Brian Clegg's Blog, page 134
September 19, 2012
Tweetness and light
The media has a very mixed attitude to Twitter. Sometimes it is given totally over the top accolades for enabling something like the Arab Spring to take place (there is no doubt it made a contribution, but equally no doubt that things would have gone ahead much the same without it). At other times it is seen as a lowest common denominator means of spreading gossip and tittle tattle.
 Why wouldn't you tweet it?I personally think it's a great way for getting and giving instant reactions. It can be genuinely interesting to see live response to a TV show, for instance, as tweets come flying in. And although I personally am not particularly interested in what people had for breakfast, say, it is very valuable as a way of highlighting something interesting or amusing. So, for instance, when I spot a van with an entertaining spelling error on its artwork, or when I recently came across a slow worm on my walk to the Post Office, Twitter was a natural way to make a quick comment.
Why wouldn't you tweet it?I personally think it's a great way for getting and giving instant reactions. It can be genuinely interesting to see live response to a TV show, for instance, as tweets come flying in. And although I personally am not particularly interested in what people had for breakfast, say, it is very valuable as a way of highlighting something interesting or amusing. So, for instance, when I spot a van with an entertaining spelling error on its artwork, or when I recently came across a slow worm on my walk to the Post Office, Twitter was a natural way to make a quick comment.
This ease can lead to problems. There was, of course, the court case for the poor guy who remarked that he was going to bomb Robin Hood airport (what a name), which should never have happened. Twitter is sounding off, worldwide light conversation, not a place to generate threats and litigation. There was also the poor Welsh councillor who was hauled up for a disciplinary hearing for tweeting I didn’t know the Scientologists had a church on Tottenham Court Road. Just hurried past in case the stupid rubs off - ludicrous over-reaction for a personal response you may or may not agree with (I do agree) but that he should have the freedom to make without harassment.
I also find that Twitter is a good, painless way for a reader to make a quick comment to an author. I would never think of emailing Stephen Fry, say, but I don't mind blasting something off to him on Twitter. He probably never sees them - but that doesn't really matter. And when I get a response from the author, as I did from a positive remark having just read one of S. J. Parris's novels featuring Giordano Bruno, it feels really good.
 Canadian bookstore purchases
Canadian bookstore purchases
Photo courtesy of Claire McCartneyAs an author myself I also receive quite a few tweets about my books - and that warm glow works both ways. I received one the other day saying Picked up your book on gravity in Chapters book store in Ottowa, Canada and 1 hour later I was still reading it! Nice - that really made my day. It's not just the nice comments, but the thought of a book I wrote making a connection in a different country - there's something heartwarming about it!
I couldn't help asking if, after reading it for an hour, the tweeter had actually bought the book - and was even more delighted to hear that not only did she do so, but she went back next day for another of my titles. And chocolate covered beaver droppings. The way you do. (Why don't our bookshops sell beaver droppings?) I've even got a photo to prove it.
So don't knock Twitter. I get really irritated with people who say 'Oh, no, I've never twitted, or whatever you call it,' wrinkling their nose as if it's something tasteless. Personally, I'm all in favour.
 Why wouldn't you tweet it?I personally think it's a great way for getting and giving instant reactions. It can be genuinely interesting to see live response to a TV show, for instance, as tweets come flying in. And although I personally am not particularly interested in what people had for breakfast, say, it is very valuable as a way of highlighting something interesting or amusing. So, for instance, when I spot a van with an entertaining spelling error on its artwork, or when I recently came across a slow worm on my walk to the Post Office, Twitter was a natural way to make a quick comment.
Why wouldn't you tweet it?I personally think it's a great way for getting and giving instant reactions. It can be genuinely interesting to see live response to a TV show, for instance, as tweets come flying in. And although I personally am not particularly interested in what people had for breakfast, say, it is very valuable as a way of highlighting something interesting or amusing. So, for instance, when I spot a van with an entertaining spelling error on its artwork, or when I recently came across a slow worm on my walk to the Post Office, Twitter was a natural way to make a quick comment.This ease can lead to problems. There was, of course, the court case for the poor guy who remarked that he was going to bomb Robin Hood airport (what a name), which should never have happened. Twitter is sounding off, worldwide light conversation, not a place to generate threats and litigation. There was also the poor Welsh councillor who was hauled up for a disciplinary hearing for tweeting I didn’t know the Scientologists had a church on Tottenham Court Road. Just hurried past in case the stupid rubs off - ludicrous over-reaction for a personal response you may or may not agree with (I do agree) but that he should have the freedom to make without harassment.
I also find that Twitter is a good, painless way for a reader to make a quick comment to an author. I would never think of emailing Stephen Fry, say, but I don't mind blasting something off to him on Twitter. He probably never sees them - but that doesn't really matter. And when I get a response from the author, as I did from a positive remark having just read one of S. J. Parris's novels featuring Giordano Bruno, it feels really good.
 Canadian bookstore purchases
Canadian bookstore purchasesPhoto courtesy of Claire McCartneyAs an author myself I also receive quite a few tweets about my books - and that warm glow works both ways. I received one the other day saying Picked up your book on gravity in Chapters book store in Ottowa, Canada and 1 hour later I was still reading it! Nice - that really made my day. It's not just the nice comments, but the thought of a book I wrote making a connection in a different country - there's something heartwarming about it!
I couldn't help asking if, after reading it for an hour, the tweeter had actually bought the book - and was even more delighted to hear that not only did she do so, but she went back next day for another of my titles. And chocolate covered beaver droppings. The way you do. (Why don't our bookshops sell beaver droppings?) I've even got a photo to prove it.
So don't knock Twitter. I get really irritated with people who say 'Oh, no, I've never twitted, or whatever you call it,' wrinkling their nose as if it's something tasteless. Personally, I'm all in favour.
Published on September 19, 2012 01:02
September 17, 2012
Nature's Nanotech #7 - Behold the Peacock
The last in the Nature's Nanotech series

There is something stunning about the colours of a peacock feather. It’s not just a simple matter of the sort of coloured pigments an artist mixes up on a palette. The colours in the feathers almost glow in their iridescence, changing subtly with angle to catch the eye. To produce this effect, the feather contains a natural nanotechnology that has the potential to transform optics when this remarkable approach is adapted for use in human technology.
Both the iridescence of that peacock’s tail and the swirly, glittering appearance of the semi-precious stone opal are caused by forms of photonic lattices. These are physical structures at the nano level that act on light in a way that is reminiscent of electronics, like the semiconductors that act to switch and control electrons, giving unparalleled manipulation of photons.

The colours of the peacock feather bear no resemblance to those of a pigment. In blue paint, for example, the pigment is a material that tends to absorb most of the spectrum of white light but re-emits primarily blue, so we see anything painted with the pigment as being blue. In the peacock feathers it’s the internal structure of the feather (or to be precise the tiny ‘barbules’ on the feather) that produce the hue.
The colouration is primarily due to internal reflections off the repeated structure of the barbule, similar to the way the lattice arrangement of a crystal can produce enhanced reflection. What happens is that photons reflected from a deeper layer are in phase with those from an outer layer, reinforcing the particular colours of light (or energies of photons) that fit best with the lattice spacing. This is a photonic lattice. These effects depend on the angle at which the light reflects, giving the typical ‘shimmer’ of iridescence.
The practical applications of artificially created photonic crystals can do much more than produce a pretty effect and striking colours. Because a photonic lattice acts on light as semiconductors do on electrons, they are essential components if we are ever to build optical computers.
These theoretical machines would use photons to represent bits, rather than the electrical impulses we currently employ in a conventional computer. This could vastly increase the computing power. Because photons don’t interact with each other, many more can be crammed into a tiny space. What’s more, one of the biggest restrictions in current computer architecture is the complex spaghetti of links joining together different parts of the structure. With photons, those links can flow through each other in a basket of light – unlike wires and circuits, photons can pass through each other without interacting, allowing more complex and faster architectures. Equally, optical switching – and in the end, a computer is just a huge array of switches – could be much faster than the electronic equivalent.
There are significant technical problems to be overcome, but the potential is great. Photonic crystals are already used in special paint and ink systems which change colour depending on the angle at which the paint is viewed, reflection reducing coatings on lenses and high transmission photonic fibre optics.
Another example of nanotechnology having a quantum effect on light is plasmonics. Something remarkable happens, for example, if light is shone on a gold foil peppered with millions of nanoholes. It seems reasonable that only a tiny fraction of the light hitting the foil would pass through these negligible punctures, but in fact in a process known as ‘extraordinary optical transmission’ they act like funnels, channelling all the light that hits the foil through the sub-microscopic apertures. This bizarre phenomenon results from the interaction between the light and plasmons, waves in the two dimensional ocean of electrons in the metal.
The potential applications of plasmonics are remarkable. Not only the more obvious optical ones – near perfect lenses and supplementing the photonic lattices in superfast computers that use light rather than electrons to function – but also in the medical sphere to support diagnostics, by detecting particular molecules, and for drug delivery. Naomi Halas of Rice University in Texas envisions implanting tiny cylinders containing billions of plasmonic spheres, each carrying a minuscule dose of insulin. Infra red light, shone from outside the body, could trigger an exact release of the required dose. ‘Basically, people could wear a pancreas on their arm,’ said Halas.
Over the last seven weeks since the first post, we have explored a wide range of the ways that nanotechnology, given a push in the right direction by nature, is starting to be important in our lives. At the moment we are most likely to come across relatively simple applications like the nanoparticles in sun block or technology making fabrics and electronics water repellent.
As our abilities to construct nanostructures improve we will see increased use of the likes of carbon nanotubes and the nano-optics described in this piece. And eventually? It is entirely possible that we will see Richard Feynman’s 1950s speculation about nanomachines come to fruition, though they are likely to be more like the ‘wet’ machines of nature than a traditional mechanical device.
When nanotechnology appears in the news it is often in a negative light. We might hear that Prince Charles is worrying about the threat of grey goo, or the Soil Association won’t allow artificial nanoparticles in organic products. But the reality is very different. Nanotechnology is both fascinating and immensely valuable in its applications. I, for one, can’t wait to see what comes next.
This series has been sponsored by P2i, a British company that specializes in producing nanoscale water repellent coatings. P2i was founded in 2004 to bring technologies developed at the UK Government’s Defence Science & Technology Laboratory to the commercial market. Applications range from the Aridion coating, applied to mobile technology inside and out after manufacture using a plasma, to protection for filtration media preventing clogging and coatings for trainers that reduce water absorption.
Image from Wikipedia


There is something stunning about the colours of a peacock feather. It’s not just a simple matter of the sort of coloured pigments an artist mixes up on a palette. The colours in the feathers almost glow in their iridescence, changing subtly with angle to catch the eye. To produce this effect, the feather contains a natural nanotechnology that has the potential to transform optics when this remarkable approach is adapted for use in human technology.
Both the iridescence of that peacock’s tail and the swirly, glittering appearance of the semi-precious stone opal are caused by forms of photonic lattices. These are physical structures at the nano level that act on light in a way that is reminiscent of electronics, like the semiconductors that act to switch and control electrons, giving unparalleled manipulation of photons.

The colours of the peacock feather bear no resemblance to those of a pigment. In blue paint, for example, the pigment is a material that tends to absorb most of the spectrum of white light but re-emits primarily blue, so we see anything painted with the pigment as being blue. In the peacock feathers it’s the internal structure of the feather (or to be precise the tiny ‘barbules’ on the feather) that produce the hue.
The colouration is primarily due to internal reflections off the repeated structure of the barbule, similar to the way the lattice arrangement of a crystal can produce enhanced reflection. What happens is that photons reflected from a deeper layer are in phase with those from an outer layer, reinforcing the particular colours of light (or energies of photons) that fit best with the lattice spacing. This is a photonic lattice. These effects depend on the angle at which the light reflects, giving the typical ‘shimmer’ of iridescence.
The practical applications of artificially created photonic crystals can do much more than produce a pretty effect and striking colours. Because a photonic lattice acts on light as semiconductors do on electrons, they are essential components if we are ever to build optical computers.
These theoretical machines would use photons to represent bits, rather than the electrical impulses we currently employ in a conventional computer. This could vastly increase the computing power. Because photons don’t interact with each other, many more can be crammed into a tiny space. What’s more, one of the biggest restrictions in current computer architecture is the complex spaghetti of links joining together different parts of the structure. With photons, those links can flow through each other in a basket of light – unlike wires and circuits, photons can pass through each other without interacting, allowing more complex and faster architectures. Equally, optical switching – and in the end, a computer is just a huge array of switches – could be much faster than the electronic equivalent.
There are significant technical problems to be overcome, but the potential is great. Photonic crystals are already used in special paint and ink systems which change colour depending on the angle at which the paint is viewed, reflection reducing coatings on lenses and high transmission photonic fibre optics.
Another example of nanotechnology having a quantum effect on light is plasmonics. Something remarkable happens, for example, if light is shone on a gold foil peppered with millions of nanoholes. It seems reasonable that only a tiny fraction of the light hitting the foil would pass through these negligible punctures, but in fact in a process known as ‘extraordinary optical transmission’ they act like funnels, channelling all the light that hits the foil through the sub-microscopic apertures. This bizarre phenomenon results from the interaction between the light and plasmons, waves in the two dimensional ocean of electrons in the metal.
The potential applications of plasmonics are remarkable. Not only the more obvious optical ones – near perfect lenses and supplementing the photonic lattices in superfast computers that use light rather than electrons to function – but also in the medical sphere to support diagnostics, by detecting particular molecules, and for drug delivery. Naomi Halas of Rice University in Texas envisions implanting tiny cylinders containing billions of plasmonic spheres, each carrying a minuscule dose of insulin. Infra red light, shone from outside the body, could trigger an exact release of the required dose. ‘Basically, people could wear a pancreas on their arm,’ said Halas.
Over the last seven weeks since the first post, we have explored a wide range of the ways that nanotechnology, given a push in the right direction by nature, is starting to be important in our lives. At the moment we are most likely to come across relatively simple applications like the nanoparticles in sun block or technology making fabrics and electronics water repellent.
As our abilities to construct nanostructures improve we will see increased use of the likes of carbon nanotubes and the nano-optics described in this piece. And eventually? It is entirely possible that we will see Richard Feynman’s 1950s speculation about nanomachines come to fruition, though they are likely to be more like the ‘wet’ machines of nature than a traditional mechanical device.
When nanotechnology appears in the news it is often in a negative light. We might hear that Prince Charles is worrying about the threat of grey goo, or the Soil Association won’t allow artificial nanoparticles in organic products. But the reality is very different. Nanotechnology is both fascinating and immensely valuable in its applications. I, for one, can’t wait to see what comes next.
This series has been sponsored by P2i, a British company that specializes in producing nanoscale water repellent coatings. P2i was founded in 2004 to bring technologies developed at the UK Government’s Defence Science & Technology Laboratory to the commercial market. Applications range from the Aridion coating, applied to mobile technology inside and out after manufacture using a plasma, to protection for filtration media preventing clogging and coatings for trainers that reduce water absorption.
Image from Wikipedia
Published on September 17, 2012 23:56
So long, farewell
 Yes, well worth savingThere was a discussion on the radio the other day about endangered species. Specifically, that old chestnut of whether it really matters if a few species go extinct.
Yes, well worth savingThere was a discussion on the radio the other day about endangered species. Specifically, that old chestnut of whether it really matters if a few species go extinct.One protagonist was arguing fiercely that it was essential to preserve every single species, though as usual, the arguments in detail were very flimsy. They came down to:
It's our (moral) duty - Essentially, because it's our fault that they're dying out, we have a duty to prevent it. I really don't know if this is true or not. I can see a good argument for not going out of your way to destroy a species (take the passenger pigeon as an example), but this isn't something we do any more. The world would be a less rich place without them - certainly true of, say, pandas. Sort of true of the 57th variety of almost identical shrew-like creature. Hard to argue for a beetle. Even harder for a bacterium.We don't know how we might benefit from them in the future - of course it's possible, but I suspect with most potential extinctions this 'okay, if altruism won't work, what's in it for me' approach is extremely low probability. We might benefit from staying in the house all day and never putting ourselves at risk from traffic. But hey.We don't know what difference their absence would make to the ecosystem - that's true, and we know that the removal/addition of some species can have devastating effects on a local ecosystem (think rabbits in Australia). But arguably, for the species that are at risk, they can't be having a big impact on their ecosystem - there aren't enough of them.Don't get me wrong, I am not suggesting we should do nothing about species we are interested in, but I really can't get behind the 'every single species should be preserved' argument. Species have always gone extinct. I know that because of our changes to the planet this is happening much faster at the moment than has been the case recently (though nowhere near as fast as in the great extinctions of the past), so I'm all in favour of putting on the brakes. But trying to save everything is crazy. We need an 80 percent solution, where I'd say that 80 percent should include the most potentially useful (to us an the environment) and the most appealing animals.
Some argue we shouldn't treat giant pandas so specially because of the 'awww!' factor. Rubbish. Given the choice, I am afraid I would save pandas over beetles and bacteria every time. Orwell might not have intended the way that some animals are more equal than others to be a positive lesson, but here it is.
Image from Wikipedia
Published on September 17, 2012 00:40
September 14, 2012
Thermodynamics? Who cares?
I was writing something yesterday for a book I'm currently working on about thermodynamics. It sounds, frankly, a bit of a dull subject. The name implies it's about the way heat moves around. And it is, sort of. It sounds like the sort of old fashioned science that dates from the age of the steam engine. And it is, sort of. Part of its origins certainly came from the need to understand steam engines better. But it is so much more.
One of the reasons for this is that surprisingly early on it was developed from thinking about engines to basics like atoms and molecules. How they interact and how we can look statistically at a whole bunch of them, because we certainly aren't going to be able to work on each one individually - there are just too many. I say 'surprisingly early' because when this theory was being developed a lot of scientists doubted that atoms existed at all, thinking they were just convenient mathematical models for working out the numbers. It was said for a long time that one of the reasons the remarkable Ludwig Boltzmann, one of the leading lights in the field, committed suicide was because there was so much opposition to his theories which were based on the reality of atoms. These days it's popular for historians of science to say his suicide was down to the depressive phase of bipolar disorder - which may be true, but it's hard to think such fervent opposition didn't make things worse.
I'm not going to drone through all four of the 'laws' of thermodynamics (terrible word to use in science, 'law' - it should be banned), but the one that is most exciting is the second law. This can be stated in a loose way as 'entropy (disorder) in a close system stays the same or increases', or 'you can't make a change in a closed system without increasing entropy' or for the steam engine enthusiasts, 'left to its own devices, heat will flow from a hotter to a cooler part of a system.' Or in the vernacular TANSTAAFL - there ain't no such thing as a free lunch.
This may all sound highly esoteric (apart from TANSTAAFL), but the second law is at the fundamental heart of existence. Every time anything changes - which, let's face it, is the interesting bit of life - the second law comes into play. It even explains teenage bedrooms - without the input of energy, disorder increases - and the eventual fate of the universe. Because the second law is so fundamental, it was the example C. P. Snow gave in his famous 'Two Cultures' ponderings as the equivalent of reading Shakespeare. He pointed out that most scientists have probably encountered Shakespeare, but very few artists have a clue about the second law of thermodynamics. Arguably they should.
The second law also produced a famous quote from one of the early twentieth century’s greatest scientists, Arthur Eddington, which I will leave you with. He said:

One of the reasons for this is that surprisingly early on it was developed from thinking about engines to basics like atoms and molecules. How they interact and how we can look statistically at a whole bunch of them, because we certainly aren't going to be able to work on each one individually - there are just too many. I say 'surprisingly early' because when this theory was being developed a lot of scientists doubted that atoms existed at all, thinking they were just convenient mathematical models for working out the numbers. It was said for a long time that one of the reasons the remarkable Ludwig Boltzmann, one of the leading lights in the field, committed suicide was because there was so much opposition to his theories which were based on the reality of atoms. These days it's popular for historians of science to say his suicide was down to the depressive phase of bipolar disorder - which may be true, but it's hard to think such fervent opposition didn't make things worse.
I'm not going to drone through all four of the 'laws' of thermodynamics (terrible word to use in science, 'law' - it should be banned), but the one that is most exciting is the second law. This can be stated in a loose way as 'entropy (disorder) in a close system stays the same or increases', or 'you can't make a change in a closed system without increasing entropy' or for the steam engine enthusiasts, 'left to its own devices, heat will flow from a hotter to a cooler part of a system.' Or in the vernacular TANSTAAFL - there ain't no such thing as a free lunch.
This may all sound highly esoteric (apart from TANSTAAFL), but the second law is at the fundamental heart of existence. Every time anything changes - which, let's face it, is the interesting bit of life - the second law comes into play. It even explains teenage bedrooms - without the input of energy, disorder increases - and the eventual fate of the universe. Because the second law is so fundamental, it was the example C. P. Snow gave in his famous 'Two Cultures' ponderings as the equivalent of reading Shakespeare. He pointed out that most scientists have probably encountered Shakespeare, but very few artists have a clue about the second law of thermodynamics. Arguably they should.
The second law also produced a famous quote from one of the early twentieth century’s greatest scientists, Arthur Eddington, which I will leave you with. He said:
‘If someone points out to you that your pet theory of the universe is in disagreement with Maxwell’s equations [the equations that describe how electromagnetism works] – then so much the worse for Maxwell’s equations. If it is found to be contradicted by observation – well these experimentalists do bungle things sometimes. But if your theory is found to be against the second law of thermodynamics I can give you no hope; there is nothing for it but to collapse in the deepest humiliation.’
Published on September 14, 2012 00:52
September 13, 2012
Science soundbites
It is popular in the scientific community to be snarky about people who talk about science in the media. Particularly if it's a science journalist or correspondent, but even if it is a full blown practising scientist, there will be much tutting, muttering and general attacking of the idiocy of the way the science is presented. I saw it happening an awful lot, for example, over the Higgs boson results - not an easy thing to explain. One scientist was very sarcastic about the analogy someone (actually a politician) used on the radio, even though it was exactly the same analogy that Brian Cox (who, after all, works at CERN when he has a day off from posing) (sorry - snark attack) had used in print.
I had a personal example of this last week. In my role as totally unpaid science correspondent for BBC Wiltshire (you pay peanuts...) I was asked in on the breakfast show to talk about ENCODE, the next generation human genome project that goes beyond the genes to look at how the rest of human DNA does all the switching of genes, and the differences in the way this operates in a wide range of cells. And I committed every error that the science moaning minnies complain about. I oversimplified, at least one thing I said was effectively wrong, and I didn't use the best analogies I could.
But. This was around a four minute slot to explain a huge scientific endeavour. I'm not a biologist. And the discussion was driven by the presenter, who inevitably was more interested in potential applications than the science itself. So not a great performance. Do I regret it? Not at all. And this is where we've got to stop moaning. The fact is, the listeners got more idea about what was going on than they would otherwise. They got a feel for the excitement, the remarkable work that was being undertaken (something they need to remember when parliament is talking about cutting science funding) and the potential for future benefits.
I honestly believe that it is better to fire people up to find out more and be supportive of science, even if what you say isn't perfect, rather than say nothing and have it drop off the agenda. It's also important to bear in mind that such broadcasts are not carefully scripted - it's all top of the head. You have to give some leeway. But even if it is scripted (or a book) I'd rather it was out there in an approachable fashion with a few errors than presented in a totally incomprehensible way by someone who totally understands the science but can't communicate, or even worse is not out there at all.
It's the Inconvenient Truth effect. Al Gore's global warming movie contained a number of unfortunate errors. But it did a lot of good. I'm not saying errors don't matter - but it's more important to communicate the gist and the feeling than to have the kind of accuracy that scientists naturally aim for. Ideally we'd have both. But this isn't an ideal world.

I had a personal example of this last week. In my role as totally unpaid science correspondent for BBC Wiltshire (you pay peanuts...) I was asked in on the breakfast show to talk about ENCODE, the next generation human genome project that goes beyond the genes to look at how the rest of human DNA does all the switching of genes, and the differences in the way this operates in a wide range of cells. And I committed every error that the science moaning minnies complain about. I oversimplified, at least one thing I said was effectively wrong, and I didn't use the best analogies I could.
But. This was around a four minute slot to explain a huge scientific endeavour. I'm not a biologist. And the discussion was driven by the presenter, who inevitably was more interested in potential applications than the science itself. So not a great performance. Do I regret it? Not at all. And this is where we've got to stop moaning. The fact is, the listeners got more idea about what was going on than they would otherwise. They got a feel for the excitement, the remarkable work that was being undertaken (something they need to remember when parliament is talking about cutting science funding) and the potential for future benefits.
I honestly believe that it is better to fire people up to find out more and be supportive of science, even if what you say isn't perfect, rather than say nothing and have it drop off the agenda. It's also important to bear in mind that such broadcasts are not carefully scripted - it's all top of the head. You have to give some leeway. But even if it is scripted (or a book) I'd rather it was out there in an approachable fashion with a few errors than presented in a totally incomprehensible way by someone who totally understands the science but can't communicate, or even worse is not out there at all.
It's the Inconvenient Truth effect. Al Gore's global warming movie contained a number of unfortunate errors. But it did a lot of good. I'm not saying errors don't matter - but it's more important to communicate the gist and the feeling than to have the kind of accuracy that scientists naturally aim for. Ideally we'd have both. But this isn't an ideal world.
Published on September 13, 2012 00:21
September 12, 2012
Idiot tiger in the tank
Those of us with any sort of scientific bent have groaned for years over the misuse of sciencey words in cosmetic adverts. Practically any cosmetic ad seems to try to do two things:
To use emotional trigger words like 'natural' to make us think the product was practically squeezed out of a fruit or leaf, rather than blended in a vast industrial complex. Also words like 'nourish' however ridiculous this is when talking about something like (dead) hair.To use words that have real meaning in science, but removed from their context. So, for instance, putting 'DNA' into the description of your product, or some wonderfully obscure compound name like pro-boswellox-retinox-B.Now an oil company has got on the bandwagon (not an entirely strange jump, since most cosmetics contain a fair amount of processed oil of one sort or another). When selling petrol, the oil companies have a real problem, because petrol is a commodity. We don't really care what brand it is, just how cheap it is. Esso's answer to this is to resort to the cosmetic world's plan B.
In recent ads, Esso makes a big thing of the fact that their new fuel (ok, petrol with a tiny bit of additive) works at a molecular level (specifically to help remove deposits). Now unlike many of the cosmetic adverts, this isn't just a use of magic words. The fuel does work at a molecular level... but then so does pretty well every chemical compound that isn't part of a larger structure. Okay there will be sub-molecular activity - hydrogen bonds, for example. And I suppose it's possible they could produce a fuel that undergoes nuclear decay and so works at the nuclear level. But otherwise how else is it going to work?
I am now going to drink my coffee. It works at the molecular level, you know. I might watch myself a classic Esso ad as I do so:

To use emotional trigger words like 'natural' to make us think the product was practically squeezed out of a fruit or leaf, rather than blended in a vast industrial complex. Also words like 'nourish' however ridiculous this is when talking about something like (dead) hair.To use words that have real meaning in science, but removed from their context. So, for instance, putting 'DNA' into the description of your product, or some wonderfully obscure compound name like pro-boswellox-retinox-B.Now an oil company has got on the bandwagon (not an entirely strange jump, since most cosmetics contain a fair amount of processed oil of one sort or another). When selling petrol, the oil companies have a real problem, because petrol is a commodity. We don't really care what brand it is, just how cheap it is. Esso's answer to this is to resort to the cosmetic world's plan B.
In recent ads, Esso makes a big thing of the fact that their new fuel (ok, petrol with a tiny bit of additive) works at a molecular level (specifically to help remove deposits). Now unlike many of the cosmetic adverts, this isn't just a use of magic words. The fuel does work at a molecular level... but then so does pretty well every chemical compound that isn't part of a larger structure. Okay there will be sub-molecular activity - hydrogen bonds, for example. And I suppose it's possible they could produce a fuel that undergoes nuclear decay and so works at the nuclear level. But otherwise how else is it going to work?
I am now going to drink my coffee. It works at the molecular level, you know. I might watch myself a classic Esso ad as I do so:
Published on September 12, 2012 00:18
September 11, 2012
Nature’s Nanotech #6 – Silk Elevators
Sixth in the Nature's Nanotech series.

Anyone who talks to young children about science knows that there are two things that really grab their attention – dinosaurs and space. While I’m not aware of any antediluvian nanotechnology, there is certainly an absolutely stunning potential space application that has some natural inspirations. (I’m aware, by the way, that the word ‘antedeluvian’ is both anachronistic and unscientific… but it’s a lovely word that we really shouldn’t lose from the language.)
 Nature has some amazing, extremely fine fibres. Take, for example, that everyday wonder, a spider’s web. The spider silk that makes up the web is a spun fibre constructed from proteins. Though light, these filaments are extremely resistant to fracture – tougher than steel. Spider silk is typically 3,000 nanometers across, but its toughness is down to its structure at the nano level.
Nature has some amazing, extremely fine fibres. Take, for example, that everyday wonder, a spider’s web. The spider silk that makes up the web is a spun fibre constructed from proteins. Though light, these filaments are extremely resistant to fracture – tougher than steel. Spider silk is typically 3,000 nanometers across, but its toughness is down to its structure at the nano level.A team at MIT discovered that the unusual strength is down to a substructure of ‘beta sheet crystals’, which hold the silk together. The linking is done by hydrogen bonds, the same kind of bonding that stops water from boiling at room temperature. Such bonds are easy to break, but the MIT scientists discovered that if they are confined to spaces just a few nanometers in span – as they are in the beta sheet crystals – they become exceedingly strong. So spider silk depends on a kind of nano-glue for its strength.
In the nanotechnology world, the equivalent of spider silk is the carbon nanotube. We are all familiar with the way carbon comes in different physical structures or ‘allotropes’ that have remarkably different properties. Chemically there is no difference between diamond and the graphite in a pencil ‘lead’ but physically one is extremely hard and the other has multiple planes that slide easily over each other making it effectively soft (although those planes themselves are surprisingly tough).
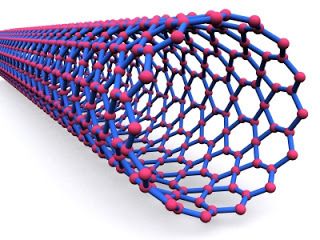 Another way to fit together a structure of carbon atoms is to form a tube. Imagine taking a plane of graphite a single atom thick (technically graphene) and folding it around to make a tubular shape. Carbon nanotubes are amongst the most amazing artefacts ever made. Though simple in structure, they are remarkable both in their strength and their other physical properties.
Another way to fit together a structure of carbon atoms is to form a tube. Imagine taking a plane of graphite a single atom thick (technically graphene) and folding it around to make a tubular shape. Carbon nanotubes are amongst the most amazing artefacts ever made. Though simple in structure, they are remarkable both in their strength and their other physical properties.Electrically they can behave as if they were a metal or a semiconductor, simply as a result of the shape of the tube. Although carbon nanotube electronics is in its infancy, there is considerable speculation about the capabilities of nanotube products. They could be used to make everything from transistors that are switched by a single electron to batteries built into a sheet of paper. But their pièce de résistance is their strength. Carbon nanotubes make spider silk look like tissue. When you compare a nanotube’s strength per unit weight with steel it comes out around 300 times greater.
All kinds of applications are possible for such a remarkable material. Nanotubes are present in the much thicker carbon fibres used to reinforce everything from tennis rackets to bike frames, but only incidentally and in small quantities. At the moment they tend to be used in random bulk combinations of many small fragments – not as strong as a set of individual aligned nanotubes, but still enough to add strength and to change electrical properties. But one potential application could totally transform the space industry.
Getting things into space is expensive. Hugely expensive. To reach a geosynchronous orbit (of which more later) typically costs around $20,000 per kilogram. But there is a hypothetical nanotube technology that once developed could deliver satellites and even people into space for around 1/100th of this cost. What’s more, rocket technology is inherently risky. You will inevitably lose some of your space missions. Yet the nanotube technology could, once established, run day after day without problem.
Imagine you were sitting on top of a house and wanted to get something up there. You could have someone attach your payload to a rocket and shoot it in your direction. But like the space launch it’s a dangerous and expensive solution. Instead you are more likely to throw a piece of string off the roof, have a basket tied to it and then haul the object up.
Now extend this picture to the Empire State Building. Your piece of string would have to be very strong, which would make it quite heavy to haul up and down, increasing the cost of the process. What might be better is to keep the string (or more likely a piece of metal) in place and have the basket haul itself up and down along the supporting structure.
Time to take another jump into that geosynchronous orbit. An object in orbit is in a very strange state. It is in free fall, dropping towards the Earth – but at the same time it is moving sideways at just the right speed so it always misses. This, incidentally, is why people float around in the International Space Station. It’s not because there’s no gravity – the Earth’s gravitational pull at its height (350 kilometres above the Earth’s surface) is around 90% Earth normal. The astronauts float because they and the station are falling. But they stay in orbit because their sideways motion means they keep missing the planet.
Because of this balance, at any particular height there is one speed that keeps you in orbit. And if you go high enough – around 35,786 kilometres up – that speed is the same as the rotational speed of the Earth, making you geosynchronous. Point the orbit in the right direction and you will stay over the same point on the Earth’s surface (this is a geostationary orbit).
So, imagine you could drop a piece of string from a geostationary satellite down to the ground. You could then just send a lift (elevator) up the string and replace all that dangerous, expensive rocketry. What you’ve got is a space elevator – and to make it work, that string needs to be made from carbon nanotubes.
Of course this is a long way in the future, though a range of companies (including, bizarrely Google) are working on the technology required. There’s no doubt that Bradley Edwards of NASA’s Institute of Advanced Concepts was being over-optimistic when in 2002 he commented ‘[With nanotubes] I’m convinced that the space elevator is practical and doable. In 12 years, we could be launching tons of payload…’ However in a more reasonable timescale – perhaps another 30 or 40 years – it is entirely feasible. And you can’t fault the scope of imagination that allows the inspiration of spider silk to transport us into space.
Next week, in the final piece in the series, we will be learning the lesson of the peacock’s tail and the amazing optics it inspires.
Images from Wikipedia and iStockPhoto.com
Published on September 11, 2012 00:27
September 10, 2012
Gauss and Newton's Apple
One of the greatest mathematicians of all time, Carl Friedrich Gauss, was painfully dismissive of the good old story that Newton dreamed up his theory of gravitation when an apple fell on his head. Gauss remarked:
Silly! A stupid officious man asked Newton how he discovered the law of gravitation. Seeing that he had to deal with a child intellect, and wanting to get rid of the bore, Newton answered that an apple fell and hit him on the nose. The man went away fully satisfied and completely enlighted.
 I'm sorry, Herr Gauss, but that picture really doesn't hold up. Look at the account of the man to whom Newton told the story of the falling apple (no mention of it hitting him), related by the historian William Stukeley:
I'm sorry, Herr Gauss, but that picture really doesn't hold up. Look at the account of the man to whom Newton told the story of the falling apple (no mention of it hitting him), related by the historian William Stukeley:After dinner, the weather being warm, we went into the garden, and drank thea [sic] under the shade of some apple trees; only he and myself. Amidst other discourse, he told me, he was just in the same situation, as when formerly, the notion of gravitation came into his mind. Why should that apple always descend perpendicularly to the ground, thought he to himself; occasion’d by the fall of an apple, as he sat in a contemplative mood.I know I've quoted this before, but I find these words absolutely fascinating, particularly given Gauss's grumpy interpretation. Firstly Stukeley was no fool. Secondly, to dismiss the apple story out of hand is to totally miss the point of the importance of stories in our understanding of the world (a frequent failing among scientists and particularly mathematicians, though not Charles Dodgson).
And finally, this isn't the story of someone fobbing off an irritating passerby, it is an old man reminiscing after dinner with a friend. I am more inclined to Stukeley's picture of events and to give Herr Gauss a firm kick up the rear.
Thanks to the Royal Society you can view Stukeley's original hand-written words in his Memoirs of Sir Isaac Newton's Life.
Published on September 10, 2012 00:01
September 7, 2012
Vive la France!
I was recently a little rude about the French habit of adding a new requirement for drivers every year. But I ought in all fairness say that practically every time I go to France I spot something and think 'That's brilliant! Why don't we do that?' And it almost always involves taking something simple and familiar and giving it a twist.
In the past it has been things like interspersing motorway service stations with cheap to build stopping places that just have loos and picnic facilities. Or making it possible to screw off crown corks on beer bottles - sheer genius.
 Boring British RDS display
Boring British RDS display
The latest was something simple but impressive. When we cross the channel we tend to press the button on the radio that finds a selection of stations, to get some authentic French sounds. And many of those French radio stations were doing something very clever.
With an RDS radio, as fitted in most cars, a typical British radio station will display something like BBC R4. To the point, useful... but not awe inspiringly informative. What the French stations were doing was changing the RDS tag with every track. So it would read something like:
RADIO X - Pink Floyd - Welcome to the Machine (1975)
Wonderful! Taking a very basic bit of technology and transforming it. Of course the RDS display can't show all this at once, but they are designed to scroll when the text is too long. My suspicion is the UK stations don't do this because it takes away one of the few advantages of DAB radio, which still has practically no penetration into the car market - rather worrying when they keep threatening to take the analogue signal away. But they should make more of RDS.
Nice one, France.
In the past it has been things like interspersing motorway service stations with cheap to build stopping places that just have loos and picnic facilities. Or making it possible to screw off crown corks on beer bottles - sheer genius.
 Boring British RDS display
Boring British RDS displayThe latest was something simple but impressive. When we cross the channel we tend to press the button on the radio that finds a selection of stations, to get some authentic French sounds. And many of those French radio stations were doing something very clever.
With an RDS radio, as fitted in most cars, a typical British radio station will display something like BBC R4. To the point, useful... but not awe inspiringly informative. What the French stations were doing was changing the RDS tag with every track. So it would read something like:
RADIO X - Pink Floyd - Welcome to the Machine (1975)
Wonderful! Taking a very basic bit of technology and transforming it. Of course the RDS display can't show all this at once, but they are designed to scroll when the text is too long. My suspicion is the UK stations don't do this because it takes away one of the few advantages of DAB radio, which still has practically no penetration into the car market - rather worrying when they keep threatening to take the analogue signal away. But they should make more of RDS.
Nice one, France.
Published on September 07, 2012 01:02
September 6, 2012
Infinity just got bigger
 One of the books I enjoyed writing most was A Brief History of Infinity, so when I got a chance to write an illustrated Introducing Infinity I jumped at the chance. It's now sunning itself in the shops for your attention.
One of the books I enjoyed writing most was A Brief History of Infinity, so when I got a chance to write an illustrated Introducing Infinity I jumped at the chance. It's now sunning itself in the shops for your attention.Part of the 'graphic guide' series it combines easy-to-absorb bite-sized chunks of text with superb illustration by Oliver Pugh (an all round nice guy). The pictures are very much part of the story, rather than being quick illustrations on the side - we worked closely together to ensure they got across the message.
As for infinity - it's one of the subjects that simply boggles the mind, but there are some great human stories from its history, whether you look back at the likes of the Ancient Greeks and Galileo, follow the calculus wars between Newton, Leibnitz and Bishop Barclay, or take on Georg Cantor and his amazing visual proofs that there is more than one type of infinity, with one bigger than another.
In the end, though, it's the mind-bending paradoxes you keep coming back to, and I've a number of them in there, from covering the number line with umbrellas, though Hilbert's infinite hotel, to the remarkable Gabriel's horn, which you could fill with just pi units of paint, but would take an infinite pot of paint if you wanted to cover its surface.
 I have to admit a particular delight for me was that Oliver included both photos and line drawings of me as a kind of narrator when there isn't a historical character in the scene. I couldn't resist including one here - which I think illustrates the style well.
I have to admit a particular delight for me was that Oliver included both photos and line drawings of me as a kind of narrator when there isn't a historical character in the scene. I couldn't resist including one here - which I think illustrates the style well.You can find out more - or buy the book from Amazon (please!) - at its web page, and it is also sharing a Facebook page with its big brother.
Published on September 06, 2012 01:40



