Carl Zimmer's Blog, page 33
February 5, 2013
Stereo Mole Noses
Never underestimate a mole.
It’s easy to have low expectations for these creatures. My own experience with moles is limited to the sight of their carcasses at my doorstep, laid there by our triumphant and generous cat. Stretched out on the cold bluestone, they look like little more than eyeless pouches of fur.
Ken Catania of Vanderbilt University has gone a long way to redeeming the mole. In the 1990s, he did some of the first studies of star-nosed moles, a species with 22 oddly finger-shaped tentacles flaring from its nostrils. He discovered that those tentacles are an exquisitely sensitive touch organ with 25,000 sense receptors. It uses this bizarre star to rapidly scan its tunnels for food, sweeping back and forth a dozen times a second. It takes a fifth of a second from encountering a piece of food for the star-nosed mole to eat it, making it the fastest eater on record in the animal kingdom. (Here’s a story I wrote about Catania in the New York Times, plus a wonderful illustration of its fast-acting anatomy.) Catania’s research landed the star-nosed mole in Guiness Book of World Records.
Recently, Catania thought it would be interesting to compare an awesomely sensitive creature like the star-nosed mole with a pathetically equipped relative. He chose the Eastern mole, the common species that I so often find dead on my doorstep.
Scalopus aquaticus seems, on paper, like a supremely mediocre mole. It has no star on the end of its nose. Other mole species that lack a star still have lots of touch sensors on their faces. But these sensors are delicate bits of tissue, and Eastern moles, adapted to life in drier, harder ground, have lost them. They are blind, and their ears are only sensitive to low-frequency sound.
So Catania started observing eastern moles search for food. Instead of stumbling around, they turned out to be, in their own way, just as impressive as their star-nosed relatives. Despite being starless and blind, they consistently headed straight for the food.
Catania decided to study the moles formally, by building a special box. One half of the box was a holding chamber, in which he could put a mole. It was divided from the other side by a wall with a closed doorway in the center. On the other side of the box, Catania drilled 15 pits, arranged in a semi-circle at equal distances from the door. Into one of those pits Catania would drop a chunk of earthworm, and then he would opened the door. The mole would squirm through and explore the new part of the box. Every time, the mole headed straight for the hidden food.
The only way that Catania could imagine the mole could manage this was by smell. But the mole’s sense of smell would have to do more than just tell it that there was a worm somewhere in the box. It would have to direct the mole to the precise spot where the worm had been placed.
How animals smell their way to food is still fairly mysterious. One method that’s been well supported by experiments is to play the hot-cold game with your nose. If you get closer to food, its smell will get stronger. If you move away, it gets weaker.
Another possibility is that the moles can smell in stereo. They can compare signals from their two nostrils to judge how far to the left or right a smell is. We use stereo for our hearing, using the differences between what our left and right ears detect to pinpoint the source of a sound. Perhaps moles can do the same with their noses.
Other scientists had found some suggestive evidence that rats could smell in stereo, but Catania was not entirely convinced. After all, ears are separated on either side of the head, but nostrils are right next to each other. How they could get a different signal across such a small distance was hard to fathom.
So Catania did a series of experiments. He used an air pressure sensor to record when the moles took a sniff. As you can see from this video (sniffs marked by white spots), sniffing was a vital element of their search.
Catania then manipulate the noses of the moles in various ways to see if he could alter their performance. In one experiment, he plugged up one nostril. Now, instead of finding their food with 100% accuracy, the moles consistently failed. And they failed in a consistent way. If their left nostril was blocked, they veered off to the right. And if their right nostril was blocked, they veered to the left.
This reliable error suggested that they did, indeed, rely on both nostrils, each providing information about the smells on each side. Catania then ran another experiment in which he inserted tubes into the mole noses. The air flowing into each nostril crossed over to the other one, where it was detected by the nerve endings there.
Now the moles got hopelessly lost. Instead of simply getting biased information that steered them off course in a consistent way, the moles were overwhelmed by confusion.
Catania concludes that moles probably use both stereo and hot-cold strategies to find food. From a distance, hot-cold works well. Closer up, where smells can get much stronger over even a short distance, the moles switch to stereo.
It will be interesting to see if other animals show such clear evidence of smelling in stereo too. Catania has some evidence that star-nosed moles aren’t so good with stereo smell, which he’s now following up on. It’s possible that eastern moles took a capacity found in many mammals and evolved it into a much more powerful form to adapt to their particular kind of life in the hard ground. I’m not too eager to have pipes shoved up my nose, but I’m awfully curious to find out if we humans share the gift of the mole.
February 4, 2013
How Pigeons Cured My Case of YAGS
In tomorrow’s New York Times, I write about what pigeons taught Darwin about evolution, and what they can teach us over 150 years later. The spur for the story is a new paper in which scientists analyze the genomes of forty different pigeon breeds to understand the molecular secrets behind their remarkable diversity. My story is accompanied by some wonderful photos as well as a podcast in which I talk to my editor, David Corcoran, about the research.
Those who have ever been in earshot of me may find this story a bit puzzling. I have ranted many times about all the hoopla that bursts upon us every time the genome of yet another species is sequenced. Eventually my rants crystalized into a self-diagnosis: I realized that I suffered from YAGS, short for Yet-Another-Genome Syndrome. I first identified this disorder here on the Loom, and when I was asked to give a keynote talk at a genome science meeting last year, I explained it at more length. You can see the lecture in this video:
I got the sense from some of the scientists I talked to afterwards that my talk came off a bit nasty. That wasn’t my intention–I was just trying to convey just how exhausted I (and some other journalists I’ve spoken to) have gotten with the endless barrage of press releases that tout new papers on newly sequenced genomes.
It is unquestionably useful for scientists to do this work. The growing database of genomes has become a new territory for biological exploration. In some cases genomes turn out to be especially messy, and in these cases the scientists involved should be commended for their high pain threshold. Some genome studies even lead to fundamentally new methods of DNA sequencing, which is all for the good.
But the mere appearance of a new genome paper is not in itself reason for major news coverage. Nor are promissory notes for how valuable the genome sequences will be in the future. (Let’s wait till the future comes before we start reporting on it, shall we?)
Yet there is, in fact, lots of news about genomes worth reporting. Genome sequencing is getting so ubiquitous that scientists can fold it into their explorations of interesting questions about life. The mere existence of one pigeon genome won’t lead me to write a story. But a study that uses forty pigeon genomes to probe the evolution of new forms? Sign me up. The same goes for my recent Wired feature about using genome sequencing to track hospital outbreaks. The antidote to YAGS, in other words, is witnessing genomes in action.
January 30, 2013
The Parts of Life
We’re made of parts. Our skull is distinct from our spine. Our liver does not grade subtly into our intestines. Of course, the parts have to be connected for us to work as a whole: a skull completely separated from a spine is not much good to anyone. But those connections between the parts are relatively few. Our liver is linked to the intestines, but only by a few ducts. That’s a far cry from the intimate bonds between all the cells that make up the liver itself, not to mention the membrane that wraps around it like an astronaut’s suit. The distinctness of the parts of our bodies is reflected in what they do. In the liver, all sorts of biochemical reactions take place that occur nowhere else. Our skull protects our brain and chews our food–jobs carried out by no other part of our body.
Biologists like to call these parts modules, and they call the “partness” of our bodies modularity. It turns out that we are deeply modular. Our brain, for example, is made up of 86 billion neurons linked together by perhaps 100 trillion connections. But they’re not linked randomly. A neuron is typically part of a dense network of neighboring neurons. Some of the neurons in this module extend links to other modules, creating bigger modules. The brain can link its modules together in different networks to carry out different kinds of thought.
The proteins that make up our cells work in modules, too. Some proteins can only work in collaboration with certain other proteins. They may need to join together to make a channel, for example, or they may help out in an assembly line of chemical reactions that breaks down a toxin. You can draw a map of these interactions by connecting lines between genes that are turned into proteins in the same situations. The modules look like dense nests of links, with a few links joining together one module to another.
We are not alone. Other animals are modular. So are plants, fungi, protozoans, and bacteria. It’s enough to make you wonder why life is universally made up of parts.
You may be able to think up plenty of reasons that seem obvious. Maybe modules do things more efficiently. Maybe too much multi-tasking slows life down too much. Maybe modules make it easier for life to adapt to new challenges, by letting one part of an organism evolve without affecting the other parts. Or maybe during evolution, modules can be easily duplicated and then tweaked to tackle a new job.
Maybe. Or maybe not. To judge the merit of such ideas, scientists put them to the test. Scientists can compare modules in real organisms to look for patterns their hypotheses predict. They can tinker with bacteria to make them more or less modular and see how they perform. Recently three scientists, Jeff Clune of the University of Wyoming, Jean-Baptiste Mouret of Pierre and Marie Curie University in Paris, and Hod Lipson of Cornell used another method that’s become increasingly popular among scientists who want to understand the parts of life: they evolved a computer network.
Clune and his colleagues created a network inspired by the network of neurons we use to see. For a retina, it has eight virtual neurons, arranged in a four-by-two grid. Each one either sees light or darkness. Like a real neuron, Clune’s virtual neurons can respond to these inputs by sending a signal to neurons in the layer below. A single neuron may receive inputs from all eight neurons, or just one. It uses certain rules to decide whether to send a signal of its own in response to the next layer down. Finally, the network funnels down to a single neuron–a virtual brain, if you will–that can switch on or off in response to information that makes its way down through all the layers.
The scientists made lots of different networks, varying which neurons were linked to which, as well as how strongly they influenced each other. And then they put these networks to a test. In effect, they asked if a specific pattern was present on the left side and whether a different pattern was present on the right side. If both were there, the eye needed to answer TRUE. Otherwise, it needed to respond FALSE.
They showed all 256 possible combinations to the networks and scored them for their accuracy. Not surprisingly, most were deeply awful. But a few were a little less awful, thanks only to chance.
Clune and his colleagues then mimicked natural selection. They selected the best-performing networks and duplicated them. They introduced a mutation-like feature to their program by randomly altering their links. Then the scientists tested the mutant networks again, and once again let the best ones produce new mutants.
Over 25,000 generations, some of the virtual eyes managed to get good–perfect in some cases. But then Clune and his colleagues threw another ingredient into the mix. They not only rewarded virtual eyes for becoming more accurate, but also for how few links they needed to do the job. It’s a plausible factor to include, because building and running more tangled networks can impose a higher cost on an organism. Neurons, for example, are big cells that require a lot of energy to build and also demand a lot of repair to keep running.
In this new environment, evolution operated differently. A lot more virtual eyes ended up recognizing patterns perfectly. They also became more adaptable. Clune and his colleagues turned the virtual eyes towards a new task: they had to recognize whether one particular pattern of four pixels was present on either the left or the right side. The minimal-wiring networks took much less time to evolve a skill at this new task than regular ones.
And there was one more difference between the two kinds of eyes–one that might tell us something about why life comes in parts. The minimal-wiring virtual eyes spontaneously evolved modules. The virtual neurons organized themselves into two networks–one on the left, and one on the right. Only at the final layer of the network do they combine their signals. In other words, a premium on minimally-linked networks spontaneously produces modules. (You can see this evolution in action in the video embedded at the end of the post.)
A skeptic might argue that modules evolved in this experiment because the problem that the virtual eye had to solve was itself modular. The two sides of the eye had to recognize its own pattern before making a final judgment. To test this possibility, the scientists evolved the eye with rewards for problems that couldn’t be broken down so neatly. For example, in one task, the eye had to determine whether there were four black squares anywhere in the eight-pixel grid. Even in these decidedly unmodular tasks, modules emerged.
Clune’s study suggests an evolutionary route to modules: as networks become more efficient, they become more modular. But once the parts of a system emerge, natural selection may then favor modules themselves, because they make living things more flexible in their evolution. Once life’s Legos get produced, in other words, evolution can start to play.
[Update 5:30 pm: Corrected description of first test]
January 29, 2013
The Jewel Wasp’s Zombie Slave: Video of My TEDYouth Talk
In November, I gave a talk at TEDYouth, an all-day event for high school students in New York, about my favorite parasite. The video of the talk is now online, along with extra education material.
January 28, 2013
Creating Young Darwins

Darwin's experimental greenhouse. Ian Capper, via Creative Commons: http://www.geograph.org.uk/photo/1976815
Charles Darwin was a DIY biologist. He was not a professor at a university; he was not a researcher at a government lab. As a young gentleman, he had the right connections to tag along on the voyage of HMS Beagle as an unofficial, unpaid naturalist. Once he came home, he spent most of his time at his country estate, where he ran decades of experiments on orchids and rabbits. He played a bassoon to earthworms to see if they sense low noises. He made painstaking observations on other species. He spent years peering through his personal microscope at barnacles. He spent afternoon following ants around his lawn. To add to his personal discoveries, he wrote to a global network of friends and acquaintances for every scrap of information he could find that seemed relevant to his theory. While Darwin took advantage of every tool a Victorian naturalist of means could get his hands on, they were quite simple compared to the equipment evolutionary biologists use today. No DNA sequencers or satellite databases for him.
The simplicity of his took kit and the grandeur of his work makes Darwin exceptional enough in the history of science. What makes him even more so is our ability, some 150 years later, to get to know his DIY biology in deep detail. Darwin described many of his projects in his books, and the University of Cambridge still has hordes of his letters on file. In recent years, this Himalaya of information has become available in searchable form online at places like The Darwin Correspondence Project and Darwin Online.
Ned Friedman, a botanist at Harvard, has come up with an intriguing way to use Darwin’s life to teach the basics of evolution. He and a team of graduate students have created a freshman seminar called “Getting to Know Darwin,” in which the students recreate ten of Darwin’s experiments and observations, spanning his life from his college days to the work on earthworms, which he carried on during his final years. To get an intimate feel for Darwin’s ideas and work, the students read his letters in which he discusses each topic. They then run experiments very similar–or in same cases, identical–to the ones Darwin ran himself.

Duck's foot with seed attached. From "Getting to Know Darwin"
Friedman has now gone the extra mile and put all the details of the class online at the Darwin Correspondence Project site. You can read about each lesson, such as the one on biogeography–the science of why species are where they are. Friedman’s students do experiments with seeds in fresh water and salt water to see how plants could get to remote islands. Some ducks’ feet obtained from a butcher shop allow students to see how Darwin figured out that birds could transport plants to new homes.
From my inspection of the site, I think it would be great not only for college courses, but for high school and even for curious families. Maybe it’s time for me to dump some seeds in some salt water…
Friedman explains the project in this video:
Darwin Resources from Darwin Correspondence Project on Vimeo.
January 27, 2013
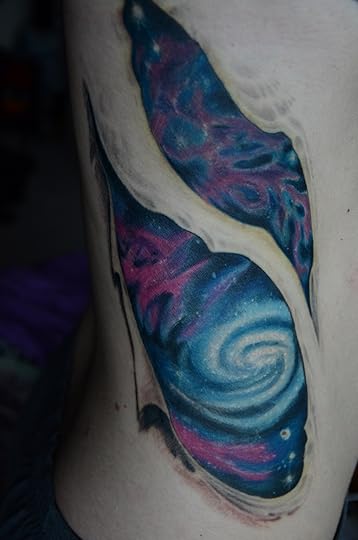
It’s Full of Stars! (Science Ink Sunday)
Valentene Peinhardt writes, “I wanted a tattoo that would embrace my love for astronomy and consciousness of being. I remember years ago realizing the fact that we were made of star stuff when I was watching an episode of Carl Sagan’s Cosmos series. Since then, my entire outlook on life has changed. Our ability to experience is so cherishable. I am not one of religion, but the feelings that I experience when I think about our origins, are not unlike spirituality.”
You can see the rest of the Science Tattoo Emporium here and in Science Ink: Tattoos of the Science Obsessed.
(Tattoo done by Steve Lemak of Quillian Tattoo in Allentown, PA.)
January 24, 2013
The Guinea Worm: A Fond Obituary
In 1996, while I was traveling in South Sudan, I visited a small hospital in Tambura. People there were sick in all sorts of ways–with malaria, sleeping sickness, and other illnesses–but one group of patients left an impression on me that I’ll never get rid of. They all stayed in a single, small narrow building. They lay on two rows of clean, thin mats on the floor. They were all clothed and were supremely bored. The men kept one pant leg rolled up to the knee. Exactly what sort of disease a sick person has can be mysterious–Is it stomach cancer? Is it HIV? It is mumps?–but there was no confusion in this room. All the patients had a short stick attached to their legs, seemingly tied by a string. That string was, in fact, an animal.
Its official name is Dracunculus medinensis. It’s commonly known the guinea worm. Measuring up to four feet long, the worms were lodged in the connective tissue inside the legs of the Tambura patients, their head poking out of a blister. The only way to get rid of the guinea worms was to wind them onto sticks, which nurses then twisted, slowly and steadily, for two weeks.
Seventeen years later, the guinea worm’s days are now numbered. In 1986, 3.5 million people suffered from guinea worm infections across Asia and Africa. In 2012, there were only 542 cases in the entire world. The vast majority of those cases–521–occurred in South Sudan. But that’s a huge improvement: in 2008, South Sudan had 3,618 cases. From 2011 to 2012, the number of guinea worm infections there dropped by 49%. If public health workers can keep up that pace, the World Health Organization expects the guinea worm to be eradicated in the next few years.
Newspapers keep an inventory of obituaries for famous people who are in their golden years, with only the date and cause of their death left to fill in when they finally pass away. The recent news about the guinea worm’s impending extinction prompted me to write an obituary for it. I will not miss it as a disease, but as an animal, it will leave a mind-boggling absence.
The guinea worms have wound themselves around human history for thousands of years. Egyptian mummies contained them. The Book of Numbers describes how the Israelites were stricken by “fiery serpents” as they wandered the desert–they, too, are believed to be guinea worms. Muslim pilgrims on the hajj suffered from guinea worm infections on their way to Medina, which led to its Latin name, which means “Little Dragon of Medina.” Greek and Persian doctors were winding the worms on sticks over two thousand years ago. It’s possible that the symbol of medicine–snakes coiled around a staff–maybe actually represent this ancient treatment.
For centuries, no one could say how guinea worms could grow inside human bodies. Some thought they spontaneously appeared. Their true nature was uncovered by the Russian naturalist Aleksej Fedchenko in the 1860s. In his early twenties, Fedchenko traveled widely in Central Asia. “Upon returning to Samarkand from a trip across the plain,” he wrote in an 1870 monograph, “I learned that the native doctor, a specialist in extracting guinea worms, already had had the occasion of removing several parasites from persons arriving in Samarkand from Bukhara.”
Fedchenko was delighted. He had studied parasitic worms as a student, and now he could do some original research. Every morning, the doctor would bring a bottle of worms to Fedchenko, who would spend all day peering at them through a microscope, making detailed drawings. One day he noticed that there were water fleas in the water. He plucked out the poppy-seed-sized animals and put them under his microscope, too. “I saw in each one several familiar looking embryos of the guinea worm,” he wrote.
Fedchenko died in 1873 in a mountain climbing accident at age 29. It was left to parasitologists who came after him to work out the bizarre life cycle of the guinea worm. The worms are born live and will squirm through fresh water for days, waiting for a water flea to notice their quick, jerky movements. For water fleas, it seems like an easy hunt, but the joke’s on them. Instead of getting a meal, they become a nursery. The guinea worm embryos drill their way out of the water flea’s digestive tract and swim around in their body cavity, feeding on the ovaries or testes of their host. Over the next few weeks, they molt twice, growing to larger stages. But the parasites will die as embryos unless the water fleas get inside of humans.
This leg of the journey occurs when a person drinks unfiltered water from a pond. The water flea plummets into the stomach, where it is dissolved by digestive juices. The guinea worm embryo, on the other hand, swims onward into the intestines and then drills its way out, crawling into the abdominal blood vessels and finally reaching the abdominal muscles over a couple weeks.
Of all the many remarkable things about the guinea worm, here is something particularly remarkable: this journey causes its victim no pain. It’s not a fleeting infection, either. It takes the guinea worms three to four months to become sexually mature. The males only get to be 4 centimeters long; the females reach 25 times that length. Most of that extra space in their bodies is taken up by their uterus. If people have both males and females in their body, they can find each other and mate. After this internal congress, the male guinea worm creeps off to some corner of the body and dies. The female, meanwhile, swells with progeny. Three million embryos begin to grow inside her.
It takes months for the multitudes she contains to grow to the point when they’re ready to leave their mother–and their human world. She begins her journey to ground, slithering through the connective tissue until she reaches her host’s leg and creeping down further towards the foot. Only now, over a year after taking in the guinea worms, does a person become aware of what’s been happening. The guinea worm mother pierces the skin from the inside and releases an irritant that creates a painful blister–”burning without cease” as one tropical disease expert once put it.
There is only one balm for this pain: water. When people splash water on the blister, the guinea worm responds by twisting into a contraction and vomiting embryos from her mouth. The pain subsides for a while, but the blister swells again until another splash of water delivers another batch of guinea worm babies. The pain lasts, off and on, for months–until the parasite’s entire uterus has emptied its millions of embryos. Some of them may be lucky enough to end up in a pond full of hungry water fleas, where they can continue the cycle.
Guinea worm infections can be fatal, but only due to human impatience. People sometimes try to yank the worm out from its blister, whereupon it retreats into the body, where it triggers an intense immune response that swells the leg. The worm dies and becomes a site for dangerous bacteria infections. Worms can also die during their journeys inside the body, and their calcified remains can cause crippling arthritis. Even in the best outcome, when the worm can be wound out of the body, the intense pain can linger for several more months. In the 1970s, doctors working in rural India would come across villages where just about every resident was bedridden, waiting to be rid of the guinea worm. For poor farmers, the infections could be economically devastating, keeping them out of their fields for months.
No one has ever invented a vaccine for guinea worms. There is no reliable drug to treat an infection. Modern medicine hasn’t gotten much better than sticking winding–the treatment that worked thousands of years ago. And yet doctors have recognized since the 1930s that the guinea worm had a rare distinction: it could potentially be eliminated from the face of the Earth. Unlike many other pathogens, guinea worms are pretty easy to track, and their life cycle’s pretty easy to block. If they can’t get into water fleas, they’re finished. And even if they do take that step, they still need to get into humans.
Some individual countries managed to eradicate guinea worm within their borders, and in 1986, the Carter Center backed an international effort to wipe them out altogether. In 21 different countries, public health workers identified the ponds where guinea worms were lurking and sprayed them with insecticide. They handed out filters for drinking water. They kept infected people away from water, so as not to allow the worms to launch a new generation. The campaign enjoyed a slow but steady success. Country after country declared itself parasite free. By the early 1990s, experts were predicting eradication within a few years. They had to keep pushing that date a few years into the future, however. The campaign turned out to be more expensive than had been expected. Poor organization in some countries stalled progress. South Sudan proved particularly difficult thanks to a long civil war. Now that South Sudan is its own country, the guinea worm campaign is moving full tilt.
The guinea worm is now one of the most endangered species on Earth. There is no hidden population of the parasites lurking in dogs or raccoons, waiting to launch a renewed attack. Those animals get infected with their own species of Dracunculus, but cannot host our little dragon. Every guinea worm still alive today had to be spewed out of a loving mother dwelling in a human body. If they do continue to become rarer and disappear, it will be only the second time in history that we eradicated a human disease, the first being smallpox.
We may still need to kick the guinea worm’s date with oblivion a few more years down the road, however. While South Sudan is now relatively peaceful, Mali has erupted into civil war and Al Quaeda-linked rebels have taken over the northern portion of the country. In the New York Times last week, Donald McNeil reported that public health teams haven’t been able to remove the worms and teach children how to avoid infection in the rebel-held territory. Officially, only seven cases of guinea worm were recorded in Mali last year, but there could be many more that have gone unrecorded. And thanks to the millions of embryos each guinea worm produces, it takes just one person to spark a massive outbreak. In 2006, McNeil writes, an infected student walked 250 miles to the northern Kidal region of Mali, where he spread the parasites to at least 400 other people. We can only hope that the civil war will end in Mali soon–not just to stop the immediate loss of life, but also to allow public health workers to stop the enormous suffering caused by diseases like guinea worms.
If we do drive Dracunculus medinensis extinct in the next few years, we will have eliminated a disease that was once spectacularly widespread. But we will also be wiping out a remarkable creature about which we actually know very little. When I went hunting for new research on the natural history of guinea worms this week, I found just about none. Most of what scientists have discovered about guinea worms, they discovered long ago. No one has sequenced the guinea worm genome. No one has used new staining technology to make its neurons light up. We don’t know how long it has infected humans, or where it came from before that. What little we do know should make us intensely curious about what we don’t know–and what we may never know.
For example, in 2002, a team of scientists at the State University of New York discovered that guinea worms make morphine. They speculate that it may use the drug to ensure a peaceful life inside its human host. The morphine may numb its host’s pain sensors, so that it can lurk unnoticed. Morphine also suppresses the immune system, which may partly explain how it is that we can carry a four-foot-long worm inside of us for over a year without experience organ rejection. Just as transplant surgeons tamp down a patient’s immune system before putting in a new liver, the guinea worm may apply its own immune-suppressing pharmaceuticals.
That’s just speculation, however, and once scientists can no longer study living guinea worms, it will probably remain so. And so will all the other secrets that the guinea worm is about to take to its grave.
(For more on guinea worms and parasites that don’t face imminent extinction, see my book Parasite Rex.)
January 22, 2013
The Strangest Cancer In the World
In thirty years, Tasmanian devils may be gone from the face of the Earth. If they do vanish, they will be wiped out in a fashion unlike any other endangered species we know of. The marsupials have developed a cancer that acts like a parasite, jumping from host to host.
In today’s New York Times, I take a look at what scientists are now learning about this strange contagious tumor, and the desperate measures they’re going to in order to protect the species from its unique devastation.
January 21, 2013
Snow Coyotes and Spirit Bears
Twenty eight years ago, the first coyotes arrived in Newfoundland. They had come a long way.
Up until the 1800s, coyotes lived mostly in the southwestern United States, and in low numbers in the Midwest. To the east and north, wolves shut them out of their forests. But when farmers and trappers exteriminated wolves in much of North America, the coyotes began to expand their range. By the 1970s, they had reached the far corners of New England. In the winter of 1985, there were reports in Newfoundland of wolf-like animals traveling across the ice to the Port au Port Peninusula. In 1987, a car hit one of the animals, and it was confirmed to be a coyote pup. The coyotes had come about as far east as they possibly could.
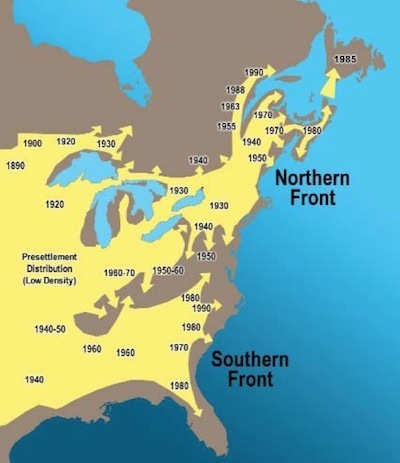
Source: http://www.env.gov.nl.ca/env/publicat...
Wildlife biologists on Newfoundland estimate that as few as three coyotes made that journey to the island in 1985. Their descendants remained rare on Newfoundland for the next two decades. But in recent years, the population has exploded. Newfoundland is now home to several thousand coyotes, which prey on caribou and scavenge moose carcasses. And starting in 2003, there have been occasional reports in Newfoundland of something truly remarkable: white coyotes.

Courtesy of Dawn Marshall
The Newfoundland Department of Environment and Conservation collects coyote carcasses as part of their research on these newly arrived immigrants. Out of the 6,000 specimens they’ve collected, six are white. The animals are not albinos, which produce no pigment at all. Instead, they’re more like Arctic foxes or polar bears, whose genes specifically turn their coats to snow, while allowing them to continue making pigment in their skin and eyes.
It’s only been in the past few years that scientists have worked out how certain genes influence the color of mammals. Pigment-producing cells called melanocytes can generate a range of hues, depending on the signals they receive. When certain proteins latch onto a receptor on melanocytes, for example, the cells produce dark pigments. If they don’t latch onto the receptor, called Mc1r, the cells make yellow or reddish pigments. Mutations to Mc1r can influence how strongly they relay their signals, and thus change the colors the cells produce. In humans, for example, variants of Mc1r produce red hair.
Dawn Marshall, a biologist at Memorial University of Newfoundland, and her colleagues, recently studied three white coyotes, sequencing their genes for Mc1r, along with two other genes known to be involved in color. They compared genes of the white coyotes to those of 59 ordinary coyotes to see if they could find any differences. And they did. They found that white coyotes carried two copies of the same variant of Mc1r. Some dark coyotes had the same variant, but only carried one copy; their other copy was a normal version. In other words, it appears that the white Mc1r gene is recessive. It takes two copies to turn a coyote white.
What makes that discovery particularly intriguing is that other scientists have seen the same mutation in Mc1r before: in golden retrievers. In the dogs, it appears to blunt the dark-pigment signals, causing them to grow light hairs.
These two findings may be no coincidence. During the coyote breeding season on Newfoundland in March 2001, a male Golden Retriever ran off with a coyote and was never seen again. It’s possible that the dog and the coyote interbred, and some of their coyote-dog hybrid pups inherited the Mc1r mutation. The coyotes that carried a single copy of the Golden Retriever gene would have looked ordinary. But from time to time, two coyotes with the gene would mate. And when a coyote pup inherited two copies of the mutant Mc1r gene, its coat became lighter. But in a coyote, these genes didn’t turn fur gold. They transformed the coyotes into snow.
There’s an eerie reflection of the snow coyotes on the other side of the continent. In the rain forests of coastal British Columbia live bears with ghostly white coats. The First Nation people who live in those forests have been familiar with these “spirit bears” for many generations. (See this 2011 National Geographic story for more information.) At first, zoologists thought that the spirit bears were a distinct subspecies of black bears. But then they started to see spirit bear mothers rearing black cubs.
Eventually, it became clear that the spirit bears were no different from black bears than red-headed people are from the rest of us. They had simply inherited two copies of a recessive gene that altered their fur. That gene–you guessed it–is Mc1r.

Spirit Bear--Nabil Harfoush via Creative Commons
Spirit bears have been around a lot longer than the snow coyotes of Newfoundland. And there are a lot more of them. Up to a quarter of the bears in some populations are white. Philip Hedrick of Arizona State University and Kermit Ritland of the University of British Columbia have studied the genetics of spirit bears to determine how they reached such high numbers.
Obviously, the bears did not pick up their version of Mc1r by mating with a Golden Retriever. Instead, the gene probably mutated spontaneously in some black bear long ago. When there was a single copy of this mutated Mc1r gene, it might have easily vanished. Each time a bear produces a sperm or an egg, only one copy of each gene ends up inside the new cell. It’s just a matter of chance. The original bear presumably passed down the gene to at least one of its cubs. From generation to generation, the frequency of the gene may have been determined by a roll of the biological dice.
Hedrick and Ritland argue that this random process–called genetic drift–could have boosted the number of spirit bears at first, but later, natural selection probably played a part. In other words, having the spirit bear version of Mc1r raised the average number of cubs that bears produced.
Animals that live in the far north often evolve white coats because it helps them blend into the snow. But spirit bears live in dense, dark forests, so it’s unlikely they get the same benefit from being white that polar bears do. One possibility is that it makes them better at fishing. When salmon return to the rivers in British Columbia, black bears and snow bears alike gorge themselves on the fish. To a salmon looking up through the water, a black bear looming overhead is far easier to see than a white bear that looks more like a cloud than a predator. As a result, scientists have found, spirit bears do much better at catching fish than their dark counterparts. Fat with fish, the spirit bears produce cubs that carry their genes.
Back in Newfoundland, Marshall and her colleagues speculate that their snowy coyotes may also be the product of both genetic drift and natural selection. If a golden retriever did indeed consort with a coyote in 2001, it did so at a time when there were still very few coyotes on Newfoundland. That would have meant that from the start, coyotes with the Mc1r variant made up a relatively large percentage of the coyote population. When the population exploded, the white variant might have exploded too. Nevertheless, the pattern of mutations in the white-fur gene hint that natural selection has been acting on the white coyotes as well. Newfoundland is hardly a snowy wasteland, nor do coyotes hunt for salmon, so it’s not clear what could drive the natural selection of white coyotes.
Marshall and her colleagues will need to take a closer look at the DNA of snow coyotes to get some more clues. And we’ll have to wait to see if snow coyotes vanish as inexplicably as they appeared–or if they become a familiar sight in the easternmost home of the coyotes.
January 18, 2013
Viruses That Make Zombies and Vaccines
This week the FDA announced that they were approving a new kind of flu vaccine. Nestled in the articles was an odd fact: unlike traditional flu vaccines, the new kind, called Flublok, is produced by the cells of insects.
This is the kind of detail that you might skim over without giving it a thought. If you did pause to ponder, you might be puzzled: how could insects possibly make a vaccine against viruses that infect humans? The answer may surprise you. To make vaccines, scientists are tapping into a battle between viruses and insects that’s raging in forests and fields and backyards all around us. It’s an important lesson in how to find new ideas in biotechnology: first, leave biologists free to explore the weirdest corners of nature they can find.
The standard way to make flu vaccines dates back to the Eisenhower administration. Scientists inject flu viruses into chicken eggs. The viruses hack the molecular machinery inside the eggs for making proteins and genes. Instead of bird molecules, the egg makes new viruses. The scientists then isolate the new viruses, kill them, and then isolate proteins from the dead viruses to put in the vaccine. Those proteins then prime the immune system to recognize proteins produced by live viruses and fight them off.
Influenza viruses grow in chicken eggs thanks to the similarities of bird and mammal biology. In fact, all the flu strains that menace us humans got their start in birds. They crossed over to our species by evolving the ability to invade our cells, replicate efficiently inside of us, and spread in our coughs.
But using eggs has a lot of drawbacks. A single egg produces a single dose, which means that a supply of tens of millions of vaccines is a massive undertaking. Making matters worse, eggs need months make new viruses, so that vaccine makers need lots of time to build up a supply before the start of a new flu season. That means that they have to decide which flu strains to use long before they can actually see which flu strains are dominating a flu season. Sometimes this scientific clairvoyance fails, and a vaccine turns out to be badly matched for a particular flu season’s strains.
A lot of scientists have been casting around for a better way. One promising idea is to take advantage of the sinister sophistication of something called a baculovirus.
Baculoviruses are sprinkled abundantly on plant leaves. They even wind up in our food. In 1973, scientists found that cabbage from grocery stores was coated in baculoviruses. A single serving contained up to 100 million of them.
Fortunately, we have nothing to fear from baculoviruses because they make insects their victims, with each strain only infecting one or a few species at most. But woe to the caterpillar that takes a bite of a baculovirus-coated leaf. The virus swiftly infects its cells and makes vast numbers of new baculoviruses. Some of the viruses spread from cell to cell. Others stay where they’re produced, manufacturing giant balls made of a protein called polyhedrin. The viruses become lodged in these balls. Between the new viruses and the new polyhedrin balls, a caterpillar can become visibly swollen with its infection.
All viruses need a way to get to a new host to escape extinction. Baculoviruses have a particularly creepy way of doing so. They produce a protein that interferes with a caterpillar’s biology, apparently making it ravenous. Normally, caterpillars will rest at the base of plants. Infected caterpillars roam day and night, feeding their inner parasites. They eventually dissolve, raining virus-packed protein balls on the leaves below. The protein balls are tough and durable, helping the viruses stay viable until another unwitting host comes munching along.
We know all this thanks to a lot of scientists dedicating their careers to these peculiar viruses. One particularly important advance they made was figuring out how to rear insect cells in a dish. Rather than having to infect an entire insect, they could now observe viruses invading individual cells. Whether any practical good would come out of all this research nobody could say at first.
But soon ideas did arise. Farmers can now use baculoviruses as a pesticide, for example. And then a less obvious application of baculoviruses turned out to be much more powerful: engineering their protein balls.
It’s fairly straightforward to engineer the genes of a baculovirus, swapping a gene from another species into its genome. Scientists figured out how to swap foreign genes for the polyhedrin gene, so that when the baculovirus infected insect cells, it made balls made out of the foreign protein instead. Evolution, in other words, had produce a remarkably efficient protein factory.
Scientists began to use baculoviruses to churn out proteins that scientists could study in large quantities. Over 500 proteins have been produced from baculoviruses, and nearly half of papers on proteins from animals and plants depended on this method. Scientists have also started to make medically valuable proteins, which can treat cancers and other diseases. And most recently, scientists have started using baculoviruses to make vaccines against other viruses.
Take papillomaviruses, which cause cervical cancer. GlaxoSmithKline identified proteins from the most dangerous papillomavirus strains that triggered a strong response from the immune system. They engineered baculoviruses with the genes for those papillomavirus proteins. The baculovirus did what it always did: it hijacked caterpillar cells and produced lots of protein balls. But then GlaxoSmithKline could isolate the proteins, stick them in syringes, and protect millions of girls and women from a deadly cancer.
The new flu vaccine, made by Protein Sciences, is produced in much the same way. Protein Sciences selected a flu protein known to trigger a strong response from the immune system–known as hemagglutinin–and engineered it into baculoviruses. The baculoviruses then infected insect cells and made hemagglutinin balls. The scientists isolated the abundant hemagglutinin and turned it into vaccines.
It’s important to note that Flublok is not terribly impressive. According to the FDA, it was about 44.6 percent effective against all circulating influenza strains, not just the strains that matched the strains included in the vaccine. It’s only approved for people between 18 and 49, and it’s got a shelf life of 16 weeks.
The drawbacks of Flublok may have less to do with being made by baculoviruses than simply the molecule Protein Science chose to engineer into them. As I recently wrote in the New York Times, other scientists are investigating the potential of other flu proteins–or even just fragments of proteins–to trigger long-lasting protection against the flu.
As a general approach to making flu vaccines, baculoviruses have some advantages over chicken eggs. They are so efficient at making protein balls, and insect cells are so small, that the process can potentially churn out more vaccines in less space. And it’s a fast process, thanks to the fast work of baculoviruses. Protein Science needed only 3 weeks to go from the genetic sequence of hemagglutinin genes to vaccine production. Baculoviruses are promising for vaccines to other diseases as well; work is underway for vaccines against HIV and malaria.
These days, it’s very easy to make fun of scientific research with no obvious practical importance. But we can’t predict where in nature we will discover the ideas that will make our lives better. It’s hard enough to believe that a virus can make a catepillar its zombie. It’s harder still to believe that this zombie-master could potentially save us from diseases.
[Thanks to Helen Branswell for some very helpful insights about flu vaccines]