Piotr Naskrecki's Blog, page 6
September 3, 2013
A song of ancient Earth

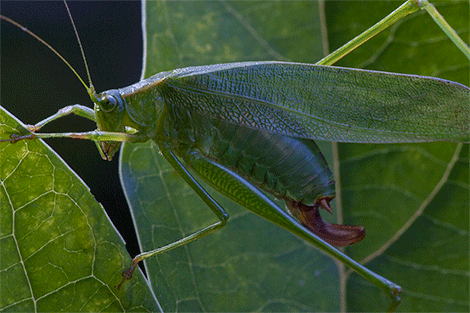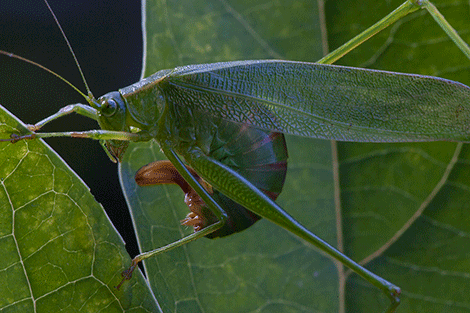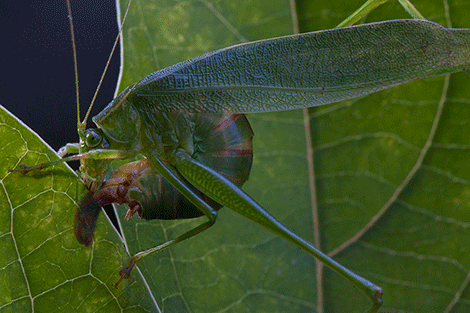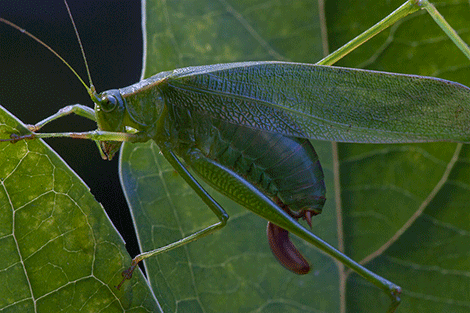
The Greater Grig (Cyphoderris monstrosa) from the Pacific Northwest.
Those who have been reading this blog with some regularity may have noticed that I find virtually all organisms equally fascinating. But some are more equal than others, and few animals and plants excite me more than phylogenetic relics. These are the last remaining members of lineages that were once dominant, or at least species-rich, but are now represented by only one or a few surviving species, species that still carry the old (plesiomorphic) versions of many of the organism’s characters. Such relics are often relegated to living in places that are inhospitable to their evolutionarily younger relatives, but are able to thrive thanks to their ability to handle extreme conditions (albeit this ability is often a relatively recent development). And though I am now in Gorongosa National Park, a place bursting with all kinds of fantastic African wildlife, before I resume my Mozambique Diary I first must recount an interesting encounter with a Mesozoic singing relic that I had only a few days before leaving for Africa.

Tall Mountain hemlocks (Tsuga mertensiana) are the preferred singing perches of C. monstrosa.
Nobody really knows who the first animal singers were. Chances are that the first acoustically active animals were aquatic — grunting placoderm fish, stridulating trilobites, or perhaps pincer-snapping eurypterid sea scorpions — they all have modern equivalents that produce sound underwater. But on land the first organisms that broke the silence were almost certainly small arthropods, whose bodies, encased in rigid plates and tubes of the chitinous exoskeleton, were perfectly suited to become percussive instruments. It is quite likely that a defensive sound production was the initial reason for the evolution of animals’ acoustic behavior, but it did not take long for them to start using sound as an effective attractant during courtship. The unquestionable leading voices of invertebrate love chorus are, and probably have always been, the orthopteroid insects – katydids, crickets, and grasshoppers. Their Permian and Triassic ancestors already had quite sophisticated sound-producing organs on their wings and must have dominated the soundscapes of the Pangean supercontinent. Wings of these fossil insects are often preserved with exquisite detail, allowing us to speculate about the kind of sound they made, and who their modern successors might be. In fact, thanks to a recent study by Jun-Jie Gu you can now listen to a Jurasic song of a katydid ancestor. A handful of direct descendants of these ancient singing insects, members of the largely extinct Mesozoic family Prophalangopsidae, or grigs, are still alive today, and their songs are quite similar to those of their long-gone forbearers.

Male Greater grigs usually call while sitting upside down directly on a tree trunk, which makes it easier for females to find them. They are highly territorial and will defend their singing perches from other males.
A few species of grigs can be found in cold, mountainous habitats of eastern Siberia and central China, and three additional ones live in the mountains of western US and Canada. Virtually nothing is known about the Asian species, but the North American ones have inspired research for decades, and we now know more about their behavior than about that of almost any other singing insect on the planet. And what fascinating behavior it is — these innocent-looking insects combine the love of freezing weather and cannibalism with a lust for virgins.
Last week I was in Seattle and decided to try my luck at finding the largest North American grig, Cyphoderris monstrosa. I had precious little time to do this and thus my only option was to look for it in the Cascades, about 70 miles W of the city. Alas, all I had to go by was a single record from 1909, with a vague description of the locality called Stampede, and I figured that it had to be the same place as what is now known as the Stampede Pass, an area covered with thick forest of hemlocks and pines on steep mountain slopes – just the right place for grigs. I got there in the early afternoon but the place did not look particularly inviting – every single road sign was pockmarked with shotgun blasts, what I took for colorful flowers on a meadow turned out to be piles of shotgun and handgun shells, and nearly all roads leading to what looked like a promising grig habitat had a “No trespassing” sign. Hoping that I will make a difficult target in a rainy night I decided to wait until dark and then search for the insects.

A closely related species, the Sagebrush grig (Cyphoderris strepitans) from Wyoming, is found low to the ground in sagebrush meadows of the Rockies.
The first grig started calling around 10 pm. His call was a high-pitched but rather pleasant warble, somewhat akin to the ring of an old-fashioned telephone. It was coming from a tall hemlock, and I had no other option but to start climbing. Thankfully, grigs are not particularly skittish, and once I located the male I had no troubles getting close to him. I recorded his call and then quickly grabbed him.
In the majority of the animal kingdom the male provides little more than reproductive cells that inseminate the female’s eggs, and hence the profusion of elaborate displays of masculine virility designed to convince the female of the male’s good genes, rather than his suitability for lasting partnership or shared investment in offspring. Usually, all the gullible female gets is a brief, entertaining show and the knowledge that she will never see the guy again. Not so in grigs. The females in these animals want to take home more than just a memory of a good time; they also demand a decent meal, something that will significantly contribute to the production of eggs. Because grigs literally live in their food, a male offering a piece of hemlock or pine, no matter how fresh and tasty, would certainly be given a cold shoulder. Having nothing else to give, the males are forced to make the ultimate sacrifice — they offer females parts of their own bodies to dine on. Like most insects, male grigs have two pairs of wings. The first pair, slightly hardened and modified into sound organs, is used to produce a song that guides the female to him. The second pair, however, is somewhat dispensable, and it has evolved into a pair of big, fleshy organs filled with the male’s blood (hemolymph), and this is what the female grigs lust for.

During mating the male grig locks the female in place on his back with an insidious trap on the tip of his abdomen. Female grigs are completely wingless.
Once a female locates an interested male, she climbs on his back and immediately starts devouring his wings. While she is busy with her cannibalistic hors d’oeuvre, the male attaches his reproductive organs to those of the female and transfers a packet of sperm. He also leaves with the female a package of nutritious carbohydrates and proteins, known as the spermatophylax, which the female consumes after the mating. It seems that the role of these nuptial gifts is to ensure that the sperm he delivered has enough time to get where it needs to, and it also precludes her from mating again with another male, at least until she is done eating. More importantly, his edible wings and the spermatophylax provide the female with nutrients that she will be able to use to produce her eggs, making the male an active and invested participant in parenthood.

This singing Sagebrush grig male has already lost his virgin hind wings and must count on the females’ inability to distinguish his call from that of a virgin male.
Although both the male and the female will soon be ready to mate again, the male now has a problem — he is not ready to retire quite yet, but he no longer has the tasty wings that attracted his first partner. Luckily for him females are not very good at distinguishing the song of an unmated male from that of one who has already lost his virgin wings (but there is evidence that the females preferentially mate with virgins). And once she climbs the back of a male and realizes that he no longer carries the tasty snack, it is too late. Male grigs have evolved an ingenious, if somewhat insidious, device on their abdomen, appropriately called the gin trap, which effectively locks the female in place and gives him extra time to transfer his reproductive cells. It takes a female a considerable amount of effort to disengage from the male, and there is plenty of time for him to pass on his genes.
I stayed in the forest until midnight, tracking, recording, and photographing grigs. By that time the temperature had dropped significantly and a freezing rain was pouring, which made for very compelling arguments to get back into the car and drive back to Seattle. But I was giddy with excitement of having found the grigs and, an unexpected bonus, not having been shot at.

A portrait of the Greater grig (Cyphoderris monstrosa).

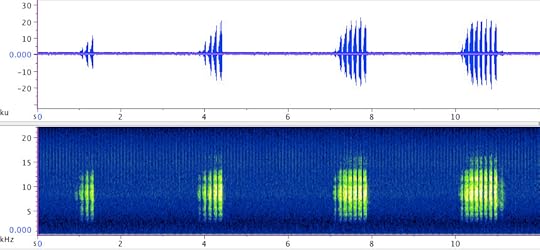
Two males of C. monstrosa calling. Click here to listen to the recording.
Filed under: Behavior, Orthoptera

August 24, 2013
Empusids

A portrait of a male empusid Idolomorpha dentifrons from Gorongosa National Park, Mozambique. This photo is a composite of four vertical frames.
In about a week I should be back in Mozambique and this blog will likely get more interesting. We have an exciting project developing in Gorongosa National Park, one that is bound to generate a lot of good data and influence biodiversity science in the country for years to come. More about it soon. But in the meantime I thought I would put a spotlight on a relatively little known group of organisms that one can easily see in Mozambique – the empusid praying mantids.
The family Empusidae is a small lineage of mantids, with only 28 described species found mostly in drier regions of Africa and a handful of additional species in southern Europe and SE Asia. It is one of the very few families of mantids known to be monophyletic, and it shows – they all share remarkable morphology that makes them stand out among other members of this singular order of insects. Two main body types are common – they are either thin and stick-like or, while still being rather spindly, the body is covered with large lobes and flaps, making them excellent mimics of dried, shriveled leaves.
The stick-like variety, such as the genera Empusa, Hemiempusa, and Idolomorpha, are usually found in grassy vegetation, where they hunt small insects, such as planthoppers and grasshopper nymphs. Earlier this year I ran across a gorgeous male specimen of Idolomorpha dentifrons on the Cheringoma Plateau of Gorongosa, but had troubles photographing it in a way that would properly convey its incredibly elongate morphology. In the end I took a series of vertical photos of its head and front legs that I stitched together in PS, and here is the result. Male empusids are unusual in having pectinate antennae, the kind usually seen in silk moths and other insects with well-developed pheromonal communication, where the female emits sex pheromones and males follow the faint scent trail. Not surprisingly, such behavior was recently demonstrated to be present in empusids (Gemeno et al. 2005. J. Ins. Behav. 18: 389-403).
The leaf-like morphology can be seen in the Devil’s mantis (Idolomantis diabolica), arguably one of the most striking and beautiful praying mantids in the world. The body of immature individuals resembles a dry, withered leaf, except for the brighter colors on the underside of the raptorial front legs. Adults turn pale green and white, and the pattern on their front legs becomes brightly red, resembling vivid petals of a flower. There is a reason for this – Devil’s mantids are specialized hunters of pollinators, such as bees and butterflies, and presumably this bright coloration fools some insects into coming dangerously too close.
Devil’s mantids are known to occur in woodland savannas of Kenya and Tanzania, but on my recent visit to the National Natural History Museum in Maputo I discovered in its entomological collection a few ancient specimens from various parts of Mozambique. One had been collected in the 1960′s tantalizingly close to Gorongosa. Knowing that there is a chance of being able to see this gorgeous creature in its natural habitat, I am now obsessed with trying to find it. I didn’t succeed on my recent visit to Mozambique, but maybe I’ll have better luck this time. Watch this space.

Nymphs of the Devil’s mantis (Idolomantis diabolica) resemble dry, shriveled leaves, which allows them to blend among the vegetation where they hunt flying insects. Interestingly, this species is not interested in slower insects and those that walk or crawl on the vegetation – the prey must be flying really fast to elicit this predator’s response. (This species has recently become popular in the pet trade, and this photo shows a captive individual.)

The Cape empusid mantis (Hemiempusa capensis) from South Africa devouring a grasshopper.

Male empusid (I. dentifrons) cleaning his pectinate antennae.
Filed under: Cheringoma, Gorongosa, Mozambique, Praying mantids


August 18, 2013
They can count, too

A male Treetop bush katydid (S. fasciata) from Woburn, MA.
In March of 1882 a little known journal that had been founded only two years prior was about to go under – nobody wanted to read it, and its owner was tired of putting any more money into it. But an enthusiastic entomologist named Samuel H. Scudder, who at that time, after many years of doing various unpaid jobs, was finally holding the prestigious position of an Assistant Librarian at Harvard University, had recognized the potential of that publication and decided to save it. He edited the journal until 1886, ensuring that it would gain prominence and a much greater readership. Thanks to his enthusiasm the journal had survived, and later became somewhat influential in the academic circles.
But in addition to his penchant for saving obscure publications from oblivion, Scudder was one of the most prolific and influential American zoologists. His list of papers and books includes 791 titles (!), and many of them deal with my favorite group, the Orthoptera. Because of his contribution to the taxonomy of orthopterans, 24 species of grasshoppers and katydids carry his name (scudderi), as does one genus, the elegant Scudderia, also known as the Bush katydid.

A female Fork-tailed bush katydid (S. furcata) ovipositing between the two layers of a leaf’s epidermis.
I always knew that one species of Scudderia was common in my backyard, the Fork-tailed katydid (S. furcata), and its short, high-pitched clicking calls can be heard almost every night from the deck of the house. But a few nights ago I noticed a different call coming from the garden, and was pleased to find a new neighbor – the Treetop bush katydid (S. fasciata). Bush katydids are graceful, delicate creatures that feed on leaves and flowers of a variety of plants, and in our garden they seem to be particularly fond of bee balm flowers and a few other ornamentals. They spend their entire lives either high in the canopy or in tall bushes, and never descend to the ground. Female bush katydids lay eggs on the vegetation, but do it in a truly masterful way. Rather than laying eggs on the surface of leaves or bark, which many arboreal katydids do, they perform the almost impossible feat of splitting the leaf in two – along its thin edge! – and insert the eggs between the two layers of the epidermis. Obviously, in order to fit there, the eggs must be really thin, and in fact they are so thin as to be virtually translucent when they are initially deposited. They harden and darken as they age, but remain remarkably two-dimensional. Well hidden inside the leaf’s tissue, eggs of Scudderia are probably at a much lower risk from parasitoid wasps, which often wipe out most of katydid egg clutches.
[image error]
The counting katydid, Scudderia pistillata – males of this species add one syllable to each subsequent “click”, and the male with the highest number of syllables has the highest chance of attracting a female. This individual is from Sanbornville, NH.
Bush katydids have also one very interesting feature – they apparently can count. Males of the Broad-winged bush katydid (S. pistillata) produce several types of call, but only one is designed to attract females. It usually starts with a typical single phrase, with only a few syllables (to the human ear these sound like a slow “chirp”), but each subsequent phrase adds one more syllable. It has been shown that females preferentially mate with males who produce the highest number syllables at the end of a calling bout. This is probably because longer phrases require more energy to produce, and thus are an honest indication of the male’s health and the ability to invest in his offspring.
Bush katydids are hardy creatures, and I sometimes find them well into late October. Their pretty nymphs are also some of the first katydids to appear in the late spring. They can be identified by their color patter, which often has vivid emerald and orange accents, and by the presence of a small “cone” on the top of their head (this “cone” disappears in adult katydids).
This is how it’s done: the female first chews off the edge of the leaf, and then uses her mouthparts to carefully guide her ovipositor in between the two layers of the epidermis.
Not surprisingly, the beautiful Scudderia is one of my favorite genera of North American katydids, and its members are deserving bearers of the name of one of America’s greatest naturalists. Oh, and the journal that Samuel Scudder saved from oblivion? It was Science.
[image error]
Nymphs of Scudderia can be recognized by their bright, emerald green coloration and the presence of a small “cone” on the head.
The song of S. pistillata – notice how the number of syllables increases in each subsequent phrase. Click here to hear it. (Based on a recording from the Singing Insects of North America.)
Filed under: Behavior, Katydids

August 13, 2013
Say’s trig

A male and a female of the Say’s trig (Anaxipha exigua) from Woburn, MA.
Yesterday my wife called me – “You need to come to Mahoney’s [our local garden center], there are tree crickets on every Holly bush.” I promptly grabbed a few containers and was there in a matter of minutes. And indeed, the place was resonating with soft, bell-like calls of dozens of crickets, but I did not recognize the species. I spent about 20 minutes looking for them, eliciting confused stares from the staff and customers, but could not locate any singing males. In desperation I shook a few bushes, and eventually a female tree cricket (Oecanthus) flew out of one. But I was not convinced that this was the genus that was singing there; the call was just not very tree cricket-like.
I returned the following evening, armed with a shotgun microphone and headphones, intent on locating the callers. The staff of the center was apparently on the verge of kicking me out after watching me waving the long microphone around the shrubs like some deranged Dumbledore wannabe, but Kristin managed to placate them and so they left me alone. But even with the ability to pinpoint each caller, finding the crickets was very tricky, and it took me almost an hour to finally catch a couple.
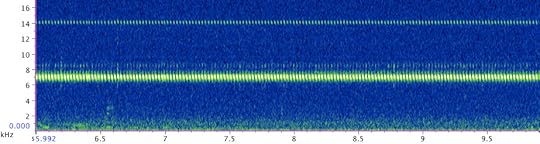
The call of the Say’s trig (click here to hear it)
The mystery insects turned out to be not tree crickets but much smaller, and orders of magnitude more agile, Say’s trigs (Anaxipha exigua), named after Thomas Say, the prolific 19th century entomologist and malacologist, and the discoverer of this species. I had never seen a Say’s trig before, and was happy to add both the recording and photos to my database of local orthopterans. Anaxipha is a large genus of the cricket subfamily Trigonidiinae, with 135 described and a bunch of yet undescribed species, found mostly in the tropical and subtropical parts of the globe. The Say’s trig, along with the Handsome trig (Phyllopalpus pulchellus), is one of the few species of the group reaching as far North as Massachusetts. The call of the Say’s trig is an almost pure tune trill, with the loud portion at exactly 7 kHz, and a softer harmonic at 14 kHz (click here to listen to the recording).
Filed under: Crickets, New England, Orthoptera


August 7, 2013
Sapo

A pair of Giant leaf frogs (Phyllomedusa bicolor) from Suriname, which I mistook for a four-eyed chimera.
It may seem counterintuitive, but many small, cryptic animals, those that blend perfectly into their environment, are much easier to find at night than during the day. Katydids, walking sticks, snakes – these animals usually spend their days absolutely still, but at night they feed and court, making their presence known through their movement. Others, including moths and spiders, can be spotted at nigh by their eyeshine, reflected in the light of a headlamp. Over the years I have learned how to recognize some of the animals by the color and size of the glow of their eyes. For example, small green eyeshine in the grass – lycosid spiders, large green in the savanna – antelopes (or cats), small orange in a tree – moths, large orange in the water – crocodiles etc. It goes without saying that the eyeshine always occurs in twos (even eight-eyed spiders display eyeshine only in their two largest eyes), or as single eyeshine, when the animal is not facing the viewer.
I was therefore quite confused when during a night walk in the rainforest of Suriname a few years ago I spotted four, large and brightly glowing orange eyes in a tree in a distance. They were moving in unison, clearly attached to one body, and by their diameter and spacing I figured that the animal had to be at least the size of a large rat. I had to find out what it was.

A female and a male of P. bicolor from southern Suriname.
Of course, once I got closer and was able to put the spotlight on the entire animal, the truth turned out to be somewhat less exciting than a four-eyed chimera, but interesting nonetheless. The strange creature was a pair of Giant leaf frogs (Phyllomedusa bicolor) in amplexus – the male firmly gripping a much larger female – moving slowly towards a branch overhanging a stream, where the female clearly intended to lay her eggs. As their name implies, these frogs are huge. I am not absolutely sure, but I believe that they might be the largest tree frogs in the world, easily reaching the length of 5 inches or so. Like other members of the genus Phyllomedusa they spend their life in the trees, and lay egg clutches on leaves overhanging forest streams or other small bodies of water.

A clutch of freshly laid eggs of P. bicolor.
And indeed, in the morning I found a fresh clutch of eggs glued to leaves over the stream. The two animals were still hanging nearby, the female noticeably thinner than the night before. I took some photos of the pair, all along wondering if I should try to lick them. In the end the smaller, more responsible part of my brain prevailed, but to this day I am curious what would have happened.
The skin of Phyllomedusa bicolor (“Sapo”) is full of potent peptides, such as phyllocaerulin, phyllomedusin, and dermorphins, all of which have incredibly powerful effect on humans. On smaller animals, such as rodents, the effect is simple – it kills them. But to something the size of a man the same compounds are not lethal. Rather, after an initial, very unpleasant reaction that includes violent vomiting and passing out, you apparently wake up refreshed, much stronger, resistant to hunger and thirst, and with heightened senses and the ability to cope with stress. The Matsés Indians of Peru and Brazil have known that for a long time, and they routinely use the extract of the Sapo skin before hunting expeditions. Unfortunately, the delivery method of the extract does not sound very pleasant, to either the man or the beast – they first burn their own skin, then peel it off (!), and only then rub in the extract, which has been obtained by, essentially, torturing the poor frog for three days. (The entire process is described in excruciating detail in a paper by Erspamer et al. 1993. Toxicon 31: 1099-1111.)

Three species of Leaf frogs (Phyllomedusa) found in Suriname (from the left clockwise): P. vaillantii, P. tomopterna, and P. bicolor. These frogs are also known as monkey frogs, on the account of their grasping hands and the ability run up vertical lianas.
But helping people get high is not the only reason why Phyllomedusa frogs have evolved this amazing pharmacopeia. I did a quick search on MEDLINE and found 167 research papers on various compounds present in their skin, including both those that serve as predator repellents (e.g., alkaloids, quinones), and a vast array of antimicrobial, antifungal, antiviral, and even cancer-suppressing peptides. These may lead to the development of new treatments for assorted ailments afflicting the humankind, and hopefully means that sooner or later I will be able to experience the positive effects of the “sapo” without having to peel off my singed skin.

Tiger monkey frog (Phyllomedusa tomopterna) from Suriname.
Filed under: Amphibians, Frogs


August 2, 2013
Scorpionflies

A male scorpionfly (Panorpa acuta) from Estabrook Woods, MA.
The Estabrook Woods near the town of Concord, MA have over the years become my favorite local place to find interesting insects and ancient plants. It is also a great location to let my dogs exercise their primeval desire to chase little furry things (always unsuccessfully) and wade in stagnant, swampy water. Recently I wrote about my failed search there for scorpionflies (although one that resulted in finding something equally interesting), but this past weekend I had much better luck, and was able to see and photograph these elusive insects in action.
Why the obsession with scorpionflies? To begin, scorpionflies (or rather their immediate ancestors) carry the distinction of being some of the oldest insects with the complete metamorphosis (Holometabola). Permian panorpoids, dating back at least 290 million years, but already shockingly similar to modern species, eventually gave rise to not only scorpionflies (Mecoptera), but also the now dominant flies (Diptera) and butterflies (Lepidoptera), plus a few smaller orders. Cretaceous fossils of scorpionflies are so similar to current species that I doubt I would be able to spot anything unusual if one flew right in front of me.

A female scorpionfly feeding on a dead moth. Unlike their close relatives, hanging scorpionflies (Bittacus), members of the genus Panorpa usually feed on insect carrion rather than trying to catch live ones.
But scorpionflies, especially those of the genus Panorpa that I have been searching for, are also incredible in their reproductive behavior. Many insects have elaborate courtship, with males (usually) displaying awesome ornaments, crazy behaviors, or feats of strength, all to impress a female. One fairly common way of wooing her is to present the female with a nuptial gift, often in the form of a prey item, or a packet of carbohydrates and protein attached to the spermatophore (the spermatophylax). Male scorpionflies, however, somehow manage to make themselves desirable to females by offering them a big glob of their own spit. For this reason they have evolved exceptionally large salivary glands, much bigger than those of the females. (This is, by the way, an excellent example of the randomness of sexually selected characters.) In most species of Panorpa a male may also get lucky if he offers the female a tasty dead insect or try to force her to mate by brutal force, but nothing beats a nice, juicy ball of spit. So enticing is this nuptial gift that males of some species of Panorpa feel confident enough to produce sexual pheromones, something that usually only females of insects do, and nothing gets a female scorpionfly more excited than a whiff of the aldehyde (2E,6Z)-nona-2,6-dienal For Men.
[image error]
A male and a female of Panorpa acuta – the male’s scorpion-like “tail” carries his genitalia and a so-called notal organ, which helps him hold the female’s wings during copulation.
A recent study (Engqvist, L. 2011. Behavioral Ecology 22: 345-349) showed an intriguing relationship between the attractiveness of the male and his investment in mating (as measured by the duration of the copulation and the quality of the nuptial gift, which in turn are directly related to the quality of the offspring). The study showed, perhaps counterintuitively to some, that the more attractive the male the less invested in the mating he is, including presenting the female with a nuptial gift of subpar quality. On the other hand, nerdy males offer both a better mating experience and a bigger nuptial gift to the females. Think about it.

A female scorpionfly breaking off a piece of the moth’s body and chewing it with her sharp mandibles.
Filed under: Behavior, Mecoptera


July 25, 2013
My crumbling beliefs

A poison arrow frog (Oophaga pumilio) next to a timber fly (Pantophthalmus sp.) on a palm leaf at La Selva Biological Station, Costa Rica.
I always assumed that there existed at least a few immutable truths about the natural world, dogmas that had no exceptions, no matter how hard you looked for them. One of them, I thought, was the rule that flies, which cartoons and children books made me believe were the favorite food of frogs, should be significantly smaller than the frogs. And I pretty much accepted this gospel, until one day I found in the rainforest of Costa Rica, sitting on a palm leaf, a frog and a fly. This singular sighting shattered my entire belief system, or at least the part that pertained to frogs and flies. Because you see, the fly was a timber fly, a member of the family Pantophthalmidae, which are the largest flies in the world, some reaching the wingspan of 100 mm. Next to one of these monsters an adult poison arrow frog looks almost like a dog next to a horse.

A female Pantophthalmus bellardii from Paloverde, Costa Rica; notice the partially retracted, telescopic ovipositor.
Timber flies are a small family, consisting of only 2 genera and 22 species, all found in the lowland rainforests of Central and South America. In addition to their unholy size they differ from other flies in that their larvae are wood burrowers, something that traditionally has been the domain of longhorns and other beetles. There are other flies that feed on wood (some Syrphidae and Asilidae), but those are incapable of drilling their own tunnels in the wood and can only use those already created by beetles or other insects.
Little is known about the behavior of adult timber flies. Nobody is really sure if they feed at this stage, and if so, on what. They have never been seen mating, although oviposition has been observed. Females have a long, telescopic ovipositor, which they use to deposit eggs in the cracks of dead and live wood, depending on the species. These insects are not common – in all my years in the tropics I have only seen them four times, but each time my faith in the unshakable laws of nature suffered a bit more. What’s next, a land arthropod as big as a cat?

A portrait of Pantophthalmus cf. pictus from Guanacaste, Costa Rica.

A female Pantophthalmus bellardii.
Filed under: Flies, Frogs


July 23, 2013
Hanging in there

A male of the common scorpionfly (Panorpa acuta) from Estabrook Woods, MA; notice the scorpion-like tip of the abdomen.
Things have been keeping me away from updating the blog, but I finally found a moment to write about one cool animal that usually shows up at this time of year in Massachusetts – the scorpionfly (Panorpa). Or at least that was the original plan. Since I had, literally, only one, rather lousy shot of this animal, I decided to visit a certain patch of vegetation where I used to see them quite frequently, and photograph some.

Hanging scorpionfly (Bittacus strigosus) from Estabrook Woods, MA.
Scorpionflies (Mecoptera) are an oddball order of insects, one of the smaller ones, with only about 550 known species (one lineage of Mecoptera has already been featured on this blog.) They are unassuming in their appearance, but what they lack in looks they amply make up for in amazing behaviors and an impressive evolutionary history. Their common name comes from the peculiar morphology of the male’s abdomen in the genus Panorpa, which displays an uncanny resemblance to a scorpion’s “tail” (telson). The difference lies in what sits at the tip of the “tail”– in scorpions it ends with a deadly stinger, in scorpionflies it carries a pair of oversized genitalia.
Last Sunday I drove to Estabrook Woods and started searching for scorpionflies. But I wasn’t having any luck – there were none and clouds of mosquitos were making me miserable. And those annoying crane flies (Tipulidae) were everywhere, flying all over as if they owned the place. In their flight pattern they were confusingly similar to scorpionflies. Suspiciously too similar.
One landed near me, and only then did I realized that those were not crane flies at all, they were indeed scorpionflies! But they were not Panorpa, the common kind. They were hanging scorpionflies (Bittacus), which up to that point I had only seen in China and Mozambique, and it did not occur to me that they might be also present in my neck of the woods.

Hanging scorpionflies (Bittacus sp.) from Sichuan, China, with moth prey.
Hanging scorpionflies do look very much like crane flies, but one major giveaway is the presence of two pairs of wings, as opposed to only one pair present in the Tipulidae and other true flies. The origin of their common name becomes immediately obvious once you see them in their hunting position – they suspend themselves from vegetation by their front legs, keeping the second and third pairs outstretched, ready to snag any insect that flies close enough. Their feet have strong muscles and the scorpionflies are able to use them in a grasping fashion, somewhat similar to the way we use our hands, by closing the fifth tarsomere against the fourth one. The downside of having such grasping hands is the scorpionflies’ inability to walk on horizontal surfaces, and thus they display only two modes of behavior – flying and hanging.

The head of Bittacus strigosus; notice the characteristic, elongated mouthparts, typical of most scorpionflies.
Species of Bittacus feed on all kinds of flying insects and are capable of overpowering seemingly stronger, more muscular ones, such as noctuid and geometrid moths. The prey is killed and devoured with their elongate, scissor-like mandibles that form an elongated rostrum. During the mating season males of some species use their freshly captured prey as a nuptial gift, offered to the female during courtship, and the larger the prey they can offer the longer and more successful the mating. But not all males play fair. A small proportion of so called “transvestite” males approach those males who already hold a prey item, and wiggle the abdomen seductively in a way a receptive female would. Often they are able to convince the honest suitor to hand over the nuptial gift, leaving the sucker confused and ashamed of himself. Other, lazier males, don’t bother with such a nuanced approach, and simply wrestle the food away from courting males, or steal it from mating pairs.

A hanging scorpionfly (Bittacus sp.) from Gorongosa National Park, Mozambique, showing the main difference between scorpionflies (Mecoptera: Bittacidae) and crane flies (Diptera: Tipulidae) – the presence of the second pair of wings.

A hanging scorpionfly in a characteristic hunting position.

A raptorial tarsus of Bittacus strigosus.
Filed under: Behavior, Gorongosa, Mecoptera


July 11, 2013
A portrait of a marine iguana
Sharlena Wood, a Canadian artist whose beautiful paintings have already been featured on this blog (here and here), did it again. Using a charcoal drawing technique she produced an outstanding portrait of a marine iguana by imaginatively reinterpreting one of my photos from the Galapagos Islands. This drawing is part of a series of portraits of endangered animals, which you should see in its entirety. And while there, look at Sharlena’s other art, you will not be disappointed.
Filed under: Art, Reptiles


July 9, 2013
Helmeted katydids

Helmeted katydid (Phyllophora boschami) from Pogera gold mining camp in the Enga Province of Papua New Guinea.
Porgera, a gold mine in the highlands of Papua New Guinea, is not a pleasant place for a biologist, especially if you are aware of the massive environmental damage of its operations, or the frequent human right violations that this mining camp is known for. But we had no choice but to sleep with the enemy, and ask for the mine’s help in helicoptering us into the remote highlands of PNG where our team was surveying animals and plants. Luckily, the heavily guarded fortress of Porgera had one redeeming quality – at night I was allowed to wander around our sleeping quarters and look for insects. This, being New Guinea, guaranteed that even a place as dismal as a mining camp was bound to reward me with something interesting. And I was not disappointed – not only did I find a lot of insects, but one of them turned out to be new to science. (Yes, I am aware of the irony of a mining camp being the type locality and the site of the only known population of Pandangraecia porgera Naskrecki and Rentz, 2010.) But the insect that really stood out in the crowd was a katydid Phyllophora boschmai, a member of a remarkable lineage of my favorite insects.

The body of helmeted katydid nymphs, like in this Phyllophora sp. from the Eastern Highlands Province of PNG, is almost entirely covered by the helmet-like pronotum.
The Phyllophorinae, known as the helmeted katydids (the names hooded katydids or box katydids are also used) are found primarily on New Guinea and nearby islands, although a handful of species venture into northeastern Australia, and a few places in the Philippines, Sri Lanka, and Taiwan. What immediately sets them apart is their size – many species are massive, and one, Siliquofera grandis, is the largest living katydid, with the wingspan of over 8 inches (20 cm)! Their morphology is also unusual – the pronotum, the part of the thorax immediately behind the head, is greatly expanded into a huge, helmet-like structure that covers the thorax and parts of the wings; in nymphs the pronotum may cover the entire body. This type of the pronotum usually indicates that the katydid is an exceptionally loud singer since the pronotum can act as a resonator and an amplifier of the call produced by the sound-producing apparatus at the base of the wings. But this is where things get interesting. If you were to peek under the huge pronotum of a helmeted katydid, you would find nothing. No sound-producing organs, no stridulatory file, nothing. These katydids cannot sing.

Some helmeted katydids, such as this Sasima versteegi from the Western Province of PNG, are huge.
It appears that the huge pronotum is there solely as a defensive mechanism, protecting the softer, more vulnerable parts of the body. Why helmeted katydids have lost the ability to sing is still a mystery, especially considering the fact that they are a highly derived group, closely related to some of the loudest singers in the family, the Mecopodinae. But the real question is, how do males and females find each other?
In katydids, the acoustic communication – attracting each other with a song produced by rubbing their wings – is the only way (that we know of) for the two sexes to get together as these insects lack pheromonal communication or any other way to send long distance signals (some katydids use substrate vibrations to communicate, but this only works over short distances). But helmeted katydids are not entirely silent, either. They have evolved unique stridulatory structures at the base of their legs that allow them to produce loud, rustling noises, which both males and females use as a defense mechanism if they happen to be assaulted by a predator (click here to listen to the defensive stridulation of a helmeted katydid). These structures, however, cannot be used to attract the opposite sex in the dense vegetation of the New Guinea rainforest, and the mechanism by which the sexes locate each other is completely unknown.

The sternocoxal stridulatory apparatus of a helmeted katydid. The first segment (coxa) of the last pair of legs is covered with dense, parallel ridges that rub agains a series of pegs on the surface of mesosternal lobes on the ventral side of the thorax. The sound produced in this way is noise-like, typical of a defensive stridulation.
But it wouldn’t be, if only I had the presence of mind to watch them more closely. While in Papua New Guinea I captured a male and a female of Phyllophora sp., and placed them in a large mesh cage. I kept looking at the pair, waiting for something to happen, but then it was dinner time and I was hungry and, long story short, when I came back the two were already relaxing with a cigarette in hand – I missed the whole thing! And it probably was quite a spectacle – I knew that they had successfully mated because the female now carried what looked like a pair of white testicles, a nuptial gift from the male. In many katydid species the male produces a similar gift, known as the spermatophylax, that the female later consumes. Why they do it is another story, but in an essence this is to prevent her from mating with another partner, and to ensure that the male’s sperm has time to enter her body. In this way the edible spermatophylax, which encapsulates a pair of small vesicles containing sperm, is indeed, from the functional point of view, a pair of autonomous testicles that the male attaches to the female.

What looks like a pair of testicles at the base of a female’s ovipositor is the spermatophylax, a nuptial gift produced by the male during mating. It consists mostly of nutritious carbohydrates and proteins, and is subsequently consumed by the female. Its presence ensures that the male’s sperm has enough time to enter the females genital opening, and effectively prevents her from mating with another partner, at least for some time. The nutrients in the spermatophylax also contribute to the fitness of his and her offspring.
And so the mechanism of the helmeted katydids’ long range communication remains a mystery. I never had a chance to watch these insects again, but hope to be able to return to New Guinea at some point, and finally document their courtship ritual, dinner be damned.
P.S. David Rentz, who also participated in the PNG katydid survey, has revised Australian Pseudophyllinae, and wrote a post about these insects on his excellent blog BunyipCo.

Although mostly diurnal, some species, such as this Phyllophora sp. from New Britain, can be found during the day on the understory vegetation.

A molting female of Phyllophorella woodfordi from the Solomon Islands.

A nymph of Sasima versteegi is a perfect mimic of mossy vegetation of the humid, lowland rainforest of New Guinea.
Filed under: Behavior, Katydids, Orthoptera


Piotr Naskrecki's Blog
- Piotr Naskrecki's profile
- 9 followers





