Piotr Naskrecki's Blog, page 10
March 18, 2013
Mozambique Diary: Back in Gorongosa
One of the first katydids I spotted was a female of Horatosphaga serrifera, an elusive species, known only from a small handful of specimens. This group of katydids is highly sexually dimorphic and males look nothing like this chunky, flightless females. [Canon 14mm, Canon MT 24EX twin light]
Yesterday I arrived in the spectacular Gorongosa National Park in Mozambique. Last time when I was here the place was dry and dusty, but now, at the end of the rainy season the luxuriant vegetation is vibrantly green and after the misery of Boston’s winter the air feels throat-soothingly humid. During the day Gorongosa’s woodlands vibrate with calls of cicadas and grasshoppers, while nights are thick with crickets and frogs, randomly punctuated with blood-curling screams of a bushbaby. On my first stroll after dark I counted eleven species of praying mantids, and immediately ran into three species of katydids that I had not seen during my previous visit. Sandy paths around the main camp of the park are Roman arenas full of carnage indiscriminately dispensed by solifugids and giant Anthia beetles. Every lightbulb along the camp’s roads is dimmed with clouds of moths, dung beetles, and ants, and under each one sits a fat toad, gorging on the seasonal manna. It is heaven.The purpose of my visit to Gorongosa is to lead a month-long survey of plants and animals of the Cheringoma Plateau, the poorly explored eastern rim of the Great African Rift Valley, of which Gorongosa is the southernmost tip. In a few weeks a large group of biologists will descend on the park, and trap, record, photograph, sample, measure, weigh, track, trace, and triangulate every plant, mammal, bird, reptile, frog, dung beetle, ant, katydid, and praying mantis living here. We will leave no stone unturned, no twig unchecked for ants, and no pile of dung uninspected for beetles. I will not be surprised if, once all the collected material is processed and identified, that we might be able to double the number of species recorded from Gorongosa, which currently stands at 1,790 confirmed animals and plants. But before this happens there is still a lot of work to do and tomorrow Marc Stalmans, Gorongosa’s chief scientist and I are leaving on a reconnaissance trip to select the survey’s camp sites.
As a scientist I am absolutely giddy with excitement about what we will find and document, and as a nature photographer I am itching to point my lens at everybody and everything that crosses our path on the Cheringoma Plateau. In preparation for this unique opportunity I had packed my brand spanking new Canon 400mm; a cool new gizmo called NeroTrigger to remotely capture elusive nocturnal animals; a battery of flashes and macro lenses; and a waterproof housing for my camera to get some shots of the underwater life. All in all, really great gear. It is thus rather unfortunate that all of it was lost on my way to Mozambique. South African Airlines gladly took my luggage and a big wad of cash for the extra piece, but somehow forgot about the delivery part of the deal. There is a big Pelican case with $12,000 worth of gear floating somewhere in the nether regions of the aviation industry, and I can only hope that at some point it will resurface and I am reunited with my beloved gear. In the meantime I will do with what I have, perhaps the limitations of my current gear will spur me to be more creative. Watch this space.
![Below my feet, carnage. A big Anthia ground beetle killed another individual and is now gorging on it favorite soft part – the ripped off genitalia. [Canon 180mm, Canon MT 24EX twin light]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785523.jpg)
Below my feet, carnage. A big Anthia ground beetle killed another individual and is now gorging on it favorite soft part – the ripped off genitalia. [Canon 180mm, Canon MT 24EX twin light]
Filed under: Beetles, Katydids, Mozambique


March 13, 2013
Spring grasshoppers
A nymph of the Green-striped Grasshopper (Chortophaga viridifasciata) found today in Woburn, MA [Canon 7D, Canon MT 24EX twin light]
Lately things have been slow on The Smaller Majority blog. This is mostly because I have been crazily busy with preparations for my upcoming trip to Gorongosa National Park in Mozambique, where I will be staying until June. While there I hope to be able to make regular updates to the blog and, if my previous trip were to be any indication of what to expect, there will be a lot of stories and pictures of really cool critters.But despite my busy schedule I simply cannot resist posting a photo of the very first orthopteran of the season, the Green-striped Grasshopper (Chortophaga viridifasciata), which Kristin found today while working in her garden. In the NE US these hoppers are usually the first ones to appear in the spring, thanks to their ability to overwinter as fairly large nymphs. Most orthopterans in our region overwinter as eggs, although there are a few other species that we should be able to see soon, including Pygmy grasshoppers (Tetrix subulata), Sulphur-winged grasshopper (Arphia sulphurea) and, a little later, field crickets (Gryllus veletis).
Green-striped Grasshoppers are quite polymorphic and green, brown, or grey individuals can be found in the same population. In the southern US there may two generations of these insects in a year, but in Massachusetts we get only one.
![An adult male of the Green-striped Grasshopper photographed in May 2012 [Canon 7D, 3 speedlights Canon 580EXII]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381131535i/4317907._SX540_.jpg)
An adult male of the Green-striped Grasshopper photographed in May 2012 [Canon 7D, 3 speedlights Canon 580EXII]
Filed under: Orthoptera


March 5, 2013
African Tuesday: Beware of the snail
A ground beetle (Carabidae: Antiinae) attacking a land snail in Atewa, Ghana [Canon 1DMkII, Canon 100mm macro, Canon MT 24EX twin light]
I had always been under the impression that snails defended themselves mostly with their calciferous shells, and that otherwise they were pretty vulnerable creatures. That changed when I ran across an interesting encounter between a pulmonate snail and a predatory ground beetle (Carabidae: Anthiinae) in the rainforest of Atewa plateau in southeastern Ghana. I did not see the very beginning of the encounter, but I imagine that the beetle attacked the snail, naively expecting no resistance other than a feeble attempt by the snail to pull itself into the shell. But he was in for a nasty surprise. For the next 10 minutes the snail systematically engulfed the beetle in a copious amount of foamy mucus that effectively prevented the beetle from getting anywhere near the vital organs of the snail. In the end the snail simply slipped down the branch in a protective cocoon of foamy mucus, while the unfortunate beetle was barely able to walk away, frantically trying to clean the mucus off its head.
A few minutes later the snail slides away protected with a cocoon of sticky mucus foam
Turns out that these kinds of encounters are very common, and some carabid beetle have evolved strategies for paralyzing their snail prey before the mollusk is able to overwhelm the predator with mucus. Snails constantly produce relatively small amounts of mucus, which helps them slide on the substrate and prevents water loss. But when attacked, many terrestrial snails respond by blowing compressed air from the pulmonary cavity into a slit between the body wall and a fold of the respiratory orifice, while exuding extra amount of mucus. Of course every defense elicits anti-defense, and some carabid beetles have evolved the ability to paralyze the snail with a single bite to the head or posterior part of the mantle, thus stopping the production of the protective mucus foam.

The defeated ground beetle will need to spend many hours cleaning itself.
Filed under: Beetles, Behavior


March 1, 2013
Coneheads
A portrait of Brown-faced Spearbearer (Copiphora hastata) [Nikon D1x, Sigma 180mm]
A fact that entomologists are well aware of, but one that usually comes as a surprise to everybody else, is that most insect species are still unknown to science, and only a relatively small portion of them have been formally named and described. According to recent estimates only about a quarter of currently living species have the distinction of being assigned official, scientific names, and millions (millions!) of species, most of them insects, remain unnamed and unseen. I kind of knew that, but I still did not expect that the very first, very common conehead katydid that I saw during my first visit to Costa Rica, unquestionably the best biologically explored Neotropical country, would turn out to be new to science. And yet it was. I subsequently named it Copiphora hastata, or the Brown-faced Spearbearer, on account of the enormous, spear-like ovipositor, which the females of this species use to lay eggs underneath thick layers of accumulated leaves on the rainforest floor.Central American Pit Bull katydid (Liromoetopum coronatum) lacks the large defensive cone on its head, but makes up for it with sharp, powerful jaws. [Canon 1Ds MkII, Canon 180mm macro]
Conehead katydids (Tettigoniidae: Copiphorini) are some of the most spectacular insects that one is likely to encounter in a tropical rainforest. They are easily recognizable by the presence of a hypertrophied fastigium of vertex or, in other words, a giant cone on the head. The function of the cone varies from species to species. In forms that live in grasslands, the elongated process on the head helps the animal blend in amongst blades of grass, and such species are some of the best plant mimics among katydids. But in arboreal species, especially those that are found high in the trees of the Central and South American rainforest, the cone is a defensive weapon, quite effectively protecting them from aerial attacks by foliage-gleaning bats (Phyllostomatidae). Thanks to this protection, coneheads are some of the few Neotropical katydids that can afford to produce long, continuous calls, which other katydids tend not to do because of the risk of being detected by hunting bats. The situation is rather different in the rainforests of Asia or Africa – those places lack the foliage-gleaning bats, and most katydids serenade to their hearts’ content, and coneheads there usually exhibit rather reduced cephalic armature.Rhinoceros Spearbearer (Copiphora rhinoceros) is an efficient predator, capable of catching and devouring other katydids and even small lizards [Canon 10D, Nikkor 17-35mm, Canon 580EX]
The diet of coneheads is quite varied – it often includes seeds, fruits, caterpillars, snails, other katydids, and even small lizards. Their mandibles are usually very sharp and powerful, and coneheads don’t hesitate to use them on potential predators, such as the fingers of an entomologist foolish enough to try to catch one. In fact, Costa Rican Rhinoceros Spearbearer (Copiphora rhinoceros) is one the few insect species that I am a little afraid to handle with my bare hands. Regardless, coneheads are still some of my favorite organisms. In Costa Rica, where I have been studying the katydid fauna for many years, I recorded nearly 60 species of coneheads, over a third of them new to science. Later this year I will be visiting Belize, and there is no doubt in my mind that the place will be teaming with spectacular coneheads. I simply can’t wait to see them.Vargas’ conehead (Podacanthophorus vargasi) is a small canopy katydid, found at middle elevations in Costa Rica [Canon 7D, Canon 100mm macro, 3 speedlights Canon 580EXI
![Brown-faced Spearbearer (Copiphora hastata), a large rainforest species that I discovered on my first visit to Costa Rica [Canon 7D, Canon 100mm macro, 3 speedlights Canon 580EXII]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381080378i/4116518._SX540_.jpg)
Brown-faced Spearbearer (Copiphora hastata), a large rainforest species that I discovered on my first visit to Costa Rica [Canon 7D, Canon 100mm macro, 3 speedlights Canon 580EXII]
Filed under: Katydids, Macrophotography, Orthoptera


February 26, 2013
African Tuesday: Heelwalkers
The first live heelwalker ever photographed – The Gladiator (Tyrannophasma gladiator) from Brandberg, Namibia, which I photographed in March 2002 [Nikon D1x, Sigma 180mm]
After ice crawlers (featured in a recent post) were officially recognized as a new order of insect in 1915, entomologists pretty much assumed that this was it, and no more discoveries of such magnitude were expected. After all, an order is a major unit of classification – elephants, turtles, and flies are examples of orders – and so it was met with great skepticism when in 2002 another new order of insects was announced to the world by a team of German and Danish scientists. These strange new animals looked remarkably similar to ice crawlers, with their elongate, completely wingless bodies. But in their biology and behavior they could not be more different. The new insects, christened with the catchy moniker Mantophasmatodea, were found on the opposite side of the globe in the scorching deserts and shrubby vegetation of southern Africa. They were fast and agile and, unlike ice crawlers, predaceous and incredibly voracious.* They also walked funny, with the tips of their feet held up in the air, earning them the common name heelwalkers.Habitat of the Gladiator on top of the Brandberg Massif in Namibia [Nikon D1x, Nikkor 17-35mm]
Through a mix of luck and pushiness I ended up on the first expedition to collect live heelwalkers in Namibia, and seeing them there, among hot rocks of the Brandberg Massif was definitely one of the highlights of my life. (I also learned something valuable that time: if you stand in the middle of a dry riverbed and hear some strange noise getting louder, run like hell, it is a flash flood coming.) In the years that followed many scientists carefully looked at the minutiae of the bodies and genes of the Mantophasmatodea, and both molecular and morphological analyses confirmed their close relatedness to ice crawlers. So close is this relationship that these two orders of insects are considered “sister groups”—two distinct lineages of organisms that diverged from a single common ancestor. As such, and considering how few species each of these orders contains, some entomologists now prefer to combine Grylloblattodea (ice crawlers) and Mantophasmatodea (heelwalkers) into a single order. When such an option was first discussed, I heard at one scientific meeting the name Gryloblattomantophasmatodea being briefly considered for the combined order. I almost wish it had been chosen, so outrageously long and yet entirely inaccurate it would have been; translated from Latin, the name means “cricket-cockroach-preying mantis-walking stick-like insect,” four groups to which neither ice crawlers nor heelwalkers are closely related!Kuduberg heelwalker (Mantophasma kudubergense) from Namibia [Nikon D1x, Sigma 180mm]
Since the initial discovery of heelwalkers in 2002, about 20 additional species have been found, mostly in South Africa where, as it turned out, in some places they were as common as dirt. How and why entomologists overlooked them for so long is a perplexing question, the answer to which appears to be surprisingly mundane. Not long ago I was in South Africa conducting a survey of katydids in the fynbos. My friend Corey and I drove along the coast, stopping every now and then to look for katydids and grasshoppers on the side of the highway. It was the end of southern winter, dry and often cold at night. Few insects are active in this season, and those that you find are often juveniles. But soon we struck gold—first one, then another, and eventually six new-to-science species of small, flightless katydids (Brinckiella) turned up in the bushes—an entire unexpected radiation of these insects. Entomologists have collected katydids in South Africa for ages; how could have they missed these? I pondered this question as I slowly scanned the vegetation looking for more insects. Suddenly, I found myself eye to eye with a chubby, green female heelwalker. She sat on a branch at eye height, motionlessly trying to blend into her surroundings. Although I knew she was a fully grown adult, she somehow looked larval. The combination of the lack of wings, stubby appearance, and the fact that she was out and about in the middle of winter, all this seemed to suggest that this insect was immature. And that was it. That was the reason these insects had escaped notice for so many years. Like our flightless katydids, similar to juveniles of species active in the spring and summer months, heelwalkers had been occasionally collected by entomologists but quickly discounted as immature forms of something else and stuffed into the darkest corners of entomological collections. Sure enough, after the publication of the official description of the first heelwalkers, many specimens were discovered in South African museums, some collected more than a century earlier. After Corey and I returned from our field trip, I paid a visit to the Iziko South African Museum in Cape Town, and there, among unidentified immature insects were dozens of the new katydids, some having sat there, unrecognized, for more than seventy years.Kuduberg heelwalker (Mantophasma kudubergense) from Namibia [Nikon D1x, Sigma 180mm]
Undoubtedly, the coming years will see new species of heelwalkers discovered and volumes of new data on their ecology and behavior published. But we may need to hurry. Like the melting and disappearing environment of ice crawlers, there are signs that heelwalkers’ habitats are threatened as well. The ancient, unique, and unbelievably rich South African plant communities of karoo and fynbos, homes of so many plant species that they earned the entire region the designation Cape Floral Kingdom, are shrinking. Development, desertification, and mining steadily strip the precious ecosystem bit by bit, literally pushing it into the ocean. Heelwalkers, with their fifteen or so known species, each restricted to a tiny patch of unique Cape vegetation, may soon be in a serious quagmire. The same Mesozoic forbearer that gave rise to ice crawlers—and their narrow ecological specialization—produced another organism that, although it occupies an entirely different niche, is also dangerously specialized. And this, as the rich fossil record of specialized lineages clearly shows us, is a recipe for extinction.A yet undescribed species of heelwalker (Sclerophasma sp.) from South Africa [Canon 1D MkII, Canon 100mm macro, 2 speedlights Canon 580EX]
[An excerpt from my book "Relics: Travels in Nature's Time Machine"]*) Entomologist Mike Ivie pointed out that ice crawlers can be equally fast and voracious, recalling his experience with these insects: “…we fed them live flies and they got them every time, and eat until they practically burst.”
Filed under: Insects, Mantophasmatodea, Notoptera


February 19, 2013
African Tuesday: Duck-faced lacewings
Spoon-winged lacewings (?Nemia sp.) from Richtersveld National Park, South Africa [Canon 1Ds MkII, Canon 100mm macro, 2 x Canon 580EX]
Thread- and spoon-wing lacewings (family Nemopteridae) are related to antlions and similarly thrive in the dry, sandy habitats. Although they are known from most parts of the world (with the exception of, sadly, North America), Africa is the real center of their diversity, and this is where over 80% of the 150+ known species are found.
These lacewings are easily recognizable thanks to their unique, extremely elongated or enlarged hind wings, reminiscent of the long plumes seen in some birds-of-paradise. The function of this unusual morphology is still not entirely known. In species with particularly enlarged hind wings their function appears to be to deter some predators by giving a false impression of the insect as much larger—and thus potentially stronger—than it really is. In species with long, thread-like wings their function may be related to the aerodynamics of the flight, and in members of the subfamily Crocinae the hind wings play a sensory function in cavernicolous habitats that these insects occupy.
Like antlions, larvae of thread-wing lacewings are predaceous, but the adult insects have more peaceful dietary preferences. They are pollen and nectar feeders, and their mouthparts are strongly modified from the typical, biting type found in their predaceous relatives. They are rather long and adapted for dipping deep into flowers, which gives their heads somewhat duck-like appearance. Interestingly, because of some species’ preference of sheltered, cave-like habitats, the larvae of these insects were first discovered in tombs of the pyramids of Giza in Egypt in early 1800′s, giving rise to a nearly mythological status of these insects.
![The head and mouthparts of spoon-winged lacewings is elongated and well-adapted for fitting into long corollas of flowers [Canon 1Ds MkII, Canon 100mm macro, 2 x Canon 580EX]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1380978692i/3646882._SX540_.jpg)
The head and mouthparts of spoon-winged lacewings is elongated and well-adapted for fitting into long corollas of flowers [Canon 1Ds MkII, Canon 100mm macro, 2 x Canon 580EX]
Filed under: Neuroptera

February 18, 2013
Slipping out of the skeleton
A time-lapse video of a male Chinese mantis (Tenodera parasinensis) undergoing his final molt. I recorded it last night over the period of 5:35 hours; this movie contains 494 individual frames taken with Canon 6D.
Note: If the quality of the video clip embedded below is poor, click here to see the uncompressed video.
Filed under: Behavior, Praying mantids, Time-lapse, Video



February 17, 2013
Mom would have been so proud
A couple of months ago tiny Chinese mantids overran my house, having hatched unexpectedly from an ootheca that was supposed to stay dormant until spring. Last night the first female of the batch successfully molted into adulthood (the first male had his imaginal molt three days earlier), thus completing the cycle that started when I adopted “Florimonde”, an old Chinese mantis that had flown into a friend’s house in Cambridge last fall.
Insect molts are one of the most amazing spectacles of the natural world and I don’t think I will ever tire of watching these incredible transformations. And although I didn’t get much sleep last night (she did not begin her molt until 6 AM), it was definitely worth it.
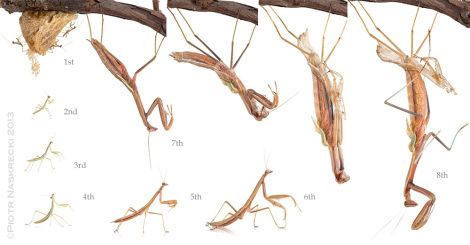
The complete developmental cycle of Chinese mantis (Tenodera parasinensis) – it began on December 4th, 2012 and ended with the final molt on February 17th, 2013.

A female Chinese mantis expanding her wings after the final (imaginal) molt.
Filed under: Praying mantids


February 12, 2013
Join me in Belize to learn macrophotography!
I am pleased to announce that I will be joining macrophotography legends Alex Wild, John Abbott, and Thomas Shahan in a tropical insect photography course in Belize later this year. The workshop will be held September 22-29, 2013. Today is the first day of registration – hurry up, it usually sells out very fast, and is limited to 20 participants.
Filed under: Macrophotography


African Tuesday: Matabele ants of Gorongosa
Matabele ants (Pachycondyla analis) returning from a successful raid on a termite colony in Gorongosa. [Canon 7D, Canon 16-35mm with an extension tube Canon EF 12 II, diffused twin flash Canon MT-24EX]
Shortly after arriving in the Gorongosa National Park in Mozambique I witnessed a puzzling phenomenon: while exploring the network of roads in the woodland savannas of the park our local driver would barely slow down to avoid hitting antelopes and warthogs, but immediately slammed on the brakes if he noticed a long column of large black ants that were streaming from one side of the road to the other. We couldn’t quite get the exact explanation for his reluctance to drive over the insects (although we were very happy about it), but eventually gathered that driving over them could bring great misfortune. In most places on Earth killing a bunch of ants carries the moral equivalency of blinking, and one might wonder why in Mozambique people would show such respect for these insects. But having met these particular ants before I immediately understood that, like so many seemingly irrational cultural oddities, this one also had a very rational explanation.I first encountered Matabele ants (Pachycondyla analis) a few years earlier in Africa, and it was a memorable experience. They are named after a particularly fierce tribe of Zulu warriors, and fully deserve this designation – a single ant delivers one of the most painful stings I have ever experienced, and just a few of them can put you out of business for most of the day. So, yes, crossing paths with Matabele ants will certainly bring great misfortune.
A column of Matabele ants streaming towards a termite mound [Canon 7D, Canon EF 14mm]
But other than their propensity for overreacting to being crushed under one’s foot, these insects are truly amazing creatures. Matabele ants are specialist termite hunters, and do so in a very sophisticated way. It all begins with a single scout leaving her underground nest and embarking on a mission of discovery. The scout wanders, seemingly aimlessly for about an hour in all directions, searching for a nest of termites of the genera Odontotermes and Microtermes. Often she finds nothing, and returns to the nest empty handed. But if she finds a termite mound her behavior changes immediately – she runs back along the shortest possible path back to the nest, leaving behind a trail of pheromones that will guide her nestmates to the source of prey. The pheromonal trail is produced by two glands in the ant’s abdomen: the pygidial gland, the secretion of which has a powerful recruitment effect on her nestmates, and the venom gland, which paints longer lasting orientation cues on the trail. Once back in the nest, it takes as little as 60 seconds to get the entire worker cast of the nest mobilized and streaming along the chemical path left by the scout towards the termite mound, which can be as far 100 m away (in human terms, it compares to walking to a grocery store located 10 miles away.)Matabele ant worker carrying a pupa [Canon 7D, Canon MP-E 65mm, 3 speedlights Canon 580EXII]
Upon arrival at the termite mound, the ants pour in through an opening marked by the scout, and begin to slaughter the termites. Within minutes, several thousand termites are dead. Each Matabele ant then stuffs her mandibles with as many limp bodies as she can carry, and the entire column runs back to the nest. In most cases the ants suffer no casualties during the raid, and the entire colony acts almost as a single, large, predatory organism, equivalent in its impact on the termite colony to a small pangolin.As I watched the column streaming in a tight formation towards the termite mound during one of many night raids that I encountered in Gorongosa, I could clearly hear the constant chatter of hundreds of workers. Bert Hölldobler and his colleagues (Hölldobler et al. 1994. J. Ins. Physiol. 40: 585-593) concluded that the sound made by the ants serves only as a warning to potential predators, and has no role as a cohesion signal to help keep the column together. But when I recorded these signals with an equipment of much greater sensitivity and frequency range than what was available to these scientists in 1994, I realized that the ants produced not one, but two types of sound, one of frequencies an order of magnitude higher than the warning signals typically heard in insects. One kind of sound was produced by rubbing together segments of the gaster, whereas the other was probably made by the ant’s mandibles. You can here the sound of the marching column here; a call of a single ant, slowed down 10 times, can be heard here – notice the knocking sound made by the mandibles, and the scraping sound made with the gaster.
In the light of the recent discoveries of acoustic communication in ants, I think that I will revisit and test in Gorongosa the idea that Matabele ants use sound to locate the column if a worker becomes separated from the rest of her nestmates. Ants may have evolved a sophisticated language of chemical compounds but when you need to shout nothing beats the good old sound waves.
[image error]
Budding myrmecologist Tonga Torcida watching a returning raid of Matabele ants (you can see Tonga assisting E.O. Wilson with ant research in Gorongosa in the new, fantastic BBC documentary “Africa”) [Canon 7D, Canon EF 14mm, diffused twin flash Canon MT-24EX + speedlight 580EX II]
Filed under: Ants, Behavior


Piotr Naskrecki's Blog
- Piotr Naskrecki's profile
- 9 followers


![One of the first katydids I spotted was Horatosphaga serrifera, and elusive species, knwon only from a small handful of specimens. This group is highly sexually dimorphic and males look nothing like this chunky, flightless female. [Canon 14mm, Canon MT 24EX twin light]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785522.jpg)
![A nymph of the Green-striped Grasshopper (Chortophaga viridifasciata) found today in Woburn, MA [Canon 7D, Canon MT 24EX twin light]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381131535i/4317906._SX540_.jpg)
![A ground beetle (Carabidae: Antiinae) attacking a land snail in Atewa, Ghana [Canon 1DMkII, Canon 100mm macro, Canon MT 24EX twin light]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785526.jpg)
![A portrait of Brown-faced Spearbearer (Copiphora hastata) [Nikon D1x, Sigma 180mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381080378i/4116513._SY540_.jpg)
![Central American Pit Bull katydid (Liromoetopum coronatum) lacks the large defensive cone on its head, but makes up for it with sharp, powerful jaws. [Canon 1Ds MkII, Canon 180mm macro]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381080378i/4116514._SX540_.jpg)
![Rhinoceros Spearbearer (Copiphora rhinoceros) is an efficient predator, capable of catching and devouring other katydids and even small lizards [Canon 10D, Nikkor 17-35mm, Canon 580EX]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381080378i/4116515._SX540_.jpg)
![Vargas' conehead (Podacanthophorus vargasi) is a small canopy katydid, found at middle elevations in Costa Rica [Canon 7D, Canon 100mm macro, 3 speedlights Canon 580EXII]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381080378i/4116516._SX540_.jpg)
![The first live heelwalker ever photographed – Gladiator (Tyrannophasma gladiator) from Brandberg, Namibia, which I photographed in March 2002 [Nikon D1x, Sigma 180mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785531.jpg)
![Habitat of the Gladiator on top of the Brandberg Massif in Namibia [Nikon D1x, Nikkor 17-35mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785532.jpg)
![Kuduberg heelwalker (Mantophasma kudubergense) from Namibia [Nikon D1x, Sigma 180mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785533._SY540_.jpg)
![Kuduberg heelwalker (Mantophasma kudubergense) from Namibia [Nikon D1x, Sigma 180mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785534._SX540_.jpg)
![A yet undescribed species of heelwalker (Sclerophasma sp.) from South Africa [Canon 1D MkII, Canon 100mm macro, 2 speedlights Canon 580EX]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1381381071i/4785535._SX540_.jpg)
![Spoon-winged lacewings (?Nemia sp.) from Richtersveld National Park, South Africa [Canon 1Ds MkII, Canon 100mm macro, 2 x Canon 580EX]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1380978692i/3646881._SY540_.jpg)

![Matabele ants (Pachycondyla analis) returning from a successful raid on a termite colony in Gorongosa. [Canon 7D, Canon 16-35mm with an extension tube Canon EF 12 II, diffused twin flash Canon MT-24EX]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1383756264i/6656299.jpg)
![A column of Matabele ants streaming towards a termite mound [Canon 7D, Canon EF 14mm]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1383756264i/6656300.jpg)
![Matabele ant worker carrying a pupa [Canon 7D, Canon MP-E 65mm, 3 speedlights Canon 580EXII]](https://i.gr-assets.com/images/S/compressed.photo.goodreads.com/hostedimages/1383756264i/6656301.jpg)

