Carl Zimmer's Blog, page 12
June 3, 2014
Talking With Sam Kean This Evening

From samkean.com
Sam Kean, the author of a couple delightful books about science (The Disappearing Spoon and The Violinist’s Thumb), has a third book out now on one of my favorite topics, the brain. In The Tale of the Dueling Neurosurgeons, Kean finds stories about kings, assassins, and other interesting people that illuminate how scientists have come to understand how the brain works.This evening at 7 pm ET, I’ll be talking to Sam about the book on Booktalk Nation, an online show. You can register to see the conversation here.
May 29, 2014
From Womb to Womb
Worldwide, women suffer an estimated 2.65 million stillbirths each year. Despite those huge numbers, we only understand some of the factors are responsible. In low- and middle-income countries (where most of the world’s stillbirths occur), diseases like malaria can put pregnant women at risk of stillbirths. In wealthier countries, the biggest risks include smoking and obesity. But these factors only go partway to explaining why some women have stillbirths, leaving many cases unaccounted for. The benefits that would come from that knowledge could be enormous.
One way to learn about reproductive health is to observe how our primate cousins have babies. And a new study on marmosets offers some hints about the causes of stillbirth. It suggests that a mother’s health during pregnant may not be the whole story. In fact, some of the risk factors may arise before mothers are even born.

Marmoset triplets. Photo courtesy Julienne Rutherford
The first thing that one notices about the white-tufted ear marmoset (Callithrix jacchus) is its wildly adorable face–a tiny visage framed by shocks of white fur. Marmosets are interesting to scientists not because they’re cute, but because of their intriguing way of having kids. While most primate females have a single offspring at a time, marmoset typically have twins. Some marmoset mothers even have triplets.
This is a tricky strategy for passing on marmoset genes. Marmoset babies can weigh between a fifth and a quarter of their mother’s weight. Imagine a 135-pound woman giving birth to two 16 pound babies–and then nursing them. The strategy only works because marmosets live in groups. A breeding female and male marmoset are attended to by helpers–usually related females that suppress their own ability to have babies while they assist the breeding female. They take turns carrying the babies and getting food. The father even helps out, too.
Despite all the help, however, female marmosets sometimes have stillbirths. Recently, Julienne Rutherford, a biological anthropologist at in Department of Women, Children, and Family Health Science at the University of Illinois at Chicago, went on a search for the factors that put a marmoset at greatest risk of having one.
She and her colleagues studied a marmoset colony at the Southwest National Primate Research Center in San Antonio, Texas. The center keeps detailed records for all the marmosets, from birth to death. Rutherford and her colleagues analyzed the reproductive history of 79 female marmosets since 1994. And when they were done with the analysis, one factor in particular jumped out of the data. Females that were born in sets of triplets are three times more likely to lose a fetus than females born as twins.
The scientists looked at the other data to figure out what was happening. The risk of stillbirth wasn’t just part of an overall problem with fertility. Triplet females were just as likely to get pregnant as twin females. It’s just that they were less likely to carry their pregnancies to a successful term.
Perhaps the result was just a shadow of a much bigger pattern. Scientists have long known that women who were low weight at birth end up at greater risk of stillbirths when they get pregnant. The same goes for other female primates. It was therefore possible that triplet female marmosets were at greater risk of stillbirths simply because triplet marmosets are smaller at birth than twins. Rutherford and her colleagues looked over the records to see if that was the case.
It wasn’t. Triplet females are born at a range of weights, and extra size offer them no protection against stillbirths. The big triplet females are also at risk of having stillbirths when they grow up.
Something must be happening in the marmoset womb that is leaving an invisible mark on triplet females for their entire life. As a female primate embryo develops, it grows the ovaries and uterus it will eventually use to bear its own young. The development of those organs is normally choreographed by the hormones that swirl around the embryo’s body. Sharing a uterus with two other embryos may disrupt that choreography. Most triplet females are born along with at least one brother, for example. It’s possible that the male hormones produced by their brothers interfered with their own development.
Since women typically only have one baby at a time, there isn’t a simple lesson in Rutherford’s research for medicine. But it may encourage scientists to to widen their search for the cause of stillbirths. Yes, the health of a woman while she’s pregnant is enormously important to a successful pregnancy. But her reproductive health may be altered before she’s even born.
Scientists have done very little research on this possibility in humans. One of the few studies looked at the legacy of the so-called “Dutch Hunger.” In the winter of 1944/45, the Netherlands suffered a famine. Many women who were pregnant at the time suffered from malnutrition. Scientists have followed their children ever since to see what effect the famine had on them before they were born. In 1997, researchers found that the women were just as fertile as women from well-fed mothers. But their children were at greater risk of stillbirth or of dying just after birth [pdf].
Given how long people live, tracing the effects of pregnancy on stillbirths is going to be slow work. Female marmosets, on the other hand, can start having babies before they’re two years old. Rutherford and her colleagues are taking advantage of the fast life of marmosets by following a number of females from birth to first pregnancy. The scientists are using ultrasound to take pictures of the marmosets’ developing reproductive systems, and measuring their hormone levels along the way.
This new research may allow Rutherford to pinpoint the reason that it’s so risky to be a triplet mother. And it may let her offer some ideas about how to make human childbirth healthier, too.
May 27, 2014
Brains, brawn, and human nature
The food you ate today for breakfast has been transformed. Your body has used some of it to generate energy and some to build new tissues. Your body controls your metabolism in a marvelously sophisticated way, channeling resources to each organ to keep it functioning. In my new “Matter” column for the New York Times, I look at how our metabolism evolved. It turns out that our brains and our muscles have an odd kind of metabolism compared to other mammals. Did we lose muscular strength to fuel a big brain? Or did we switch our muscles to a different kind of metabolism, which let our brains burn brighter? The answer’s not clear yet, but the research is pretty cool. Check it out.
May 21, 2014
Another Kind of Brain
In December, I blogged about an animal most people have never heard of–the comb jelly. It’s a gorgeous, mysterious creature that just might belong to the oldest lineage of animals alive today. Today, over at National Geographic News, I’m reporting on a new study of the comb jelly that suggests it’s even more interesting than that. Unlike all other animals with a nervous system, it seems to have evolved nerves and a brain all its own. It even has its own special neurochemical language. If true, it’s about as close to an alien intelligence that we can encounter here on Earth. Check it out.
May 20, 2014
Introducing Carl’s Banned-Word Scanner
I’ve taught writing semi-regularly over the past few years. Over that time, I’ve come to realize that one of the biggest challenges in learning how to write about the natural world is to learn how to skillfully wield beautiful, plain language . Scientists and scientists-in-training often lard their writing with jargon, rather than looking for a conversational equivalent. This addiction to jargon can leave a piece of writing sterile. It can mystify everyone except the experts–which is a bad strategy if you aspire to write for the public. An addiction to jargon can even create catastrophic misunderstandings. Readers may apply a non-technical definition for a word that a scientist uses with a very technical meaning in mind. (Think of “theory” as a hunch.)
All writers, scientist and non-scientist alike, can be tempted by clichés and other useless constructions. Clichés like “Holy Grail” are just lazy surrenders to the challenge of inventing fresh phrases. And “miracle cure” is really just a cynical promise of false hope.
So I’ve gotten very persnickety about the individual words and phrases that students choose. I’ve built up a list of “banned words” that I’ve come across in assignments and which I never want to see in class again. It’s not that those words are absolutely wrong in terms of their meaning. It’s just that writers–both new and veteran–should try to do better. My index of banned words was a pretty modest enterprise–just a blog post that I updated from time to time (either from assignments or from suggestions from weary readers). But recently I got a chance to turn it into an interesting experiment.
The opportunity came to me thanks to Charles Best. Best is a former public school teacher who founded the philanthropy site Donors Choose, where you can give money for supplies requested by public school teachers. Wearied of dealing with tired, redundant, or pretentious language in writing, he decided to launch a web site called Irregardless. It allows people to crowd-source a list of words and phrases that writers should avoid, explain why, and offer alternatives.
What’s interesting about this site is that you can use it in a number of interesting ways. You can just read through the entries. You can pick out a list made by someone in particular. Author Reza Aslan explains why he loathes “essentializing the sacred,” for example.
You can also check your own writing. Choose the “check your writing” box, and paste text into the field that appears. You can choose to run your writing by all the tips, or just use a style guide. Best asked me to set up a science writing guide, and so I’ve poached my banned words, along with other good sources (like this paper). If you’re interested, check out Carl Zimmer’s Science Writing Guide at http://irregardless.ly/carlzimmer
You’ll see any flagged words highlighted in your text, with comments and suggestions appearing next to them. It can even distinguish between different uses of a word (I dislike “access” as a verb.)
Both Irregardless and my own style guide are works in progress. If you find any bugs, let the owners of the site know. And remember that you can add your own tips too. If you think I need to add a particular word to my own guide, let me know (this blog post’s comment thread is a good place). I can’t promise I’ll dislike it too, but it’s always worth learning about a word that sets someone on edge.
May 15, 2014
Why Are Our Marshes Dying?
Marshes are dying off, and scientists are trying to figure out what’s killing them. In my new “Matter” column for the New York Times, I look at a new experiment that points to a surprising culprit. Check it out.
May 14, 2014
Attack of the Monster Turtles
My family and I were trapped once in our house by a terrorizing turtle. Last week, I told the saga of that day–and of my lifelong obsession with strange animals–at Story Collider, an evening of live story-telling about science. The recording is now online, and so you can listen to it here. May you have many peaceful encounters with turtles in your life.
May 13, 2014
Seeing The Branches for the Tree
There is a scientific picture waiting to be drawn. Someone has to do artistic justice to the evolutionary tree of life.
Back in 1837, Charles Darwin sketched out a tree of life in a notebook as a way to visualize his idea that different species share a common ancestor. In the generations since he published The Origin of Species, biologists have tried to draw trees that distill the actual relationships between living things.
As I wrote in 2012, the discovery of molecular biology gave scientists a better telescope for looking back through evolutionary history at the branches of the tree of life. Our DNA is an historical archive, storing a wealth of information about our kinship with the rest of life. In the 1970s, the biologist Carl Woese attempted the first sketch of the tree of life–a tree including the biggest groups of species. Woese argued that life consisted of three great branches–what he called domains. Those domains were typically referred to as bacteria, archaea, and eukaryotes–the last being our own.

Three-domain tree. Wikipedia. High-resolution version here: http://bit.ly/threedomains
This picture is straightforward and bracing. Straightforward, because you can see its overall structure clearly. Bracing, because you can see your place in it. The length of the branches corresponds roughly to evolutionary distance. Humans and oak trees share the same tuft. For the most part, life’s diversity is microbial.
But this picture now appears to be wrong. A number of studies now suggest that the tree of life does not have three domains. Eukaryotes evolved from a lineage of archaea, which merged with a species of bacteria. In other words, we descend from a colossal hybridization. I blogged about some of this research in 2012, and this February, Ed Yong published a fantastic longer piece in Nautilus that included more recent research.
Today, three biologist offered an updated look at the evidence in Nature Reviews Microbiology. James McInerney, Mary O’Connell and Davide Pisani argue that the evidence in favor of the three-domain tree has been steadily diminishing, while the evidence for a merger has been gaining strength.
Maybe I have an overactive visual cortex, but when I read things like this, I think to myself, “What should I be seeing?” And perhaps I’m even more primed to ask that question because I’ve written textbooks about evolution, where pictures are invaluable for conveying the gist of complicated concepts. So I was intrigued that McInerney and his colleagues used this picture to illustrate their piece. (A bigger version is here. That “chloroplast origin” is a wonderful tale in itself: how the algae that gave rise to plants gained the ability to capture sunlight for energy. For the full story, read Eating the Sun.)
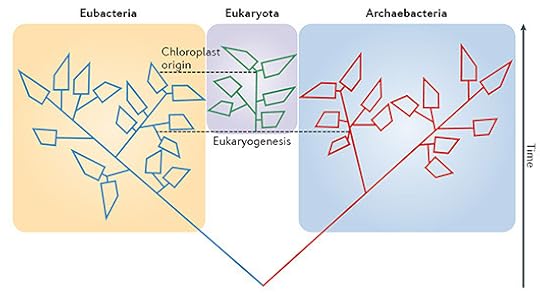
McInerney et al Nat Rev Microbiology 2014
It’s a beautiful picture, but…well, I’m not sure it quite works. It still has a three-ness to it, despite the fact that McInerney and his colleagues call on us to ditch the whole concept of three domains. Part of the problem may be that Darwin was only half right when he championed the tree of life as a metaphor for evolution. Mathematically speaking, a tree is a graph made of splitting lines. But the full story of evolution appears to be a graph that doesn’t just split, but also joins together into rings and other shapes (see this paper [pdf] for details). It becomes harder to slice life up into neat groups when it keeps joining together.
I’ll probably use this figure in the next edition of my textbook, but I hope some visionary artist comes up with a new way to look at life.
May 9, 2014
The Case for Junk DNA
Genomes are like books of life. But until recently, their covers were locked. Finally we can now open the books and page through them. But we only have a modest understanding of what we’re actually seeing. We are still not sure how much our genome encodes information that is important to our survival, and how much is just garbled padding.
Today is a good day to dip into the debate over what the genome is made of, thanks to the publication of an interesting commentary from Alex Palazzo and Ryan Gregory in PLOS Genetics. It’s called “The Case for Junk DNA.”
The debate over the genome can get dizzying. I find the best antidote to the vertigo is a little history. This history starts in the early 1900s.
At the time, geneticists knew that we carry genes–factors passed down from parents to offspring that influence our bodies–but they didn’t know what genes were made of.
That changed starting in the 1950s. Scientists recognized that genes were made of DNA, and then figured out how the genes shape our biology.
Our DNA is a string of units called bases. Our cells read the bases in a stretch of DNA–a gene–and build a molecule called RNA with a corresponding sequence. The cells then use the RNA as a guide to build a protein. Our bodies contain many different proteins, which give them structure and carry out jobs like digesting food.
But in the 1950s, scientists also began to discover bits of DNA outside the protein-coding regions that were important too. These so-called regulatory elements acted as switches for protein-coding genes. A protein latching onto one of those switches could prompt a cell to make lots of proteins from a given gene. Or it could shut down the gene completely.
Meanwhile, scientists were also finding pieces of DNA in the genome that appeared to be neither protein-coding genes nor regulatory elements. In the 1960s, for example, Roy Britten and David Kohne found hundreds of thousands of repeating segments of DNA, each of which turned out to be just a few hundred bases long. Many of these repeating sequences were the product of virus-like stretches of DNA. These pieces of “selfish DNA” made copies of themselves that were inserted back in the genome. Mutations then reduced them into inert fragments.
Other scientists found extra copies of genes that had mutations preventing them from making proteins–what came to be known as pseudogenes.
The human genome, we now know, contains about 20,000 protein-coding genes. That may sound like a lot of genetic material. But it only makes up about 2 percent of the genome. Some plants are even more extreme. While we have about 3.2 billion bases in our genomes, onions have 16 billion, mostly consisting of repeating sequences and virus-like DNA.
The rest of the genome became a mysterious wilderness for geneticists. They would go on expeditions to map the non-coding regions and try to figure out what they were made of.
Some segments of DNA turned out to have functions, even if they didn’t encode proteins or served as switches. For example, sometimes our cells make RNA molecules that don’t simply serve as templates for proteins. Instead, they have jobs of their own, such as sensing chemicals in the cell. So those stretches of DNA are considered genes, too–just not protein-coding genes.
With the exploration of the genome can a bloom of labels, some of which came to be used in confusing–and sometimes careless–ways. “Non-coding DNA” came to be a shorthand for DNA that didn’t encode proteins. But non-coding DNA could still have a function, such as switching off genes or producing useful RNA molecules.
Scientists also started referring to “junk DNA.” Different scientists used the term to refer to different things. The Japanese geneticist Susumu Ohno used the term when developing a theory for how DNA mutates. Ohno envisioned protein-coding genes being accidentally duplicated. Later, mutations would hit the new copies of those genes. In a few cases, the mutations would give the new gene copies a new function. In most, however, they just killed the gene. He referred to the extra useless copies of genes as junk DNA. Other people used the term to refer broadly to any piece of DNA that didn’t have a function.
And then–like crossing the streams in Ghostbusters–junk DNA and non-coding DNA got mixed up. Sometimes scientists discovered a stretch of non-coding DNA that had a function. They might clip out the segment from the DNA in an egg and find it couldn’t develop properly. BAM!–there was a press release declaring that non-coding DNA had long been dismissed as junk, but lo and behold, non-coding DNA can do something after all.
Given that regulatory elements were discovered in the 1950s (the discovery was recognized with Nobel Prizes), this is just illogical.
Nevertheless, a worthwhile questioned remained: how of the genome had a function? How much was junk?
To Britten and Kohne, the idea that repeating DNA was useless was “repugnant.” Seemingly on aesthetic grounds, they preferred the idea that it had a function that hadn’t been discovered yet.
Others, however, argued that repeating DNA (and pseudogenes and so on) were just junk–vast vestiges of disabled genetic material that we carry down through the generations. If the genome was mostly functional, then it was hard to see why it takes five times more functional DNA to make an onion than a human–or to explain the huge range of genome sizes:
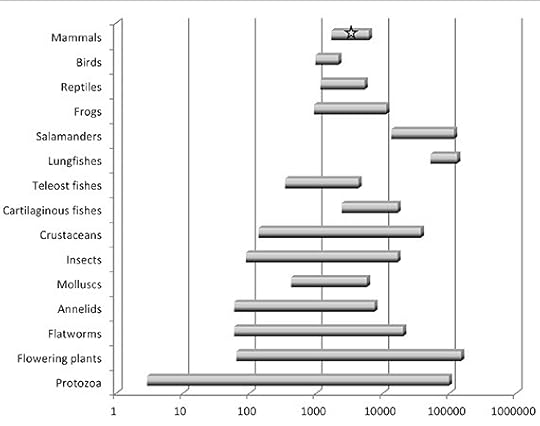
From Palazzo and Gregory, PLOS Genetics 2014. Size of genome is in thousands of bases. The star marks humans
In recent years, a consortium of scientists carried out a project called the Encyclopedia of DNA Elements (ENCODE for short) to classify all the parts of the genome. To see if non-coding DNA was functional, they checked for proteins that were attached to them–possibly switching on regulatory elements. They found a lot of them.
“These data enabled us to assign biochemical functions for 80% of the genome, in particular outside of the well-studied protein-coding regions,” they reported.
Science translated that conclusion into a headline, “ENCODE Project writes eulogy for junk DNA.”
A lot of defenders of junk have attacked this conclusion–or, to be more specific, how the research got translated into press releases and then into news articles. In their new review, Palazzo and Gregory present some of the main objections.
Just because proteins grab onto a piece of DNA, for example, doesn’t actually mean that there’s a gene nearby that is going to make something useful. It could just happen to have the right sequence to make the proteins stick to it.
And even if a segment of DNA does give rise to RNA, that RNA may not have a function. The cell may accidentally make RNA molecules, which they then chop up.
If I had to guess why Britten and Kohne found junk DNA repugnant, it probably had to do with evolution. Darwin, after all, had shown how natural selection can transform a population, and how, over millions of years, it could produce adaptations. In the 1900s, geneticists turned his idea into a modern theory. Genes that boosted reproduction could become more common, while ones that didn’t could be eliminated from a population. You’d expect that natural selection would have left the genome mostly full of functional stuff.
Palazzo and Gregory, on the other hand, argue that evolution should produce junk. The reason has to do with the fact that natural selection can be quite weak in some situations. The smaller a population gets, the less effective natural selection is at favoring beneficial mutations. In small populations, a mutation can spread even if it’s not beneficial. And compared to bacteria, the population of humans is very small. (Technically speaking, it’s the “effective population size” that’s small–follow the link for an explanation of the difference.) When non-functional DNA builds up in our genome, it’s harder for natural selection to strip it out than if we were bacteria.
While junk is expected, a junk-free genome is not. Palazzo and Gregory based this claim on a concept with an awesome name: mutational meltdown.
Here’s how it works. A population of, say, frogs is reproducing. Every time they produce a new tadpole, that tadpole gains a certain number of mutations. A few of those mutations may be beneficial. The rest will be neutral or harmful. If harmful mutations emerge at a rate that’s too fast for natural selection to weed them out, they’ll start to pile up in the genome. Overall, the population will get sicker, producing fewer offspring. Eventually the mutations will drive the whole population to extinction.
Mutational meltdown puts an upper limit on how many genes an organism can have. If a frog has 10,000 genes, those are 10,000 potential targets for a harmful mutation. If the frog has 100,000 genes, it has ten times more targets.
Estimates of the human mutation rate suggest that somewhere between 70 to 150 new mutations strike the genome of every baby. Based on the risk of mutational meltdown, Palazzo and Gregory estimate that only ten percent of the human genome can be functional.* The other ninety percent must be junk DNA. If a mutation alters junk DNA, it doesn’t do any harm because the junk isn’t doing us any good to begin with. If our genome was 80 percent functional–the figure batted around when the ENCODE project results first came out–then we should be extinct.
It may sound wishy-washy for me to say this, but the junk DNA debates will probably settle somewhere in between the two extremes. Is the entire genome functional? No. Is everything aside from protein-coding genes junk? No–we’ve already known that non-coding DNA can be functional for over 50 years. Even if “only” ten percent of the genome turns out to be functional, that’s a huge collection of DNA. It’s six times bigger than the DNA found in all our protein-coding genes. There could be thousands of RNA molecules scientists have yet to understand.
Even if ninety percent of the genome does prove to be junk, that doesn’t mean the junk is unimportant to our evolution. As I wrote last week in the New York Times, it’s from these non-coding regions that many new protein-coding genes evolve. What’s more, much of our genome is made up of viruses, and every now and then we have harnessed those viral genes to carry out a job for our own bodies. The junk is a part of us, and it, too, helps to make us what we are.
*I mean functional in terms of its sequence. The DNA might still do something important structurally–helping the molecule bend in a particular way, for example.
May 8, 2014
Our Planet Seethes With Antibiotic Resistance–And Has For A Long Time
We’re in a medical crisis, as bacteria that can resist antibiotics become more common. But, as I write in my new New York Times column, antibiotic resistance isn’t just limited to the doctor’s office. Bacteria all over the world have genes that make them resistant to our best drugs–bacteria living in caves, in ice, in the ocean floor, and in the dirt outside your door. And they probably had those genes long before doctors started using antibiotics.
Scientists have long considered the rise of antibiotic resistance one of the most striking examples of evolution in our own time. Our growing appreciation for antibiotic resistance beyond disease-causing bacteria doesn’t change that. After all, scientists can observe antibiotic resistance evolve from scratch in laboratory experiments. What the new research does is give us a richer, planet-wide glimpse of that evolutionary process.



