with tendrils. Under the fungus’s spell, the ant climbs up a plant and clamps down on the underside of a leaf. This movie shows the ant in its last hours, shot upside down for clarity. The fungus shoots a spike of spores out of the ant, which can then rain down on unfortunate ants below.
The second video shows how the white butterfly wasp takes over the cabbage butterfly caterpillar. A female wasp inserts her stinger into the host and injects dozens of eggs. They grow inside the caterpillar, which continues munching on leaves–leaves that now fuel the growth of the parasites inside. They chew their way out all at once, and yet they don’t kill the caterpillar. They prevent it from bleeding to death by thickening its bodily fluids and seal up their exit holes with bits of their own tissue. The caterpillar recovers from this strange birth and then spins a cocoon for the wasp larvae. It then sits atop its parasitic brood, fending off any animals that try to get at the wasps. At the end of the movie, you see the most dreaded of these enemies: another species of wasp that only lays its eggs in the larvae of the white butterfly wasp.
If you’re in Connecticut, you can come hear me talk about these puppet masters at the Westport Library on Saturday at 4 pm. And if you still crave more, check out my book Parasite Rex.
October 24, 2014
Flu and Ebola: How Viruses Get Around
A couple viruses are waving hello to the United States right now. Flu season is about to kick off, while people have been diagnosed with Ebola not just in Texas, but in New York. But there are some important differences between the two viruses that I explore in an article in today’s New York Times. Most importantly: there’s no evidence that Ebola spreads through the air like the flu.
October 21, 2014
Translation: A New Episode of Radiolab
The good folks at Radiolab have a new episode out. It’s on the many different senses of the word translation. The show ranges from vision-sensing tongue vibrators to high-level diplomatic misunderstandings. At the end of the show, I talk to Jad Abumrad about the most fundamental translation of all: the process by which our cells turn information in our DNA into proteins. Here’s the embedded episode. And for more, see my recent story for Nautilus.
October 15, 2014
Ebola, Good Germs, and More Tales of the Invisible
This morning I stopped by WHYY in Philadelphia to talk about Ebola and other news from the microscopic realm on “Radio Times.” Here’s the hour-long conversation I had with Marty Moss-Coane.
October 14, 2014
Ebola’s Past and Future
I have a story in the news section of today’s New York Times on the past and future of Ebola. There is so much anxiety and curiosity about the virus that it seemed like an opportune time to check in with a bunch of evolutionary biologists who study Ebola–as well as other viruses. In my piece, I make two basic points: 1) the scientists I’ve spoken to don’t think that the virus currently spreading around West Africa (and beyond) is some freakish mutant, and 2) it’s very unlikely that during this outbreak it will transform into some fundamentally different–and more dangerous–pathogen.
Reporting a story like this is a bit like dipping a bucket into a burbling fountain. There’s just too much to capture in a single article–especially a standard 1000-word newspaper piece. Fortunately, the Internet provides us with infinite overflow space.
So here I’d like to just expand on a couple of the more compressed points in my story. And I’ll extend an invitation to you, dear reader, to use the comment section below to post any questions my story raises in your mind. I’ll do my journalistic best to answer them in updates to this post. (I’ll also note any revisions I have to make to the post to correct any errors I’ve made.)
1. Ebola is 20 million years old? How do you know? Viruses are terrible at leaving fossils, but they can leave their imprint on their hosts.
Every now and then, a virus will insert some of its DNA into its host’s genome, and that viral DNA gets passed down their descendants for millions of years.
Our own DNA is riddled with viral DNA, which makes up at least 8 percent of the human genome. Most of it has mutated into useless baggage, but some has been transformed into useful genes (useful to us, that is). I’ve written here about how virus proteins are essential for our placentas to develop.
The presence of the same virus at the same spot in two different host species can give a clue to its age. That’s because the virus must have infected the common ancestor of the two species.
In recent years, scientists have been finding DNA from the lineage of viruses that includes Ebola (called filoviruses) in mammal genomes. Last month, they published an especially interesting study on this fossil virus material. They found the same viral DNA at the same spot in two species of rodents–hamsters and voles. And this DNA is more similar to Ebolavirus than to its closet relative, Marburg virus. Hamsters and voles share a common ancestor that lives roughly 20 million years ago, and so that means that Ebola viruses had split off from Marburg virus relatives at least that long ago.
There’s even some evidence that some of this Ebolavirus DNA is performing some useful jobs in its mammal hosts. It would be fascinating to learn more how this deadly virus has been domesticated.
2. Hasn’t Ebola gone airborne already? There’s been some suggestive evidence in the past, but scientists have questioned whether transmissions that seemed like they were airborne were just the result of short-range droplets–not long-range aerosols. Here is a report from July in which Canadian researchers tested out a bunch of nasty viruses for how easily they could be transmitted by air between monkeys. “In the current study,” they write, “two NHPs [non-human primates] were lethally infected with EBOV [Ebola virus], and no EBOV virus or antibodies to EBOV GP [a virus surface protein] were detected in the neighbouring uninfected NHPs for up to 28 days after the challenge date.” Here is a lengthy blog post from Heather Lander on this study and previous ones. And here is virologist Vincent Racaniello with more thoughts on the issue.
October 11, 2014
On Superiority
I’m writing this at my house in central Connecticut. Twenty thousand years ago, this spot was buried under a mile of ice.
About thirteen thousand years ago, after the ice thinned and retreated, plants swept over the bare land. They came from the southern United States, and they established the same kinds of forests and swamps that had grown in Connecticut in earlier periods between the Ice Ages.
But today, many of the most abundant plants around my house today come from distant continents–plants like honeysuckle. And Japanese barberry. And ground ivy. And the knotweed. And on and on: you can read a list of invasive plants in Connecticut here.
It’s a story repeated around the world. As ships cross the oceans and planes soar through the air, they deliver species to places that would probably never get to on their own. And sometimes they thrive amazingly, beating back the native species.
This week in the New York Times, I write about new research on why invasive species thrive. Some scientists argue that native ecosystems have to be weakened for aliens to take hold. Some favor the idea that alien species gain an advantage by leaving their parasites and predators behind. But a pair of ecologists, Jason Fridley and Dov Sax, argue that something else may be going on. It may simply be that the invasive species are superior.
Using the word “superior” is a risky thing to do, because it can trigger lots of troublesome and irrelevant associations…
@carlzimmer @nytimesscience You mean with traits having more survival value? “Biologically superior” is so 1930′s.
— MICHAEL SAUKA (@tc99mman) October 9, 2014
But the word is correct–Fridley and Sax use it repeatedly in their paper–so I’ll stand by it even if it means I have to clarify things.
Fridley and Sax have drawn on some remarks by Darwin to develop what they call the Evolutionary Imbalance Hypothesis. They argue that species in different parts of the world have adapted to similar physical conditions. East Asia is a lot like Connecticut, for example, in terms of its climate. The species in both places have evolved, as natural selection improved their ability to survive, grow, and reproduce. It’s conceivable that a species in one place might get better at all that than a species somewhere else. It would be, in other words, superior. That doesn’t mean that this species would be some kind of Platonic ideal, or that it was a kind of Aryan paragon. It simply produced more offspring under identical conditions than another species.
Fridley and Sax hypothesized that some places might act as nurseries for superior species. These would be stable regions where evolution could play out longer than other places. As I describe in my column, they found evidence that these places–like East Asia–do in fact exist, and they send lots of invasive species to other regions. Like Connecticut. With ice sheets sweeping down from time to time, Connecticut and the rest of the northeastern United States may have been home to an ecosystem of–sorry to say–inferior species.
As I noted in my column, this hypothesis has some gloomy implications for those who want to preserve native species. Healing a native ecosystem may not be enough. Introducing an alien species’s parasites to its new range may not be enough. That’s because fighting invasive species may, in effect, be fighting against millions of years of evolution.
And this leads to a prickly question. One of the things that make ecosystems worth saving is the services they provide us with. If a superior species swoops in, it may do a better job at some of those services than native ones. An alien plant might draw more carbon dioxide out of the atmosphere and store it away in the soil, for example. Maybe we’ll be better off with an invaded ecosystem? I may not be fond of the barberry infiltrating the New England forests, but maybe our decisions about managing the wilderness have to be based on more than aesthetics.
October 1, 2014
The Central Park Zoo Hidden From View
In 2003, an army of 350 scientists and volunteers swept out across Central Park. Their mission, called a BioBlitz, was to find as many species as possible over the course of 24 hours. At the end of the day, they had compiled a catalog of 836 species of plants and animals.
It’s impressive that Central Park–an 843-acre island in an ocean of Manhattan concrete–can play host to so many species. But that’s hardly a complete inventory of the biodiversity of the place. Along with its plants and animals, Central Park is home to invisible wildlife too.
The ground swarms with invertebrates, fungi, and a wealth of microbes. This underground diversity–especially the microbes–has been very hard to explore, not just in Central Park but around the world. For one thing, you have to dig. For another, you can’t usually can’t tell the species apart with the naked eye. It’s possible to distinguish between the five species of turtles in the Central Park’s Turtle Pond just by looking at them. But if you dig up a patch of dirt by the pond and look at the bacteria it contains, they might well look like just a bunch of rods and spheres. The diversity of microbes is instead a matter of chemistry. They have evolved a staggering range of ways to break down molecules and grow on them.
In recent years, scientists have developed powerful new tools for measuring that diversity. Rather than looking at feathers or stripes, they look at DNA.

Each dot represents a place where scientists scooped up dirt to look for DNA. From Ramirez et al 2014
A team of researchers has now used this approach to carry out a sort of MicroBioBlitz in Central Park. They marched their way systematically through the park, and every fifty 50 yards or so, they stopped, bent down, and scooped up some dirt. All told, they gathered 596 scoops. Back at their lab, they threw out everything from those scoops except for the DNA. And then they plucked out just one particular stretch of that DNA. To be more precise, they plucked out different versions of that stretch, each carried by a different species.
The scientists then looked at the sequence of each of those versions. In some cases, the DNA turned out to be identical to a known species, or nearly so. In other cases, the sequence was very different. A peculiar sequence of DNA told the scientists that it came from a species that’s new to science.
The results were, to be blunt, pretty insane. All told, the scientists identified over 167,000 species.* That’s about thirty times more species that all the mammals on Earth–everything from fruit bats to walruses, from musk ox to marmosets.
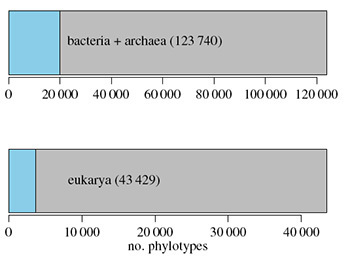
Diversity. Blue: known phylotypes (similar to species). Gray: unknown. Ramirez et al 2014
This chart shows just how ignorant we are of the life even in Central Park. In each chart, the blue bar shows number of species in the park that are already known to science. The gray bar shows the ones that don’t match anything we know of. The top chart shows bacteria and another group of microbes called archaea. (Archaea are single-celled microbes that, like bacteria, keep their DNA floating loose inside their cell.) The bottom chart shows our own branch of the tree of like, the eukaryotes. Eukaryotes include not just animals and plants, but fungi, amoebae, and other protozoans. In both cases, the unknown dwarfs the known.
The more you drill down into these numbers, the more insane they get. Even the “known” species in these charts aren’t very well known at all. For the most part, scientists have never seen the organisms from which they come. They’ve only fished out the same DNA segment from another sample.
The geography of Central Park’s microbes is also mind-boggling. It’s not as if all 167,000 species were present in every sample of the soil. Instead, different species showed up in different places. On average, each sample had about 7000 bacteria and archaea, and 1250 eukaryote species. When the scientists compared the diversity in the samples, they found that any pair picked at random shared only 19.3% of their bacterial and archaeal species, and just 13.5% of their eukaryote species. Even neighboring sites were no more similar to each other than ones on opposite ends of the park. And as the scientists scooped up more dirt, they kept finding more species. So the true number of species in Central Park is probably far higher. (It’s also worth noting that there are probably a lot of other microbes living on the trees, in the ponds, and in other places in the park the scientists didn’t even touch.)
Now, it might have turned out that many of the species in Central Park were closely related to each other. There are many, many species of beetles, for example. Perhaps Central Park only had microbial versions of beetle, and no microbial ants or termites.
That’s not how things turned out. Central Park has the microbial ants and the termites, too. In fact, its microbes span much of the tree of life. You’d get a similar span of species if you took the same number of soil samples from around the world, from jungles to deserts.
Some of the results of the study may be the result of Central Park’s own peculiar history. Its soil contains a high level of species that are considered potential human pathogens. In their report, the scientists hasten to note that this doesn’t mean you’re especially at risk of getting sick in the park. But it may be a sign of the presence of lots of people nearby.
For the most part, though, Central Park may just be a typical plot of microbial habitat. Soil, as a rule, is just rife with microbial richness on a scale we can barely understand. It’s loaded with dying plant matter and the remains of dead animals. Its particles and tunnels and other features make soil an incredibly complex environment, where microbes can specialize in all sorts of ways of making a living. It may turn out that the only thing that makes Central Park unusual is the many holes that scientists have dug there.
*I’m going to use the term species here for convenience. Determining exactly what a species is can be tricky for microbes, and the scientists only presented distinct lineages that might or might not be separate species–what they call phylotypes. Here’s a piece I wrote on why this whole problem is so challenging.



