Carl Zimmer's Blog, page 9
September 28, 2014
Pines and Viroids: On The Deep Past And Imminent Future of Life
Recently I’ve been writing a fair amount about plants–what they can tell us about the deep history of life, as well as what life will be like for them in the near future.
Tomatoes, dahlias, and many other cultivated plants can fall victim to a strange infection. The pathogen is not a fungus or a bacterium or even a virus. Instead, it’s a naked snippet of genes known as a viroid. A team of scientists is convinced that viroids are relics of the earliest stages of life on Earth, a form of life that evolved before the dawn of DNA. For the rest of the story, see my column in the New York Times.
Viroids will still be a fact of life for plants in the next century–but so will a rapid shift in the climate. What will happen to plants when the temperature in their current range changes? Will they be able to colonize places further from the equator where they can still thrive? Or will they be unable to get there fast enough? In another piece for the New York Times, I investigate the question by looking at one plant in particular, the magnificent whitebark pine. Check it out.
September 22, 2014
Two new videos: where new genes come from and where new biologists come from
Recently I had the pleasure of working on two videos that are now online. I’ve embedded them under the fold.
First up is an animation from TED-ED. I worked with them on a piece explaining where new genes come from, based on some of my articles (such as this and this).
Next is a fun conversation I had on Huffington Post Live with a sharp 11-year-old boy named Cody who wanted to talk about Parasite Rex. I’m hoping my book eventually leads him to find a new way to fight malaria. (No pressure!)
Two new videos: where new genes from and where new biologists come from
Recently I had the pleasure of working on two videos that are now online. I’ve embedded them under the fold.
First up is an animation from TED-ED. I worked with them on a piece explaining where new genes come from, based on some of my articles (such as this and this).
Next is a fun conversation I had on Huffington Post Live with a sharp 11-year-old boy named Cody who wanted to talk about Parasite Rex. I’m hoping my book eventually leads him to find a new way to fight malaria. (No pressure!)
September 19, 2014
What Slipped Disks Tell Us About 700 Million Years of Evolution
From Zimmer and Emlen, Evolution: Making Sense of Life
There’s a unity to life. Sometimes it’s plain to see, but very often it lurks underneath a distraction of differences. And a new study shows that there’s even a hidden unity between our slipped disks and the muscles in a squirming worm.
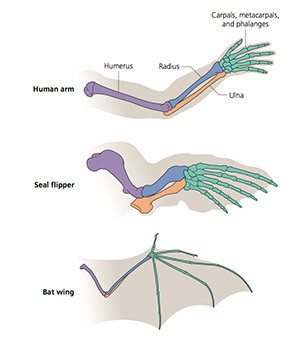
Scientists call this unity “homology.” The British anatomist Richard Owen coined the term in 1843, sixteen years before Charles Darwin published The Origin of Species. Owen defined homology as “the same organ in different animals under every variety of form and function.” For example, a human arm, a seal flipper, and a bat wing all have the same basic skeletal layout. They consist of a single long bone, a bending joint, two more long bones, a cluster of small bones, and a set of five digits. The size and shape of each bone may differ, but the pattern is the same regardless of how mammals use their limbs–to swim, to fly, or to wield a hammer.
Darwin argued that homology was the result of evolution. The common ancestor of humans, seals, bats, and other mammals had a limb which became stretched and squashed in various contortions. And over the past 150 years, paleontologists have found a wealth of fossils that help document how the tiny paws of Mesozoic mammals diversified into the many forms found in mammals today.
But Darwin wasn’t just out to explain the evolution of mammals. He saw a kinship across the entire living world. And that’s where things got complicated. Anatomists in Darwin’s day could find no clear counterparts to many of the traits in our own bodies in distantly related animals.
It turns out the homology is there, but you just need the right eyeglasses to see it.
Recently, Detlev Arendt, a biologist at the European Molecular Biology Laboratory, and his colleagues investigated the evolution of an important but overlooked feature in our bodies, known as the notochord. It’s a stiff rod of cartilage that develops in human embryos, running down their back. Later, as the spine develops, the notochord transforms into the disks that cushion the vertebrae (and sometimes slip later in life, causing much grief).
Other mammals also develop a notochord as embryos. And so do birds, reptiles, amphibians, and fish. Even our closest invertebrate relatives, such as lancelets, have notochords. All animals with a notochord belong to the same group, known as the chordates.
Unlike most vertebrates, lancelets keep their notochord into adulthood, using it to stiffen their bodies when they swim. Early chordate fossils also have a lancelet-like anatomy. So it’s likely that 550 million years ago, the notochord evolved in chordates first, and then the skeleton evolved later. In fish, the spine took over the body-stiffening job, but the notochord still had other work left to do. In the vertebrate embryo, the notochord releases chemical signals that tell the surrounding cells whether they should become nerves, blood vessels, or other tissues.
Arendt and his colleagues wondered how the notochord first evolved. Squid don’t have a notochord. Neither do clams, or cockroaches, or tarantulas. The notochord, in other words, seems to be unique to chordates. So where did it come from? Did it emerge right at the dawn of chordates, or did it have deeper origins?
The scientists decided to tackle these questions by looking at the genes in notochord cells. In a developing vertebrate embryo, notochord cells switch on a unique combination of genes. The scientists wondered if the genetic signature of a notochord cell might be lurking in animals that have no notochord.
They started their search in ragworms, ocean-dwelling relatives of the more familiar earthworms. Worms (or to be more precise, annelids) are an ancient lineage that split off from our own ancestors long before the notochord evolved. There’s nothing in their squishy bodies that you would mistake for a notochord.
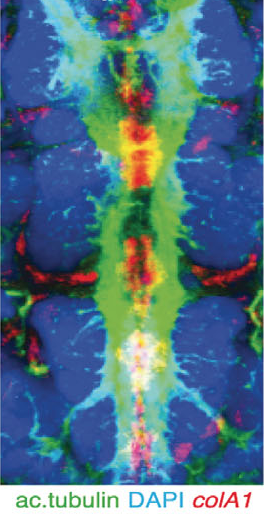
The axochord. Colors correspond to activity of genes listed in the figure. Lauri et al, Science 2014
Arendt and his colleagues added chemicals to ragworm larvae to make cells glow if they were using notochord genes. The larvae lit up like Christmas trees.
The cells using notochord genes formed a strip running from the head to the tail of the ragworm larvae–in much the same arrangement as our own notochord. When the rag worm larvae matured, the scientists found, the strip developed into a cord of muscle.
The worms need this cord–which the scientists dubbed the axochord–to move around. When the scientists destroyed the axochord with a laser, the ragworms could no longer swim. And once Arendt and his colleagues discovered the axochord in ragworms, they looked for it in other animals that lack a notochord. They found signs of axochords in a number of other invertebrates.

The marine worm Platynereis has a muscle (red) which develops in the same place and has the same genetic signature as the notochord (blue) that develops into our spinal discs. Credit: Kalliopi Monoyios
This study reveals the homology of notochords and axochords, but it also does something more. It helps us go back in time. Ragworms and humans share a common ancestor, along with other animals that have brains, heads, tails, and distinct left and right sides. Collectively all these animals are known as bilaterians. The emergence of bilaterians some 700 million years ago was a tremendous evolutionary event, giving rise to a huge diversity of animal forms and even changing the chemistry of the oceans and atmosphere.
Evolutionary biologists would love to know what the first bilaterians looked like, and to understand how they gave rise to such different animals today. One of the biggest surprises in the history of biology has been the discovery that bilaterians share a deep homology in the genes that build their bodies. Fly eyes and human eyes may look different, for example, but the same network of genes helps build both kinds. Or take the front and back of our bodies. In us (and other chordates), the main nerve cord runs down the back and our digestive tract runs down the front. In a fly or in many other bilaterians, the arrangement is vice-versa. But all bilaterians use the same genes to tell the two sides apart.
These discoveries let scientists develop hypotheses about what the first bilaterians looked like. They may have already had a head, tail, brain, and eye-like senses for example. And the new study hints that they may have had a precursor of our notochord. Our own cartilage notochord turns out to be a peculiar variation on the bilaterian body plan. Other bilaterians have an axochord–basically, a notochord made of muscle. So it’s possible that 700 million years ago, our first bilaterian ancestors had an axochord made of muscle, which they might have used to swim. The descendants of these first bilaterians diverged into many body forms. In a few lineages, Arendt and his colleagues argue, the axochord was transformed into structures that look dramatically different today. We belong to one of those lineages.
The new study suggests that the signals that our notochord cells received changed slightly, switching from muscle to cartilage. This is actually easier than it may sound, since embryonic stem cells are incredibly versatile. In fact, we can fall victim today to such anatomical mix-ups. Last year, for example, I wrote a story for The Atlantic about a rare disease called FOP that switches muscles to cartilage, which then turns to bone. It takes a single mutation to produce this disease.
Only 1 in 2 million people get FOP. Slipped disks are a far more common disorder. As many as a third of people may end up with a disk bulging out of place. That’s the kind of risk you run when your ancestors’ notochord evolves into spine cushions, and then, much later, your ancestors start walking around upright. If you ever find yourself laid up in bed because your notochord fails you, try distracting yourself by reflecting on its long evolutionary history reaching back over half a billion years, and the unity it shares with worms wriggling through the sea.
(Here’s a video showing the axochord in 3-D)
(Update: changed title to better reflect story)
September 5, 2014
The Erotic Endurance of Whale Hips
Buried deep within the body of a whale, underneath the heaps of muscles and tendons, lie some little, lonely bones. They are whale hips–and they are one of the stranger examples of evolution’s transforming power. Perhaps kinkier is a better word.
Some 54 million years ago, the ancestors of whales and dolphins were four-legged mammals. Their anatomy was well-adapted for moving around on land, including their hips. Here, for example, is an ancient member of the whale lineage, called Indohyus. The hip bones of these early whale relatives had scoops where the balls of their femurs could be tucked away. They had shelves where leg muscles could anchor. While the hips themselves were made up of a cluster of bones, they were fused together, and they were also joined tightly to the spine. Those firm connections allowed the animal to hold its body up against gravity, and use the forces generated by its legs to propel its body forward.

Indohyus reconstruction. Thewissen et al. Nature 2007. 450, 1190-1194
Over the course of about ten million years, the ancestors of today’s whales moved into the water. They evolved seal-like bodies with stout limbs; later, their forelegs became flippers and their hind legs dwindled away. They lost their fur and their nostrils migrated from the tip of their head to above their eyes, where it became a blow hole. (I wrote about this transition in my book At the Water’s Edge.)
By 40 million years ago, the walking whales were long gone. In their place were species like Dorudon atrox. As you can see from this diagram, its body looks a lot like a living whale. And like a living whale, its hips have shrunk and become separate from its spine. Unlike today’s whales, however, Dorudon still had well-developed hind legs–albeit tiny ones.

Dorudon. From M. Uhen, Annu. Rev. Earth Planet. Sci. 2010. 38:189–219
Within a few million years, those leg bones were pretty much gone, too. The diagram below shows a series of hip bones from the whale lineage, going from terrestrial species (Indohyus and Pakicetus) to more aquatic ones (Ambulocetus) to totally aquatic (Basilosaurus, which looked a lot like Dorudon) to living whales.
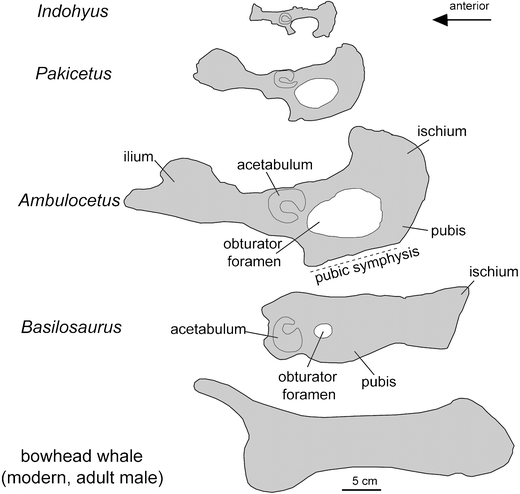
Whale pelvic bones. From Thewissen et al 2009, Bioscience. dx.doi.org/10.1007/s12052-009-0135-2
All that remained were a tiny set of hip bones. They lay far away from the rest of the skeleton. They no longer fused to each other, and they no longer had the distinctive scoops and shelves that used to be essential for walking.
When we see whale hips at the end of this long evolutionary history, they make more sense. They are vestiges of the terrestrial history of whales. But the fact that they still linger is also puzzling. If hips were adaptations for a vanished life, then why haven’t they vanished altogether?
A new study in the journal Evolution helps to answer that question. Far from being abandoned by evolution, whale hips are still evolving today. While they may not be essential for walking, they still matter a lot to whales. (A quick editorial note: this post gets NSFW from here on out.)
To see why, we have to go back to those hips of land mammals. They are important for walking on land, but they serve other purposes, too. Among other things, they anchor muscles that control the sex organs. If these muscles are anesthetized in men, for example, they have a hard time gaining an erection.
As whale hips stopped mattering to walking, they didn’t stop mattering to having sex. In male whales, the pelvis controls the penis with an especially elaborate set of muscles. In some whale and dolphin species, these muscles make the penis downright prehensile.

Dines et al, Evolution in press
Jim Dines of the Natural History Museum of Los Angeles and his colleagues have recently been studying how the sex life of whales drives the evolution of their hips. If a male animal can fertilize more eggs than other males, his genes may become more common over the generations. This process–known as sexual selection–can lead to all sorts of baroque adaptations in animals. Dines and his colleagues wondered if whale hips are also experiencing sexual selection.
Sexual selection gets stronger as the competition between males gets more intense. In some species, males fight battle for the opportunity to mate with females, and they often evolve big weapons like horns or oversized claws. In some species, the competition takes place inside the females, because a single female may mate with several males. Any strategy that lets one male’s sperm do better than another’s may become more common.
Females can make sexual selection even more intense. In some species, they have evolved elaborate reproductive organs that let them choose which male’s sperm she will fertilize her eggs with. Those female adaptations may drive the evolution of even more elaborate male organs that can overcome them.
One of the best ways to see sexual selection in action is to compare different species. As I wrote on the Loom a few years back, different species of ducks and other water fowl have huge penises and equally huge reproductive tracts. The longer the penis, the more maze-like the reproductive tract. This pattern suggests the birds are trapped in a sexual arms race.
Another way for males to increase their success is to produce more sperm, so as to overwhelm the competition. Scientists have tested this possibility by comparing primate species where males compete a lot with each other to species where there is very little competition–in other words, where the primates are monogamous. They’ve found that in the more promiscuous species, males have bigger testicles. (We humans have moderately large testicles, suggesting we’ve experienced moderate sperm competition.)
Dines and his colleagues decided to take a similar approach to whales and dolphins. They studied pelvic bones from 29 different species, and compared their dimensions to their mating systems. Some of the species the scientists looked at, like the franciscana dolphin, are monogamous. Other species are more promiscuous. Marine biologists once observed two male Northern Right whales mating with a female at the same time, for example.
A pattern emerged from their analysis–the kind of pattern you’d expect from sexual selection’s fingerprints. The more promiscuous a species was, the bigger its pelvis bones tended to be. The scientists also found that as whales evolved to become more promiscuous, their pelvic bones changed shape. These changes weren’t part of some general change to their skeleton, however. The ribs near the hips didn’t show the same patterns of size and shape change.
Dines and his colleagues can’t say what the change in the shape and size of pelvic bone does to a whale. That would require a level of intimate observation of whale sex that is simply impossible. But they have been able to get some clues by looking at other parts of male whale sexual anatomy. The whales with big hip bones also tended to have big testicles and big penises. This pattern may mean that hip bones are evolving as part of a bigger system. Whales with more competition may be using bigger hip bones to control a longer penis to deliver more sperm to females. (The new study only considers how whale hips may be selected in males. That doesn’t necessarily mean they have no function in female whales, which have hips too. Or perhaps they are the female equivalent of male nipples, carried along for the ride. For now, the subject is too mysterious for scientists to say anything firm about it.)
“Far from being mere relics of a terrestrial past,” Dines and his colleagues conclude, “cetacean pelvic bones are targets of sexual selection.” In fact, the only reason that we can still see these strange vestiges may be that they still matter to evolution, and in the most intimate way imaginable.
[Update: Added sentences about hips in females.]
September 4, 2014
Coffee: Millions of Years of Poison and Brain Manipulation
Many people think of coffee simply as an absolute necessity in the morning. But it’s also a fascinating piece of natural history. Here we have a plant that produces a potent chemical–caffeine–that can snap our brains to attention in low doses and kill us in big doses. Why on Earth would some Ethiopian bean go to such great lengths? For my Matter column this week in the New York Times, I take a look at a new study that offers some answers.
The study is, in fact, the sequencing of the coffee genome. Normally, I’m very leery of genome papers–I come down with a disorder I’ve dubbed YAGS. But sometimes the scientists who sequence a genome also discover some interesting things in it. Such is the case with coffee: the scientists were able to test some hypotheses for how caffeine evolved. The scenario they propose is an elegant combination of gene duplication and convergence. And it can help scientists to figure out exactly what kind of benefits caffeine offers plants that could fuel its evolution not just in coffee, but in other species like tea and cacao as well. Check it out.
August 31, 2014
Catching up: A Hundred Years Without Passenger Pigeons, and the Secrets of the Puppet Masters
If you’re looking for something to read this weekend, here are a couple pieces I’ve written in the past few days:
1. Epigenetics are cool. Mind-controlling parasites are cool. Epigenetics+mind-controlling parasites=Very cool. That equation is the subject of my latest column for the New York Times.
2. Tomorrow marks the 100th anniversary of the extinction of the passenger pigeon. In honor of that event, I’ve written a piece for National Geographic News about why its demise still means so much to scientists a century later.
For more on the passenger pigeon, check out this previous post from the Loom, as well as this feature I wrote last year for National Geographic. A number of other writers are also marking tomorrow’s anniversary–for example, Elizabeth Kolbert at the New Yorker, David Biello at Scientific American, and John Fitzpatrick in the Sunday Review section of the New York Times.
August 27, 2014
Evolution’s Baby Steps
If you explore our genealogy back beyond about 370 million years ago, it gets fishy. Our ancestors back then were aquatic vertebrates that breathed through gills and swam with fins. Over the next twenty million years or so, our fishy ancestors were transformed into land-walking animals known as tetrapods (Latin for “four feet”).
The hardest evidence–both literally and figuratively–that we have for this transition comes from the fossil record. Over the past century, paleontologists have slowly but steadily unearthed species belong to our lineage, splitting off early in the evolution of the tetrapod body. As a result, we can see the skeletons of fish with some–but not all–of the traits that let tetrapods move around on land. (I wrote about the history of this search in my book At the Water’s Edge; for more information, I’d suggest Your Inner Fish, by Neil Shubin, who discovered Tiktaalik, one of the most important fossils on the tetrapod lineage.)

Updated reconstruction of Tiktaalik. Image courtesy of John Westlund, University of Chicago.
You can get a feeling for how fish became tetrapods by looking at a fossil like Tiktaalik, shown here. These days, a lot of scientists are turning up clues about how a fish turned into this kind of creature, and how this kind of creature turned into creatures like us.
Along the way, a lot of genes changed. The genes in the egg of a fish encode the molecules that will produce the fins, gills, and all the rest of a fish’s body. A different set of genes will produce a tetrapod. These days, scientists are finding some of the mutations that reprogrammed fins into feet.
But a new study in Nature puts a fascinating new wrinkle on our origins story. It suggests that our fishy ancestors already had the potential to develop the beginnings of a tetrapod body. They just needed some time on land to bring it out.
The authors of the new study, three scientists at McGill University in Montreal, studied fish called bichirs (Polypterus). Bichirs are the living remnants of a very old lineage of fishes, which split off from other fish lineages some 400 million years ago. While they mostly live in lakes and rivers, they will sometimes crawl across dry land with their fins. They can even sustain themselves on these journeys by breathing through primitive lungs. Here’s a video of how they walk.
The McGill researchers saw some intriguing parallels between bichirs and early tetrapod relatives like Tiktaalik. Bichirs use their front pair of fins to lift their head and the front end of their trunk off the ground. They then push the back end of their trunk in order to propel themselves forward. Tiktaalik may have moved in a similar fashion.

Credit: Antoine Morin
But bichirs spend relatively little time on land. The McGill scientists wondered what would happen if they forced the fish to grow up out of the water. To find out, they reared eight bichirs in a terrarium with a pebble-strewn floor. To prevent the bichirs from drying out, the scientists installed a mister to keep their skin moist. The fish grew for eight months, clambering around their terrarium instead of swimming.
Then the scientists examined these fish out of water. They found that eight months on dry land (or at least moist land) had wreaked profound changes to the bichirs.
For one thing, they now walked differently. Overall, they were more efficient. In each step, they planted their fins on the ground for less time, and they took shorter strides. Instead of flapping their fins out to each side, they placed their fins under their bodies. Their fins slipped less when they pushed off of them. They made smaller movements with their tails to go the same distance as a bichir raised underwater. Aquatic bichirs walk on land with an irregular gait. The terrestrial bichirs, on the other hand, walked more gracefully, planting their fins in the same spot relative to their bodies time after time.
The bichirs probably developed this new walking style in large part through learning. Growing up on land, they had more opportunity to test out their moves and to perfect the best ones. But it wasn’t just their brains that changed on land. Their bodies changed, too.
The McGill scientists examined the bones of the terrestrial bichirs and compared them to normal aquatic ones. They found striking differences in the bones, especially in the shoulder region. Some of the bones had become less tightly connected to each other, giving the bichirs more room to swing their fins as they walked. By contrast, their bones corresponding to our collarbones became bigger and more strongly braced, letting the animals resist gravity and lift their bodies higher.
It was the experience of walking that changed the fish bones. The forces exerted on them changed how the bone cells grew, leading them to take on new shapes. What’s especially intriguing about these changes is that they’re a lot like the changes paleontologists have documented in tetrapod fossils.
Over millions of years, our fishy ancestors evolved looser connections between some shoulder bones, enabling their legs to sweep bigger motions and also starting to separate the head from the neck. They also evolved stronger support systems for their trunk so that they could lift themselves out of the muck. It’s as if the bichirs are replaying evolution in their own lifetime.
This is not the first time that biologists have found tantalizing parallels between the experiences of individual animals and long-term evolution. The environment in which animals grow up can steer the development of their bodies, and then evolution can follow suit.
In 2008, for example, scientists raised stickleback fish on two different diets. One group of fish ate bloodworms squirming around at the bottom of their tanks. The other fish ate shrimp scooting around in the open water. The bloodworm-eating fish had to clamp down on the blood worms to eat them, while the shrimp-eating ones just needed to sneak up on their prey and swallow them with a quick slurp.
The result of these different movements was different heads: the bloodworm-feeders had short, wide mouths, and the shrimp-feeders had long, narrow ones.

Wund et al, American Naturalist 2008 http://www.jstor.org/stable/10.1086/5...
These sticklebacks, which are abundant in the ocean, have repeatedly colonized lakes. As they’ve adapted to their new freshwater home, they’ve evolved over and over again into two distinct populations. Some of them scrounge on the lake bottoms for prey, while the others zip around the open water. And time and again, they’ve evolved wide short mouths, or narrow long ones. Evolution has followed the path of experience.
The ability that animals and plants have to develop differently in different conditions is known as plasticity. It’s possible that plasticity opens the door to new paths that evolution can then take. When organisms find themselves in a new environment, they develop in a way that helps them cope with their new surroundings. Their descendants may acquire mutations that encode that anatomy in their genes. Eventually evolution takes them beyond where plasticity alone could take them.
This kind of experience-led evolution, known as genetic assimilation, might have helped take our ancestors out of the water. The forerunners of tetrapods might have been forced to scoot over dry land more than their ancestors–to flee predators in the water perhaps, perhaps to mate, perhaps to get to other streams and ponds. Their underwater genomes gave them the plasticity to grow into halfway decent walkers, like bichirs do today. And then subsequent mutations gave them an improved anatomy. They didn’t have to grow into walking: their genes had now taken over the job, and our ancestors were ready to walk on.
August 15, 2014
Microbes as Poisoners and Puppet Masters
I’ve been on something of a microbial jag recently. For my past two columns for the New York Times I’ve explored the creepy biochemical sophistication of bacteria.
First, I took a look at the outbreak of toxic bacteria that shut down Toledo’s water supply a couple weeks ago. A lot of people don’t realize it, but those microbes have been spewing out these toxins for about three billion years–for reasons that scientists are still trying to figure out.
Then I wrote about the chemicals that our own microbiome releases, and the ways they can affect our behavior. Some scientists don’t think those changes are just random side effects. Instead, our microbes may be trying to manipulate us for their own benefit, eating certain foods or getting close to other people (also known as hosts).
Check them out!
August 14, 2014
Taking the Yuck Out of Microbiome Medicine
I can still remember the shock I felt when I heard about fecal microbiota transplants for the first time. It is not the sort of thing you forget.
At a microbiology conference, a scientist was giving a lecture about the microbiome–the microbes that live harmlessly inside of us. She described one unusual case she was involved in where a doctor named Alexander Khoruts used the microbiome to save a patient’s life. The patient had taken antibiotics for a lung infection. While the drugs cleared that infection, they also disrupted the ecology of her gut, allowing a life-threatening species of bacteria called Clostridium difficile to take over. The pathogen was causing horrific levels of diarrhea. Khoruts couldn’t stop it, because it was resistant to every antibiotic he tried.
So Khoruts decided to use an obscure method: the fecal transplant. He took some stool from the patient’s husband, mixed it with water, and delivered it to her large intestines like a suppository. In a matter of days she was recovering.
Since I first heard about these transplants in 2010, they’ve hit the big time. Last year, a team of Danish and Finnish doctors reported clinical trials in which the transplants 94 percent effective against C. difficile. It appears that some species in the transplant from a healthy gut will grow quickly and outcompete the pathogen, returning a sick person’s intestines to its former state. Scientists have been exploring using fecal transplants for other disorders of the gut, along with conditions beyond the gut, such as diabetes and obesity.
But there are many obstacles left to putting fecal transplants into widespread practice. For one thing, the FDA is very cautious with this kind of living medicine. For another thing, fecal transplants are conceptually crude. Doctors simply give a patient a random sample of hundreds of different species from a healthy person’s gut, assuming that at least some of them will restore the patient to health. When the patients get better, they can’t say precisely why.
And then there is the yuck factor. In 20102, scientists conducting a survey about attitudes towards feccal transplants, politely summed up the problem this way: “patients recognize the inherently unappealing nature of FMT.”
But now there’s a potentially promising development in the quest to harness the microbiome. At an American Gastroenterological Association conference in Chicago this weekend, researchers will be describing how they cured C. difficile not with a fecal transplant, but with a pill full of bacterial spores.
The pill is the work of a small Boston-area company called Seres Health. They came up with a combination of certain harmless microbe species that naturally live in our gut. These species all form spores, which are rugged enough to survive inside a pill. Once they reach the warm refuge of the gut, they pop out of their spores and multiply. In previous studies, Seres researchers showed they could treat C. difficile infection effectively in mice and hamsters. (Technology Review described the company’s efforts in this article from last December.)
Recently, doctors at the Mayo Clinic, the Miriam Hospital in Providence, and Massachusetts General Hospital ran a clinical trial on people to see if the pills from Seres were safe and effective. They gave the pill to fifteen people. The results were striking: the overall cure rate was 100 percent. (The detailed abstract pdf is here.)
I contacted Khoruts to see what he thought of the study. “It looks very promising,” he told me.
But Khoruts also raised a few caveats. He pointed out that the authors excluded very sick patients from the study because of the risk of adverse events. So the 100 percent cure rate might be higher than it would be in the real world.
Khoruts also pointed out a few potential problems with taking a pill full of spores as opposed to getting stool from a donor. Scaling it up to industrial production will require making sure that the factory stocks don’t get contaminated by strains of bacteria that would harm patients, for example.
In those factory stocks, Khoruts pointed out, the microbes will continue to evolve and adapt to their surroundings. If they become too well adapted to life in a factory, they may not do as well inside of people’s bodies.
Nor does the initial report on these pills actually explain how these particular species are conquering C. difficile. I’m sure that the fifteen people who were cured of these awful bugs aren’t clamoring for a detailed mechanistic explanation of what happened when they swallowed the pills.
But if scientists are going to rationally design microbiome treatments for a lot of different conditions, they’re going to have to open this microbial black box.



