Carl Zimmer's Blog, page 20
October 24, 2013
Naming Our Ancestors: My New Column for the New York Times
Last week, scientists published a study of five 1.8 million-year-old hominid fossils. They may reveal profound lessons about a crucial chapter in our evolution: how our ancestors changed from bipedal apes to a more human-like lineage–in other words, the emergence of our genus, Homo. So what name do we give these skulls? What species do they belong to? It’s no simple matter naming our hominid ancestors, and that difficulty tells us something intriguing about their biology. And that’s the subject of my “Matter” column this week in the New York Times.
October 23, 2013
How Many Cells Are In Your Body?
A simple question deserves a simple answer. How many cells are in your body?
Unfortunately, your cells can’t fill out census forms, so they can’t tell you themselves. And while it’s easy enough to look through a microscope and count off certain types of cells, this method isn’t practical either. Some types of cells are easy to spot, while others–such as tangled neurons–weave themselves up into obscurity. Even if you could count ten cells each second, it would take you tens of thousands of years to finish counting. Plus, there would be certain logistical problems you’d encounter along the way to counting all the cells in your body–for example, chopping your own body up into tiny patches for microscopic viewing.
For now, the best we can hope for is a study published recenty in Annals of Human Biology, entitled, with admirable clarity, “An Estimation of the Number of Cells in the Human Body.”
The authors–a team of scientists from Italy, Greece, and Spain–admit that they’re hardly the first people to tackle this question. They looked back over scientific journals and books from the past couple centuries and found many estimates. But those estimates sprawled over a huge range, from 5 billion to 200 million trillion cells. And practically none of scientists who offered those numbers provided an explanation for how they came up with them. Clearly, this is a subject ripe for research.
If scientists can’t count all the cells in a human body, how can they estimate it? The mean weight of a cell is 1 nanogram. For an adult man weighing 70 kilograms, simple arithmetic would lead us to conclude that that man has 70 trillion cells.
On the other hand, it’s also possible to do this calculation based on the volume of cells. The mean volume of a mammal cell is estimated to be 4 billionths of a cubic centimeter. (To get a sense of that size, check out The Scale of the Universe.) Based on an adult man’s typical volume, you might conclude that the human body contains 15 trillion cells.
So if you pick volume or weight, you get drastically different numbers. Making matters worse, our bodies are not packed with cells in a uniform way, like a jar full of jellybeans. Cells come in different sizes, and they grow in different densities. Look at a beaker of blood, for example, and you’ll find that the red blood cells are packed tight. If you used their density to estimate the cells in a human body, you’d come to a staggering 724 trillion cells. Skin cells, on the other hand, are so sparse that they’d give you a paltry estimate of 35 billion cells.
So the author of the new paper set out to estimate the number of cells in the body the hard way, breaking it down by organs and cell types. (They didn’t try counting up all the microbes that also call our body home, sticking only to human cells.) They’ve scoured the scientific literature for details on the volume and density of cells in gallbladders, knee joints, intestines, bone marrow, and many other tissues. They then came up with estimates for the total number of each kind of cell. They estimate, for example, that we have 50 billion fat cells and 2 billion heart muscle cells.
Adding up all their numbers, the scientists came up with…drumroll…37.2 trillion cells.
This is not a final number, but it’s a very good start. While it’s true that people may vary in size–and thus vary in their number of cells–adult humans don’t vary by orders of magnitude except in the movies. The scientists declare with great confidence that the common estimate of a trillion cells in the human body is wrong. But they see their estimate as an opportunity for a collaboration–perhaps through an online database assembled by many experts on many different body parts–to zero in on a better estimate.
Curiosity is justification enough to ponder how many cells the human body contains, but there can also be scientific benefits to pinning down the number too. Scientists are learning about the human body by building sophisticated computer models of lungs and hearts and other organs. If these models have ten times too many cells as real organs do, their results may veer wildly off the mark.
The number of cells in an organ also has bearing on some medical conditions. The authors of the new study find that a healthy liver has 240 billion cells in it, for example, but some studies on cirrhosis have found the disease organ have as few as 172 billion.
Perhaps most importantly, the very fact that some 34 trillion cells can cooperate for decades, giving rise to a single human body instead of a chaotic war of selfish microbes, is amazing. The evolution of even a basic level of multicellularity is remarkable enough. But our ancestors went way beyond a simple sponge-like anatomy, evolving a vast collective made of many different types. To understand that collective on a deep level, we need to know how big it really is.
October 21, 2013
Genomes From Birth: My New Piece for Slate
Genetic testing for individual disorders was once the brave new world, but now it’s a familiar routine of having a baby. But what about looking over a baby’s entire genome? As the price for sequencing DNA crashes, it’s becoming a real possibility. At Slate, I write a new study in which the genomes of 240 babies will be sequenced, and researchers will see whether how that avalanche of data affects their medical care. Check it out.
October 18, 2013
Nature’s Double Con
Mimicry is one of the eeriest feats of evolution. An insect doesn’t know what a leaf looks like, and yet some species have evolved to resemble leaves down to the finest details. Their mimicry emerges from the ruthless cycle of evolution. The ancestors of leaf insects produced lots of genetic variation, thanks to mutations and mating. Some of that variation affected how they looked. Birds have been feasting on the insects for millions of years, and their victims have tended to be the easiest ones for them to see against their leafy background. The insects that were harder tended to survive and reproduce. Over time, evolution acted like a sculptor, turning an ordinary insect body into a shape that blended in with the surrounding leaves.
But the mimicry of leaf insects, as cool as it may be, is simple stuff compared to what’s evolved in other species. The leaf insect mimics just one other living thing: a leaf. But there’s another kind of animal that can mimic two different species, and for entirely different effects.
That animal is the cuckoo. Instead of building their own nests, female cuckoos slip into the nests of other birds and lay their own eggs. They’re parasites, although not of the sort we may be familiar with, like a tapeworm that glides into our gut and exploits us from within. Cuckoos exploit the parental care of their hosts.
When a host bird returns after a visit from a cuckoo, it may recognize an alien egg in its brood and knock it out of the nest. But often it won’t. That’s because the cuckoo is very good at mimicking its host species. The shells of their eggs often closely match those of the species they exploit. Unable to tell the difference between the parasitic eggs and their own, the host birds will care for them all.
When the cuckoo chicks are born, they often will throw the other eggs out of the nest. It will even kick out the host’s chicks, to starve to death on the ground. The murderous baby birds then open their mouths like any chick hungry for food. In some cuckoo species, chicks look remarkably like those of their foster parents. They even sing like their hosts. All this mimicry triggers responses in the host birds to feed the cuckoo chicks as if they were their own. Remarkably, the host will keep feeding the cuckoo long after you’d think it should notice something’s wrong. For example:

Reed warbler feeding cuckoo fledgling. Source: PBS Science Now http://www.pbs.org/wgbh/nova/sciencen...
But it turns out that cuckoos mimic a second kind of animal to succeed in their parasitic ways. They don’t just pretend to be their hosts. They also pretend to be their hosts’ enemies.
In many species of cuckoos, the adult birds develop stark stripes on the feathers covering their belly. The barred pattern looks remarkably like that of hawks and other raptors.

African harrier hawk. Photo copyright Gabriel A. Jamie.
Why should cuckoos look like hawks–and why is the similarity most striking on their belly? Consider the fact that in order for a baby cuckoo to fool its foster parent, its mother first has to get into the nest to lay her eggs. The host bird doesn’t just sit by and let this happen. Instead, it will often try to chase off the cuckoo intruder.
Cuckoos have evolved tricks to short-circuit this defense. In some species, a male cuckoo will fly around a nest, luring the host bird into a chase. While the nest is unguarded, his female partner slips into the nest and drops her eggs.
Given the evolutionary arms race in which cuckoos and their hosts are locked, one explanation for those hawk-like bars comes knocking loudly at the door. What if the cuckoo has evolved to look like a hawk?
A host bird looks up and sees barred feathers zooming into view. There’s no time to mull a decision–if the host bird doesn’t instantly fly away, it will be killed by the hawk to whom those stripes must belong.
Scientists have tested this idea in several ways. In some experiments, they’ve built cuckoo models and presented them to host birds. The birds are more likely to attack a model without bars than one with them.
But most of this work has been carried out on just one pair of birds–the common cuckoo and the Eurasian sparrowhawk. Even if this one cuckoo species is pretending to be a raptor, it’s hard to know if the many other cuckoos that live across the Old World are pretending, too.
And there are other potential explanations for cuckoos to be striped. Cuckoos will lurk in trees before springing on a victim’s nests, waiting for the right moment to swoop in. Maybe the stripes serve as camouflage.
Two biologists at the University of Cambridge, Thanh-Lan Gluckman and Nicholas Mundy, decided to test the predator mimic hypothesis in a new way. Other animals, they knew, have evolved to look like dangerous models. The English naturalist Henry Bates was the first scientist to discover this phenomenon as he studied butterflies in the Amazon in the late 1800s. Some of the butterflies were poisonous, and their bright markings warned off birds. Bates noticed that other butterflies that were harmless had similar markings. These so-called Batesian mimics could ward off birds without putting in the extra energy to store toxins in their tissues.
What’s most striking about the butterflies Bates discovered is the fine detail of the mimicry. The poisonous butterfly species in one part of the Amazon look different from the species in other parts. Each mimic species has evolved to look identical with the poisonous species that overlaps its range. That’s probably because birds recognize the markings of the poisonous butterflies that they encounter–either through instinctive reactions, or by learning from bitter experience. There’s no advantage in looking like a poisonous butterfly from Peru if you’re a harmless butterfly in Venezuela.
If cuckoos are indeed mimicking raptors, then they are also Batesian mimics. They are pretending to be an enemy of their hosts–in this case, a predator rather than poisonous prey. So Gluckman and Mundy wondered if, like Batesian butterflies, the cuckoos match their local raptors, too.
To find out, the scientists plunged into the collection of bird specimens kept by the Natural History Museum and took photographs of the barred bellies of a number of cuckoo species. The scientists also took photos of the raptors that live alongside them. Rather than eyeball the patterns, the scientists ran the pictures through a computer program. Taking into account how bird vision works, the scientists transformed the images to what the birds would see. They then transformed the feather patterns from each species into mathematical representations. It was then possible for the scientists to calculate how similar the patterns were to each other.

Barred feather photos transformed as birds would see them. Photo courtesy of Thanh-Lan Gluckman
All the cuckoos, Gluckman and Mundy found, strongly resembled a species of raptor that shared their range. None resembled a species outside its range. While this tight link was striking, Gluckman and Mundy investigated some alternative explanations for its existence.
Perhaps it was due not to cuckoos mimicking local raptors, for example, but cuckoos and raptors both adapting to the same ecology. Maybe living in dim jungles or bright savannas influences how any bird’s stripes evolve. But when Gluckman and Mundy looked at cuckoos that lived in similar ecosystems, they didn’t find similar stripes.
Instead, it looks as if cuckoos are indeed Batesian mimics. They are evolving to look like local raptors to scare off their hosts, so that they can lay eggs in their nests.
This new study is just one of a growing number revealing just how deep the sophistication of mimicry can go. Mimics don’t just mimic one feature in another species. They can be multi-dimensional in their disguises. An ant known as Protomognathus uses mimicry to turn other species of ants into its slaves. The queen invades a colony and releases chemicals that prompt the workers to treat her as one of their own. The queen’s offpspring have the look and smell of their host’s, tricking the workers into feeding them.
As I marveled about the double-con of cuckoos, I couldn’t think of another animal that pulls a two-species scam. I asked Gluckman if she knew of any. She didn’t, but asked around her lab.
Someone drew our attention to the Spicebush Swallowtail butterfly. As a catepillar, it avoids getting eaten by birds by taking on the appearance of bird droppings. When it gets bigger, it takes on the appearance of a snake. And when it develops into an adult, it mimics a poisonous butterfly.
Which just goes to show, when you wonder whether something exists in the natural world, usually the answer is yes.
October 17, 2013
On the Origin of Ants–From Wasps
Growing up on a small farm, I was able to get to know the insects that lived on the property pretty well. Some I liked, and some I hated. I hated the mud dauber wasps that built organ-pipe shaped cavities for their eggs on the side of our chicken coop and always seemed poised to sting me. On the other hand, I became fond of ants; they hypnotized me with their affable industry, hauling food back to their nests or moving larvae to a new home.
In my “Matter” column today for the New York Times, I take a look at a new study that has produced an evolutionary tree of ants and their relatives. I was surprised to find that those mud dauber wasps look to be closely related to ants. In fact, the authors of the study argue, ants started out as a similar kind of predatory wasp. And in those waspish origins may lay the roots of the remarkably complex societies of ants. Check it out.

Mud dauber wasp. Photo by Jaxo S via Creative Commons: http://flic.kr/p/9SHhE9
October 14, 2013
What Dolly Wrought: Retro Report Looks at Cloning
Last month I blogged about Retro Report, a new outfit that produces deeply researched videos that bring history up to date. I found today’s report especially interesting, as it explores some of the same material I wrote about in my piece for National Geographic about de-extinction in April. The Retro Report team looks at the sensation caused back in 1997 by Dolly, the cloned sheep. All of the scare-mongering about armies of zombie clones has blotted out people’s understanding of cloning’s actual history, its disappointments, and its big impacts today.
I’ve embedded it below; you can also see it here.
October 10, 2013
Pointing At the Minds of Elephants: My Column This Week For the New York Times
Earlier this year, I wrote about a simple way to probe the mind of a dog: point to something and see if the dog understands your intent. Dogs generally do, and that’s remarkable. Many species, including our closest ape relatives, do a bad job of interpreting a pointed hand. This week in my “Matter” column for the New York Times, I look at a new study that suggests we add another species to the elite list of animals that understand pointing: elephants. Do elephants learn the meaning of pointing from humans? Or do these social behemoths use their trunks to point things out to each other?
October 9, 2013
Evolution in Color: From Peppered Moths to Walking Sticks
The color of an animal can determine whether it lives or dies. If it’s easily spotted by predators, it may well become a meal. Hidden nicely against its background, an animal can escape its enemies for another day.
The particular colors on an animals are determined partly by the genes its gets from its parents. That means that genes that hide animals can spread thanks to natural selection, leading to the evolution of exquisite camouflage. But that’s not to say that the animal kingdom has settled on a perfect, fixed palette. You can find mismatched individuals. Over the course of generations, a whole population can flicker between mismatched and well-matched.

White form of peppered moth. Via Wikipedia

Melanic form of peppered moth. Via Wikipedia
The most famous example of mismatched colors first came to light in the 1950s. Coal smoke had darkened England’s trees, so that light pepper moths, once blended nicely against bark, now stood out against the smudgy background. A dark form of peppered moths, once rare, became common. Researchers suspected that natural selection was the reason why, and they tested that idea by putting dark and light moth models on trees. Birds quickly attacked the mismatched ones, as had been predicted.
The photos from these experiments became a staple of textbooks. But doubts arose about the research. Real peppered moths often don’t sit on tree trunks with wings extended, for example. Creationists called the whole phenomenon a fraud and a reason to question evolution itself.
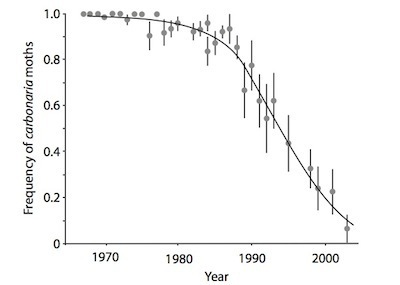
Decline of dark peppered moths around Leeds, England. From Thompson, “Relentless Evolution”
But the evidence in favor of natural selection on peppered moths continued to accumulate. For one thing, Britain and other countries cleaned up their air in the late 1900s, and trees went from dark to light. Now natural selection’s balance shifted: black became a liability. And, as you’d predict, the dark moths went from common back to rare again.
To see if predators were the instrument of their disappearance, biologist Michael Majerus launched a massive study in 2001. He died before he could publish the experiment; it only came out last year, completed by some of his fellow scientists, in Biology Letters. (It’s free to read.)
Majerus released 4864 moths, some dark and some light, and then observed how they landed on trees and how likely they were to escape being eaten by birds. Each day, he found, dark moths were nine percent less likely to survive. Since the moths only live for a few days in the wild anyway, that difference was enough to quickly drive dark moths from common to rare if they didn’t match their background.
The story of the peppered moth is striking, but it’s also a story of civilization. As we humans developed different ways of producing energy–first dirty, then clean–we altered the environment in which animals evolve. Their colors tracked our history. The story of the peppered moth still leaves us to wonder about the evolution of color in the natural world, beyond the dark Satanic mills.
Thousands of miles away from England’s peppered moths, a species of walking stick insects called Timema cristinae lives in the hills of southern California. A new study shows that they feel the pressure of evolution on their color, too. In fact, the evolution of color is so powerful that it doesn’t just affect the walking sticks. It affects the diversity of their entire ecosystem.
The new study, carried out by Patrik Nosil of the University of Sheffield and his colleagues, took place in the hills outside of Santa Barbara. When the scientists beat the bushes in those hills, they find Timema cristinae insects with two color patterns. Some are solid green, while others have white stripes running up their bodies. Depending on the bush they inspect, they may find mostly green bugs, mostly striped ones, or a mix of the two.

A striped form of walking stick (A) is often found on narrow-leafed bushes. A solid green one is often found on wide-leafed bushes. From Farkas et al 2013, Current Biology. Illustration by Rosa Ribas
It’s no surprise that they can find a mix of the insects. Walking sticks don’t have wings, so they live mostly on a single bush their whole life. But when a new generation of walking sticks emerges, some of the insects will disperse to a different bush.
But why are there two such different forms in the same species? The ecologist Cristina Sandoval first recognized that the answer had to do with the bushes that the walking sticks live on. Each pattern may do a good job of protecting a walking stick from birds–just as long as it is living on the right bush.
One species of bush that the insects live on has thick green leaves. A solid green walking stick blends right in with that foliage. Another species of bush grows needle-like leaves. The white stripes on some walking sticks divides into green strips, making them look like thin leaves.

A striped walking stick hides among thin leaves. Photo by Mortiz Muschick
If the walking sticks get on the wrong bush, however, they lose their disguise. Against the thin leaves, the solid green insects leap out. Against the big leaves, the pale stripe of the other walking sticks looks out of place.
Nosil and his colleagues have been studying how these different kinds of camouflage play out in the California hills. A map of the insects and the bushes bears out this idea. In places where there are lots of thick-leaves bushes, the walking sticks are mostly solid green. In places where the thin-leaved bushes dominate, most of the insects have a white stripe. But if a thick-leafed bush is surrounded by thin-leaved ones, it will have many mismatched insects. That’s a pattern you’d expect from the combination of bird-driven natural selection and insects moving among neighboring bushes.
To put this idea to a thorough test, Nosil, his student Tim Farkas, and their colleagues studied 186 bushes in the California hills. They caught every walking stick insect on the bushes to do a population census. They found that when the insects were well-matched to the bush, their numbers were high. When they were badly matched, the population was much lower. That pattern makes sense if the birds are picking off the insects that are standing out against the bushes.
Nosil and his colleagues then altered the populations of walking sticks on each bush. To some bushes, they added 200 well-matched insects. To others, they added 200 mismatched ones. They waited a month–during which time the insects fed on the bushes and birds fed on the insects–and then returned to see how things had gone.
On the bushes with mismatched insects, the populations were half what Nosil found on the bushes with the well-matched ones. Birds presumably swooped in and feasted on the easy-to-spot walking sticks.
But the effects did not stop there. Instead, they rippled out to other species. Nosil and his colleagues didn’t just count up the walking sticks on the bushes–they also tallied the caterpillars, spiders and other invertebrates. And they found a stark change in their numbers too. On the bushes with mismatched walking sticks, the other species dropped by half as well.
Not only did the numbers go down for all the species, but some species disappeared altogether from bushes. On bushes with extra camouflaged walking sticks, on the other hand, other species thrived.
Nosil and his colleagues also found that they altered the bushes themselves with the experiment. Walking sticks chew on leaves, and so it stands to reason that bushes with fewer walking sticks ended up getting chewed less. But the scientists wondered how much damage the plants suffered from other insects, which pierce or suck the plants instead of chewing them. They found that bushes full of mismatched walking sticks suffered less of this kind of damage, too. In other words, the bushes benefited from mismatched walking sticks because they wiped out all the species feeding on them.
It was surprising that getting rid of walking sticks would also lead to the devastation of other species. The disappearance of walking sticks could have led to an increase in other animals, because there would be less competition for food. To understand why this was’t happening, Nosil repeated the experiment all over again, but with a twist. He and his colleagues put chicken wire over some bushes so that birds couldn’t get to them.
On the bushes that were left unprotected, the scientists found the same result: walking sticks and other species were wiped out when the walking sticks were mismatched, and the bushes benefited. But the chicken wire wiped out those results on the enclosed bushes. There was no difference between bushes with mismatched and well-matched walking sticks.
That experiment established that it’s the birds that are responsible for all the effects the scientists had seen. They were attracted to the mismatched walking sticks, because walking sticks are so abundant on the bushes. And then stayed on the bushes to feast on the other prey. When the birds cleaned off all the animals from the bushes, they protected the bushes.
Adding extra walking sticks to bushes mirrors the different combinations of walking insects you can find in nature. Nosil argues that as evolution acts on the walking sticks, it shapes the diversity of other species in the hills of California. To understand why there are a given number of species in one place, scientists may need to understand the rapid evolution going on there. The power of color evolution, it appears, reaches far beyond our eye.
[10/10: A few corrections, including name-checking Sandoval]
October 4, 2013
Breathe in the Mystery: My New York Times Column on Oxygen
One of the most important things that makes Earth special is that the abundant oxygen in its atmosphere. How it got that way is a surprisingly complicated story that’s just coming to light. It’s a story I delve into for this week’s “Matter” column in the New York Times. Check it out.
When Science Goes Wrong And Ways to Fix It: Video of My Lecture At Vanderbilt
Yesterday I had the pleasure of delivering the Flexner Discovery Lecture at Vanderbilt to a packed room. My talk was entitled, “When Science Goes Wrong and Ways To Fix It.” I talked about some of the struggles the science community is facing with poorly replicated research and misconduct, and how we science journalists can make things worse by seizing on research to make huge claims. The video (complete with slides) is now online. Check it out.



