Piotr Naskrecki's Blog, page 8
May 31, 2013
Mozambique Diary: The Abominable Frogman

Some of the frog species recorded by MO Roedel during the Cheringoma biodiversity survey.
If frogs tell their children scary stories, one of them certainly must be about the Abominable Frogman, a strange, giant creature that comes in the middle of the night to snatch unsuspecting frogs and take them away. And, like so many scary stories, this one would have a kernel of truth in it, for the Frogman does exist and his name is Mark-Oliver (MO) Roedel.
MO is the leading expert on African frog biology and systematics, based at the Museum für Naturkunde in Berlin, and we were extremely fortunate that he was able to join our biodiversity survey of the Cheringoma Plateau in Gorongosa. Every evening, as soon as the sun started to set, MO donned his headlamp and rubber boots, stuffed his pockets with plastic bags and a digital recorder, and headed out for a nightly amphibian hunt. He was a discerning stalker – after over 20 years of experience in Africa MO knows the call of almost every frog on the continent, and was able to identify scores of Gorongosa species without ever laying eyes on them, and only the rarest or unusual calls attracted his full attention. So good was he at recognizing different call patterns that he could pinpoint an unknown, possibly new to science species of a tiny reed frog from a 100 m away, in a cacophony of hundreds of other frogs rattling on at the same time. He would then slowly stalk it through the shallow pond, completely oblivious to the water pouring into his boots which were always an inch too short, and grab the poor animal with a swift flick of his wrist.
As MO’s frog collection continued to grow it was easy to be startled by the sight of what looked like tiny humans, their arms and legs outstretched and fastened with pins, marinating in a tray of formalin on the table of our laboratory tent; the macabre illusion was completed by a small toe tag attached to each specimen. But appearances aside, the small number of specimens that were collected during the survey were sacrificed for a very good reason. Positive identification of frog species is often impossible in the field as it requires detailed examination of minute skeletal characters or other features that cannot be gleaned from a live, wiggling animal. And identifying an organism is the first, most important step in an effort to protect it.

The Eye of Sauron from the Lord of the Rings movies seems to have been inspired by that of the Yellow-spotted tree frog (Leptopelis flavomaculatus).
During a visit to the Ministry of Tourism in Maputo I learned of a terrifying possibility of reclassification (degazetting) of some of Mozambique’s national parks to allow for industrial development, mining mostly, to take place within their boundaries. Such downgrading was to be justified by the notion of those areas’ seemingly low biodiversity, the result of both the civil war and subsequent poaching and illegal logging. Without getting into the politics of such potentially disastrous decisions, I am convinced that our survey and every species we documented prove that national parks in Mozambique are bursting with life, often sheltering organisms that can be found nowhere else. Each name of an orchid, a mouse, or a frog that we can add to the list of species living in a national park adds weight to the argument that we must protect such areas at all cost. Luckily, Gorongosa is under no threat of being reclassified, but other, less developed parks may be. After hearing about our Cheringoma survey, the Head of the Department of National Parks and Reserves immediately saw its incredible value, and urged us to do it in all national parks in Mozambique.

MO was not the only one interested in catching the colorful reed frogs (Hyperolius marmoratus)
Some of the frogs collected by MO may turn out to be new to science. We found a gorgeous, silver and black Kassina deep in limestone caves of Gorongosa, and this species is quite likely undescribed. Some Hyperolius may be new as well, as is an interesting, big-eyed Leptopelis. Each is a potential endemic, and thus a strong argument for Gorongosa’s protected status. All in all, MO and the rest of the herpetological team documented 33 species of frogs, more than tripling the number of species of amphibians known from the park. And thus the bad news is that the Abominable Frogman does exist and sneaks up on frogs at night. But he is actually their best friend and defender.

MO and a rather angry, and very deadly, Twig snake (Thelotornis capensis)
Filed under: Amphibians, Cheringoma, Frogs, Gorongosa, Mozambique


May 28, 2013
Mozambique Diary: Manticora redux

The head of a Manticora larva blocking the entrance to its burrow.
Some weeks ago I wrote about the Monster Tiger Beetle (Manticora) that I had found in the savanna of Gorongosa. These insects are powerful predators, hunting grasshoppers and other small invertebrates using their enormous mandibles. The larvae of Manticora are similarly carnivorous, but rather than actively pursuing their prey the way their parents do, they are sit-and-wait predators. At that time I had not been able to see or collect Manticora larvae, but tonight I finally managed to snag one.
Like other tiger beetles, the larvae of Manticora hunt from the safety of their narrow, nearly vertical burrows in the sand. Their soft body is safely tucked inside the tunnel, and the only thing that is visible on the surface is a large, heavily sclerotized head and pronotum, both of which form a shield that blocks the access to the burrow. The mandibles of a Manticora larva are pointing upwards so that any insect unlucky enough to step on the head is instantly grabbed by its leg and pulled underground. Imagine walking down the street and stepping on one of those round metal plates that cover sewer manholes, only the plate turns out to be the head of monster, and you are instantly sucked underground – this is what it must feel to a cricket or an antlion as it is being dragged by Manticora.

Only the head and pronotum of a Manticora larva are heavily sclerotized, while the rest of the body is soft and safely tucked inside the burrow. Notice the anchor-like structure on the 5th abdominal segment.
I found a small aggregation of Manticora larvae on one of the sandy paths in the Chitengo camp and watched them for a little while. The aggregation consisted of 10 larvae of different ages, the smallest ones with the head diameter of about 5 mm, the largest 10-12 mm wide. A nearby lamp was attracting a lot of insects, and every 20-30 seconds an insect would invariably land near one of the Manticora burrows. The larvae clearly used their eyes to locate the prey and stretched out as far as they could out of the burrow to be ready when an insect gets really close. If the insect got within a couple of millimeters of the head, the mandibles snapped around the insect’s leg and the victim was instantly pulled underground. Interestingly, the larvae were clearly able to assess their chances of success: if the potential victim appeared to be too large to be pulled inside the burrow, rather than catching the insect’s leg the larva would flick its head and toss the insect away.

This cricket is done for – a Manticora larva grabbed its front leg and is dragging it down the burrow.
It took me a while to figure out how to extract one of the larvae out of its burrow. At first I tried digging, but the tunnels turned out to be very long, and the hard, caked soil made digging very difficult. Eventually, I used the insect’s own voracity to catch it – I gently touched the head with the forceps, and when the mandibles snapped around it I grabbed the head and pulled the larva out. It was not easy as the 5th abdominal tergite of the larva is modified into a large, spiny structure that effectively anchors the animal in its burrow. The larva’s morphology reminded me of marine polychaete worms that use a similar tactic for catching prey from the confines of their burrows.

A Manticora larva, just like its parents, is a voracious killing machine.
Filed under: Beetles, Behavior, Gorongosa, Mozambique


May 21, 2013
Mozambique Diary: On the benefits of being lazy

A Gorongosa crocodile sliding into the Urema River.
Since I needed for one of my book projects a few shots of the famous Gorongosa crocodiles, which rank among the largest in Africa, I asked for help from Bob Poole, a man with considerable crocodile experience. Bob is a legendary National Geographic cameraman who shot, among other NatGeo titles, “Africa’s Lost Eden” and “War Elephants.”
We decided to set up a blind near one of the crocodiles’ basking beaches on the night before, arrive when it was still dark and sneak into the hide, and photograph the animals from there. But we both felt a bit lazy, and in the end did not leave the camp until the late morning, when the sun was already up.

Bob Poole holding all that remains of his hide, and his tripod dragged into the river by a crocodile.
When we got to our spot we found an empty beach, with not a trace of the hide. Or rather, all that we found were traces of the hide, which had been ripped off its stakes still embedded in the ground, and dragged under water by an enormous crocodile. Luckily, the beast did not take Bob’s tripod, which we found in the mud nearby.
I am not a morning person, and never in my life did I feel more grateful for that. Had we been in the hide before dawn as planned, there is no telling how our little adventure would have ended. I think I will continue sleeping in.

Tracks of the crocodile that took, and most likely ate, our hide.
Filed under: Gorongosa, Reptiles


May 20, 2013
Mozambique Diary: The Lizard Quest

Harith Farooq holding a Rock monitor (Varanus albigularis). These enormous lizards are some of the largest reptilian predators of Gorongosa, surpassed only by fully grown rock pythons and crocodiles.
Sitting on the dusty floor of a makeshift laboratory tent Harith Farooq carefully folded a piece of fine, steel mesh into a foot-long cylinder, then weaved in a stretch of a thick wire along its edge. Finally, he carefully attached a neck of an empty water bottle to one of the ends and looked at the contraption in his hands with deep concentration. “Something is still missing”, you could almost hear him think, “but what? A battery? A fork? Some gasoline, perhaps?” His gaze shifted to a stack of paper mouse traps covered with thick, sticky glue, the kind that was meant to immobilize any animal unlucky enough to step onto it. “Bingo!” – Harith picked one up and squeezed it into the tubular apparatus. “The perfect leezard trap”, he announced proudly.

Swynnerton’s amphisbaenian (Chrindia swynnertoni), a subterranean blind lizard, found only in Gorongosa and a small surrounding area.
For the last few days Harith, a Mozambican scientist from the University of Lúrio in Pemba and his colleague MO Roedel from Berlin, two herpetologists participating in a biodiversity survey of the Cheringoma Plateau in Gorongosa, had been trying to catch some of the many lizards found in the Nhagutua Gorge, the site of our first camp. Alas, the sneaky reptiles proved to be extremely difficult to catch by hand, which prompted Harith to come up with an alternative solution. As the survey progressed his traps kept growing larger and more complex, combining both natural materials (rocks, sticks, bark) and man-made objects – a plastic sheet, twine, wire and, of course, steadily increasing amounts of glue. The one thing that they all had in common was their total inability to capture even a single reptile.

Flap-necked chameleons (Chamaeleo dilepis) are common in the savanna woodlands of the Cheringoma Plateau
The strangest part was that Harith was incredibly good at catching reptiles, or any other organisms, without the need for additional accessories. I had never seen anybody catching, with their bare hands, a giant centipede, a solifugid, or a deadly spitting cobra, but Harith caught them all, while carrying a casual conversation. In the end, during the Cheringoma survey he and MO collected 47 species of lizards and snakes, effectively quadrupling the number of reptiles known from Gorongosa National Park.
Within a three week period in Gorongosa our team of biologists was able to document the presence of all nine families of lizards that occur in southern Africa. Among them were some real gems, including an entirely blind, subterranean lizard, the Swynnerton’s amphisbaenian (Chrindia swynnertoni). These tiny reptiles, known only from a handful of specimens recorded around Gorongosa, spend their entire life underground, leading a lifestyle remarkably similar to that of earthworms, and feeding on termites and ant larvae.

Thunderbolt lizard (Nucras sp.), one of the fastest animals found in Gorongosa.
On the opposite end of the lizard spectrum, two species of giant monitors (Varanus) turned out to be quite common on the Cheringoma Plateau. One day Harith walked into the camp carrying a live Rock monitor (V. albigularis) the size of a goat, which he had captured by throwing himself on top of the gargantuan animal, barely overpowering it with the help of two other people. The reptile’s snout was still covered with blood of the last victim, probably a bird or a small child, by the looks of it, and gazing into the monitor’s eyes made me realize how grateful I was that our species appeared long after the era of dinosaurs had passed. We released the beautiful creature after examining it for the presence of external parasites, which the lizard had none, proving its excellent health condition.

Plated lizard (Gherrosaurus major) was one of the most exciting finds of the survey.
Almost every day our herpetological team, which also included a Mozambican student Francisco Domingos, recorded something new and exciting. Often it was a tiny brown frog that differed from all other frogs by the presence of a slightly enlarged corner of the left supraocular cuticular fold, which was enough to make our herpetologists prance and giggle with excitement like little girls. But at other times it was a vine snake that could kill you with a half a drop of its venom, or a spiny rock lizard that defends itself by squeezing into rock crevices and inflating its body like a balloon. The survey found charismatic chameleons, among them the famed pygmy chameleon of Mt. Gorongosa, unquestionably the cutest lizard in Mozambique, and blindingly fast lacertid lizards with flame orange tails, which looked like tiny thunderbolts zipping across the ground.
The survey officially ended yesterday, and Harith is on the way back to Pemba. Data collected by him and the rest of the herpetological team will be added to the ever growing Gorongosa biodiversity database, a powerful tool that helps manage the restoration efforts in the park. I was sorry to see the members of the team depart, but having witnessed Harith handle cobras and puff adders as if they were harmless puppies I was relieved to see him leave the park, still alive and well. All things considered, a gash in his finger, courtesy of a pouched rat, followed by a nip from a giant scorpion hardly count as injuries.

The male Gorongosa girdled lizard (Cordylus mossambicus) looks like an alligator wearing an orange T-shirt. These spectacular reptiles are found only in a small area around Gorongosa and the neighboring Chimanimani Mountains of Zimbabwe, and are threatened by habitat loss and overcollecting for pet trade.
Filed under: Cheringoma, Gorongosa, Reptiles


May 17, 2013
Mozambique Diary: Alipes

A strange chimera, the feather-legged centipede (Alipes sp.)
A couple of weeks ago I was ripping slabs of bark off an old fallen log, an activity that to me ranks among the most pleasurable things one can do, right up there with unwrapping Christmas presents. There is always a chance of finding something incredible – a beautiful cerambycid beetle, a colony of Pyramica, a ricinuleid. But then I pulled off a big chunk of bark and caught a glimpse of an animal that made me think that I am having a stroke. For a split of a second I saw what clearly appeared to be a large centipede, nothing unusual about it, only this one had two long feathers attached to the back of its body. Before I could get a really good look, it jumped off the log and disappeared in a tangle of branches on the forest floor. “I must be really dehydrated” was the only explanation I could come up with, and I made an active effort to forget about the whole thing.
But the strange chimera turned out to be very real. Last night, while rummaging around the camp at night, I found another one. The animal is indeed a centipede, a member of the mysterious genus Alipes (“feather leg”), closely related to scolopendras, and found only in parts of eastern Africa. Its last pair of legs is modified into large, feather-like paddles, the function of which is unclear. According to some sources the “feathers” can vibrate to produce a rustling sound, but I find it unlikely as they are quite soft and very flexible. This animal is also unusual among centipedes in possessing distinct longitudinal ridges on its tergites (most species have the dorsum smooth and shiny). Otherwise it behaves like a typical scolopendra, always trying to bite you and ripping to shreds any animal that it can sink its fangs (forcipules) into. And if anybody knows more about this amazing animal I would love to hear it.
[image error]
A closeup of the “feathers” of Alipes. Their function in this centipede remains a mystery.
Filed under: Chilopoda, Gorongosa, Mozambique

May 13, 2013
The Greatest Show on Earth, happening now

The best time to see Atlantic horseshoe crabs (Limulus polyphemus) is on the nights of the full and new moon in May and June.
I am still in Mozambique, and will be here for a few more weeks, but I simply must take a quick break from describing African nature to highlight a spectacular phenomenon that is taking place right now along the eastern coast of North America – the mass spawning of the Atlantic horseshoe crabs (Limulus polyphemus). Watching these magnificent animals is to me one of the most beautiful natural events that one can witness, and I encourage everybody living on the East Coast to take a trip to the beach this and next month (this year the best time to see them are nights of May 24th, and June 9th and 23rd.) What follows is a short excerpt from my book “Relics” (Chicago University Press 2011), describing my experience of watching horseshoe crabs on the beaches of the Delaware Bay.
“As hundreds of biting flies did their best to drain us of every drop of blood, my friend and fellow photographer Joe Warfel and I stood on the beach, waiting for the spectacle to begin. The sun grew dim, and the high tide was nearing its peak. There were a few people on the beach when we first arrived, but by now they had all disappeared, and we were the only witnesses to what was about to unfold. I started to tell Joe how strange it was that nobody else stayed to watch, but swallowed a fly and decided to quietly enjoy the rest of the evening. First came the big females. Nearly all had males in tow. In the dimming light we could see spiky tails of hundreds more as they tumbled in the waves, trying to get to the dry land. By the time the sun fully set, the beach was covered with hundreds of glistening, enormous animals. Females dug in the sand, making holes to deposit their eggs, nearly 4,000 in a single nest, while the males fought for the privilege of fathering the embryos. Fertilization in horseshoe crabs is external, and often multiple males share the fatherhood of a single clutch. Equipped with a pair of big, compound eyes (plus eight smaller ones), capable of seeing the ultraviolet range of the light spectrum, male horseshoe crabs are very good at locating females even in the melee of waves, sand, and hundreds of other males.

Delaware Bay is the best place in the world to see these magnificent animals. On a good night one could easily see 100,000 horseshoe crabs.
Horseshoe crabs have been around longer than most groups of organisms that surround us now. A recent discovery in the fossil deposits of Manitoba, an interesting little creature named Lunataspis aurora, proves that horseshoe crabs quite similar to modern forms were already present in the Ordovician, 445 million years ago. By the time the first dinosaurs started terrorizing the land in the Triassic (about 245 million years ago), horseshoe crabs were already relics of a long-gone era. And yet they persisted. Dinosaurs came and went, the Earth changed its polarity and climate many times over, but horseshoe crabs slowly plowed forward. Yet during this time they changed surprisingly little. Species from the Jurassic were so similar to modern forms that I doubt I would notice anything unusual if one crawled in front of me on the beach in Delaware. Somehow horseshoe crabs had stumbled upon a lifestyle and morphology so successful that they were able to weather changes to our planet that wiped out thousands of seemingly more imposing lineages (dinosaurs and trilobites immediately come to mind.) But despite claims to the contrary by creationists and other lunatics, they kept evolving. Modern horseshoe crabs, limited to three species in Southeast Asia and one in eastern North America, differ in many details from their fossil relatives. We know, for example, that many, if not most of fossil horseshoe crabs lived in freshwater, often in shallow swamps overgrown with dense vegetation, and some might have even been almost entirely terrestrial. Currently only the mangrove horseshoe crab Carcinoscorpius rotundicauda from the Malayan Peninsula routinely enters rivers, and is the only species to lay eggs in fresh or brackish water.
[image error]
Even Sir David Attenborough, a man who probably witnessed more natural spectacles than any other human being, is fascinated by the spawning of horseshoe crabs. Here he demonstrates the improper way of holding a horseshoe crab (never hold them by their telson) while on the beach in Delaware during the filming of the BBC series “Life in the Undergrowth”.
The following morning Joe and I found the beach covered with horseshoe crab eggs. Well-rested and ready to start a bright new day the flesh-piercing flies attacked us with a renewed enthusiasm. Flailing our arms and swatting dozens at a time we went about flipping crabs stuck on their backs in the sand, and started to look for particularly big clutches of eggs. Although females burry the eggs in the sand, the returning tide washes out many of them. Freshly laid eggs are small, not larger then half a grain of rice. Surprisingly, the eggs grow as they develop, eventually becoming more than twice as large. This, of course, is impossible. The “growth” is an illusion, the result of the production of an external, thin membrane by the developing embryo. A fully developed egg, which at this stage has spent two weeks in the sand, resembles a tiny glass aquarium, with a petite horseshoe crab twirling inside, impatient to break the walls of its miniature prison. Once free, the larva (or at least the lucky ones) catches a wave back into the ocean and will spend about a week floating freely, before settling on the bottom of the shallow shore waters to begin life akin to that of its parents.[…]“

Tiny horseshoe crab larvae, known as the trilobite larvae, twirling in their aquarium-like egg shells. Soon they will break free to begin a short pelagic period, after which they settle on the bottom of the ocean to begin a lifestyle similar to that of their parents.

Just like their distant relatives, scorpions, horseshoe crabs display green fluorescence under the ultraviolet light.

Atlantic horseshoe crabs on the Prime Hook Beach near Milford, Delaware.
Filed under: Behavior, Invertebrates, Macrophotography, Xiphosurida


May 10, 2013
Mozambique Diary: I have fallen and I can’t get up

Sunrise over Nhagutua Gorge in Gorongosa National Park
The best part of traveling with a group of biologists in a place like the Cheringoma Plateau is the impossibility of ever being bored. Not only can you witness hilarious and exotic injuries (where else do people get bitten by bats?), but every day brings new discoveries of things you never thought existed. At our first camp, which was located on the rim of a deep, stunningly beautiful limestone gorge called Nhagutua, our team’s myrmecologist Leeanne Alonso found a colony of what is arguably the most amazing ant species on the planet. Leeanne was collecting ants running on the trunk of a large Knobthorn (Acacia nigrescens), and on a whim pulled off a piece of bark from the tree. To her surprise she found underneath a nest of tiny, yellow ants, which she immediately recognized as members of the genus Melissotarsus.

Leeanne Alonso collecting Melissotarsus ants from an acacia tree
A few things make Melissotarsus stand out among other ants. Unlike other species, their adult workers retain the ability to spin silk (a characteristic typical of many ant larvae), which is produced from a gland on the underside of the head and spun with special brushes on the tarsus. The brushes pull the viscous fibers from the silk spigots, stretch, shear it, and line the wood corridors with it. The silk lining presumably helps prevent the invasion of the colony by predators, and may help keep the corridors free from fungi and other pathogens.

Melissotarsus ants are incapable of walking or even standing on flat, smooth surfaces, and immediately fall on their back, unable to right themselves up.
Melissotarsus workers never leave the nest to forage outside, for two important reasons. For one, they have a strongly reduced sting, which makes them highly vulnerable to attacks by other ants. In fact, minutes after Leeanne had opened their nest it was invaded by Crematogaster and Pheidole ants, which quickly wiped out all workers and larvae present in the exposed corridors. But even more importantly the ants could not forage outside their narrow corridors even if the world outside was safe and friendly, for the simple fact that they cannot walk. Yes, these six-legged insects are incapable of walking or even standing on flat surfaces outside the narrow confines of their nest. Inside the narrow wood corridors they move in a way reminiscent of a rock climber squeezing between two vertical walls by pressing the back and knees against the wall (a move known as stemming). Their first and third pair of legs points down, like in all other insects, but the second pair of legs is usually held up, with the feet pressing against the ceiling. I placed a few individuals on the flat surface of my portable photo studio, and they immediately fell on their back and flailed their legs helplessly.
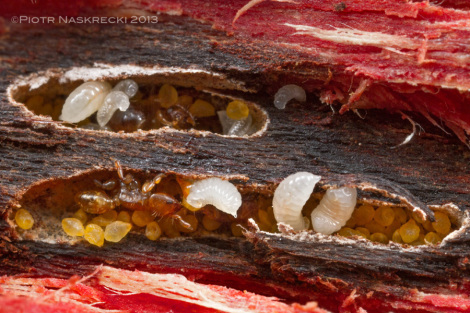
Opened corridors of the Melissotarsus (probably M. emeryi) colony within the wood of Knobthorn (Acacia nigrescens). The yellow objects are diaspidid scale insects, which the ants raise for their meat.
Since these insects cannot leave their nest inside the tree, they are forced to obtain all their food there. They do so by raising scale insects, mostly of the family Diaspididae. Symbiotic relationships between ants and scale insects are not uncommon – many ant species tend and protect them in exchange for honeydew produced by these floem-feeding insects. Melissotarsus also collect honeydew from some species of scale insects and groom them constantly, removing wax from the insects’ bodies and preventing the formation of the protective shield that scale insects are typically covered with. But the bulk of their food comes not from the sugary water exuded by the scales (in fact, most of the species they raise don’t produce honeydew), but rather from slaughtering the scale insects for meat. This, as far as I know, is the only example outside of human societies of an organism raising another species for its flesh, and adds animal husbandry to the already impressive list of seemingly human skills (e.g., architecture, farming, solar navigation) that ants had evolved long before our species first appeared on Earth.
(You can see photos of another species of Melissotarsus on Alex Wild’s blog.)

A Melissotarsus worker walking through a narrow passages of her nest; notice the second pair of legs, which is pressed against the ceiling of the corridor.

A winged, reproductive male of Melissotarsus. These are the only members of the colony capable of walking on flat surfaces.
Filed under: Ants, Behavior, Gorongosa, Mozambique


May 8, 2013
Mozambique Diary: Heroes, and what bugs them

Jen with her exciting catch
One early morning, while on the Cheringoma Plateau, Jen “the Mammal Lady” Guyton came running into the camp. “We caught something really good in our traps!”, she announced breathlessly, sending the media team that was filming our expedition into a state of frenzied excitement. Everybody rushed to see the mystery animals, whose identity Jen refused to reveal. We scrambled through a spiny thicket and arrived at a place where she had set a large trap the night before. My imagination was running wild, and I was really hoping for a pangolin, or at least a baby aardvark. But the animal I saw was neither of these. At first, in the dim light I couldn’t quite make out the large mammal in the cage, and my first thought was of a small kangaroo, which would have been exciting if rather unlikely. “It is a pouched rat!”, Jen said proudly, and I felt all my hopes deflate like a punctured tire.
But my disappointment turned out to be grossly unjustified. To begin, the animal was actually very pretty. It was the size of a small cat, with a sweet face and slow, deliberate movements, quite unlike those of a house rat or any other rodent that I had ever seen. When Jen handed it a snack, it calmly ate it. Its tail was thick, half black and half white, which is a characteristic coloration of this species. Pouched rats (Cricetomys gambianus) are members of the family Nesomyidae, and thus not closely related to the rats that infest human houses, and derive their name from their ability to store food in large pouches in their cheeks. They can reach the weight of about 2 kg, and in some parts of Africa are hunted for their tasty meat.

Pouched rat (Cricetomys gambianus)
In Mozambique, however, pouched rats play a very different role. The civil war of 1975-1992 has left many areas of the country infested with land mines, resulting in human casualties, and forcing people to abandon their land and houses. The removal of mines is an arduous process that normally requires the use of metal detectors or highly trained sniffer dogs. But in the late 1990′s Frank Weetjens, a Belgian mine-removal specialist, realized that pouched rats could make great mine detectors – they have an unparalleled sense of smell, and their calm nature and intelligence makes them easy to train. His organization APOPO set up a rat training camp in Tanzania, where young pups of pouched rats are taught to detect the smell of 2,4,6-trinitrotoluene (TNT), the main ingredient of land mines, and signal its presence to the trainer in exchange for a snack. After a 250-day long training the rats are flown into Mozambique, where they are used to systematically de-mine large areas still too dangerous for people to inhabit. Their efficiency in detecting land mines is nearly 100%, and their use in Mozambique has already allowed thousands of families to return to their homes and farmlands. It is not surprising then that in Mozambique they are known as Hero rats, a designation they fully deserve. In an even more amazing development, the pouched rats are now being used in Africa as sensitive and efficient diagnosticians of tuberculosis, a disease that affects many rural populations. Their incredible sense of smell allows them to differentiate between sputum of a healthy and an infected person at a rate and accuracy much higher than those afforded by traditional medical tests.

Epizoic earwig (Hemimerus sp.) on the fur of a pouched rat
All this would be enough to make me worship pouched rats, but the animal we caught had a hidden entomological bonus. We had placed it a large container to take a few photos, and then we noticed dozens of large, brown insects running on its fur. One look and I immediately recognized them as something I had only read about before – they were parasitic earwigs! Earwigs are not insects that are usually thought of as something to be found on other animals, but members of the suborder Hemimerina are specialized parasites of rodents. They lack the large abdominal forceps of their free-living relatives and they are blind. Unlike other earwigs, they are also viviparous. On the body of the pouched rat they moved with an amazing speed, and their legs acted like clasps, allowing them to cling to hair and virtually swim through the mammal’s fur.

Epizoic earwigs (Hemimerus) lack the clasping cerci of their free-living cousins
Adult parasitic earwigs (Hemimerus) have many nymphal characteristics, such as the lack of clasping cerci, wings, and wing musculature, suggesting that they are paedomorphic (reproductive stages that retain larval morphology.)
From the pouched rat’s perspective, having the earwigs is probably not too bothersome. These insects feed only on dead flakes of skin of the mammal, and thus act more as helpful exfoliants, rather than true parasites, and I also imagine that they provide an occasional snack.
All in all, the pouched rat in the trap turned out to be far more interesting than a baby aardvark would have ever been. (But I am still hoping to see one.)

An adult epizoic earwig (Hemimerus sp.)
Filed under: Dermaptera, Gorongosa, Insects, Mammals, Mozambique


April 14, 2013
Mozambique Diary: Golden bats

A colony of Lander’s horseshoe bats (Rhinolophus landeri) – notice the orange hairs in the armpits of the flying male.
Tomorrow marks the first official day of the Gorongosa Biodiversity Survey on the Cheringoma Plateau. All participating scientists are arriving, and the following morning we will depart for the first, northernmost site. But even before we get to those remote and unexplored areas, some of us have been already collecting interesting data.
Earlier today Jen Guyton, the expedition’s bat and rodent specialist, discovered a large colony of bats in an old, abandoned concrete water tank on the outskirts of the Chitengo Camp. It was too good of an opportunity to learn something new about bats of Gorongosa to pass by. Armed with a large butterfly net Jen had descended deep into the dark and rather odoriferous structure, and soon emerged triumphant with half a dozen bats fluttering in the net. She immediately identified them as Horseshoe bats (Rhinolophus), members of the family Rhinolophidae.

Two color morphs of Lander’s horseshoe bats (Rhinolophus landeri) found in Gorongosa.
These mammals get their common name from the characteristic, horseshoe-shaped noseleaf, an intricate structure on their faces that is the source of their echolocation signals. The ultrasonic signals of horseshoe bats are unusual in their relatively long duration and constant frequency, as opposed to more typical, short signals of shifting frequency found in most other insect-feeding bats.
Our bats turned out to be Lander’s horseshoe bats (Rhinolophus landeri), and the Chitengo colony had two color morphs of this species, one of which had a beautiful golden fur, which reminded me of that of the Amazonian Lion Tamarin. Lander’s horseshoes appear to feed mostly on moths, and can be identified among related species of the genus by tufts of distinct orange hair in the armpits of adult males (see photo).
In the coming days and weeks we will undoubtedly see more bat species and other amazing organisms. I will try to post updates from the field as often as I can, but it remains to be seen if my cellphone modem works in the areas were we will do our work. Stay tuned.

Mammalogist Jen Guyton examining a freshly caught Lander’s horseshoe bat.
Filed under: Gorongosa, Macrophotography, Mammals, Mozambique


April 12, 2013
Mozambique Diary: Is this tortoise broken?

An adult Hingeback tortoise (Kinixys belliana) from Gorongosa. What looks like a wound on its carapace is a flap of skin that allows the shell to close and protect the hind legs and tail.
Some time ago I was driving in Gorongosa when I noticed a large tortoise laying in the middle of the road, stuck upside down in the mud. The animal was alive but had what appeared to be a large wound in posterior part of its cracked carapace. There were fresh tracks of a civet all around it in the mud, and I assumed that the civet found an injured tortoise (I didn’t think a civet could do it itself) and was hoping to eat it. I pulled the animal out of the muck and only then recognized what I was looking at – the tortoise was not injured at all, and what I took for a wound was a flap of skin that connected the front part of the shell with its movable back. Because it was not an ordinary tortoise, but a Hingeback tortoise (Kinixys), a member of a remarkable group of reptiles that are capable of closing shut the back of their shells to protect the hind legs and the tail. This, combined with the head tightly pulled into the front of the shell and blocked by its heavily armed front legs makes the hingeback tortoise virtually immune from attacks by smaller predators. Judging by the dense trail of civet tracks around the tortoise it seemed that the mammal had spent a lot of time in frustration, unsuccessfully trying to get to the soft parts of the reptile.

A juvenile Hingeback tortoise; its hinge is not yet developed.
Although the movable carapace is a great way to protect the rear part of the body, the flap of skin that connects the two components of the carapace is a favorite location for another enemy of the tortoises to attack – the ticks. Every tortoise I found in Gorongosa carried several huge, heavily armed ticks (mostly of the genus Amblyomma), whose body shape and sculpturing was surprisingly similar to that of the tortoise. Carrying a bunch of blood-sucking ectoparasites is no fun, but the burden of being drained by them is probably particularly heavy on smaller, younger animals. A few days ago I found a tiny Hingeback tortoise, one whose hinge was not yet fully developed, and it carried on its leg one of the largest ticks I have ever seen. It was as if I had a parasite the size of a football permanently attached to my body. I removed the tick from the tortoise, put one of the animals into a vial of alcohol, and applied some antiseptic to the other and let him back on his merry way.

An enormous tick (Amblyomma sp.) on the tortoise’s leg.
Hingeback tortoises are quite common in Gorongosa, and I see them often as they cross the network of trails. But across Africa their numbers are declining as the result of habitat loss and collecting for the pet trade. Every year about 20,000 of these animals are exported to be sold in pet stores in the US and Europe, and Mozambique alone sends out about 3,000 of these animals every year (this is the official quota allowed by CITES, the actual number is undoubtedly higher). Luckily, recently the importation of most of Hingeback species was banned in the US. The reason – ticks. Some of the ticks carry a disease which, while not harmful to the reptiles, is often fatal to cattle – the Heartwater disease, caused by rickettsia Ehrlichia ruminantium. And so, the same parasite that makes the tortoises’ life miserable may in the end help them survive in the wild. I guess you never know who your true ally is.

Tortoise tick (Amblyomma sp.) actually looks like a tortoise, and its opisthosma is almost as hard as the reptile’s shell.
Filed under: Arachnida, Gorongosa, Mozambique, Reptiles


Piotr Naskrecki's Blog
- Piotr Naskrecki's profile
- 9 followers



