Carl Zimmer's Blog, page 35
December 31, 2012
Happy New Year from the Loom!
Whew! Tomorrow, January 1, 2013, marks two weeks since I started writing here at National Geographic’s Phenomena. I’m in a holiday lull at the moment, but after New Year’s, I’ll rev back up to full speed. I’m looking forward to a delightful year in blogging in 2013, and hope you’ll join me for the ride.
December 29, 2012
Tectonic Skin (Saturday Science Tattoo)
Anna writes, “I’m a geology student at UCSC and I got to thinking what tattoo would really encapsulate the major, regardless of what I do with it. Plate tectonics is the driving force behind all geologic processes; it’s crazy to me how young the science is. So I got a tattoo, a map of the world with plate boundaries and the direction of their motion, along with a 3D image of a cross section of a subduction zone.”
You can see the rest of the Science Tattoo Emporium here and in Science Ink: Tattoos of the Science Obsessed.
December 28, 2012
Of Men, Navigation, and Zits
How, you may be asking yourself, is a good sense of direction like a bad case of acne?
Over many decades, psychologists have measured the minds of men and women, looking for similarities and differences. Reliable results are notoriously hard to come by, because it can be very easy to find differences where none really exist. If you decided in 1970 to look at the fraction of scientific and medical Ph.D. awarded to women–under 5 percent–you might conclude that women’s brains just weren’t suited to the task. Today, that figure has reached about 50 percent. Women’s brains haven’t evolved over the past 40 years. Their social environment has.
Yet a few differences between the sexes do seem to hold up to scrutiny. One is spatial abilities. If men look at an object, for example, they are slightly faster at guessing what it would look like if it were rotated 180 degrees. There are plenty of women who do better than individual men. But overall there’s a stasticially significant difference in their average performance. This kind of difference carries over from one culture to another. It’s even detectable in babies.
What accounts for the difference? Some scientists argue that it is an adaptation. Obviously, the evolution of the human race hasn’t hinged on being able to turn a stack of blocks around in our heads. But spatial abilities can have some far more practical benefits. If you can picture a landscape clearly in your head, you are less likely to get lost in it. People who score high on spatial ability tests also tend to do well on navigation tests. And in some studies (but by no means all), men do better at finding their way through a new place–be it a university building or a forest.
But why should men be better at women at navigation? Some researchers argue that we have to look at the different roles of men and women over course of our evolutionary history. Women spent most of their time in small ranges, either caring for children or gathering food. Men, on the other hand, had to rove over much bigger ranges to hunt game. They benefited from a better sense of navigation because they were at a greater risk of getting lost or failing to find game.
Whenever we reflect on human evolution, it pays to compare our species to other animals. And in the case of spatial abilities, the comparison is fascinating. Almost a century ago, the psychologist Helen Hubbet found that male rats could get through a maze faster than females. The difference can also be found in a number of other species.
If the “home range” explanation accounts for the difference, then you’d expect that species with a big difference between male and female home ranges should have a big difference in their spatial abilities. Some studies seem to bear that prediction out. Scientists have long been intrigued by meadow voles and their close relatives, the pine voles. Male meadow voles mate with lots of females, and have to defend a large home range against other males. Pine voles, on the other hand, are monogamous, and the males unsurprisingly have home ranges no bigger than their mates. When scientists look at their spatial abilities, they find that male meadow voles–the ones with big ranges–score much better than females. In pine voles, there’s very little difference.
QED? Not quite.
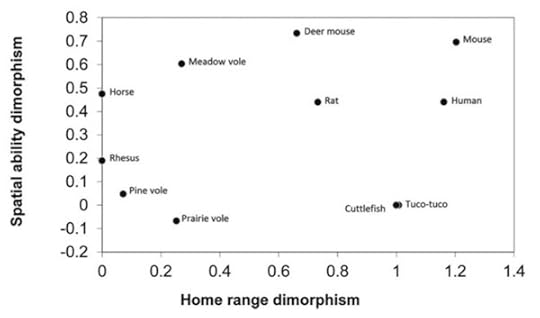
Two species are not enough to make a meaningful comparison, and so Edward Clint, a psychologist at the University of Illinois, and his colleagues recently made a larger comparison. They gathered data from studies on 11 species, including humans, voles, rats, and even horses and cuttlefish. They compared the differences in home ranges and spatial abilities. In the latest issue of Quarterly Review of Biology, they published their results, which can be summed up with this graph.
If the home range explanation was correct, you’d expect the points to fall along a line going from the lower left corner to the upper right. If you only look at the pine vole and the meadow vole, that indeed is what you find. But if you look at all 11 species, they’re all over the map. There is no correlation whatsoever.
Clint and his colleagues propose a different explanation: male spatial ability is not an adaptation so much as a side effect. Males produce testosterone as they develop, and the hormone has a clear benefit in terms of reproduction, increasing male fertility. But testosterone also happens to produce a lot of side effects, including male pattern baldness and an increased chance of developing acne. It would be absurd to say acne was an adaptation favored by natural selection. The same goes for the male edge in spatial ability, Clint and his colleagues argue. They note that when male rats are castrated, they do worse at navigating a maze; when they are given shots of testosterone, they regain their skill.
There are many sorts of behavior that have been shaped by natural selection. But it’s always important to bear in mind that what looks like an adaptation may be nothing of the sort.
[Image: Reinhard Dietrich, Wikipedia]
December 24, 2012
The Quantum Earthworm
When things get small–like millionths-of-an-inch small–they get very interesting. The ordinary rules of physics we’re used to fade back as the oddness of quantum physics looms large. Engineers have taken advantage of this fact by fashioning tiny bits of matter, known as quantum dots, that behave in all sorts of useful ways. For example, quantum dots made from cadmium telluride will respond to ultraviolet light by giving off a flash of visible light–the color depending on their size. If you attach certain molecules to cadmium telluride quantum dots, they will latch onto certain targets, making it possible to detect trace amounts of substances ranging from pesticides to cancer cells.
As versatile as cadmium telluride quantum dots are, however, they’re not easy to make. It’s especially tedious to fashion them so that they’re not toxic to living cells, since both cadmium and tellurium are nasty metals. In the latest issue of Nature Nanotechnology, a group of scientists at Kings College London offer a remarkably easy way to make them.
The scientists started with some dirt, into which they mixed cadmium chloride and sodium tellurite. Then they dropped earthworms into this polluted soil. The earthworms did what earthworms do: they sucked the dirt into their mouth and pushed it down the length of their bodies, digesting the nutrients and excreting waste out their back ends.
After eleven days, the worms were still happily grazing through the dirt, despite its Superfund-scale pollution. The scientists cut the animals open and searched for metals inside their bodies. They found that the cadmium and telluride the worms had eaten had broken away from their original molecular partners and had combined into cadmium telluride. In other words, the worms had manufacture quantum dots.
When the scientists flashed the dots with ultraviolet light, they gave off a green glow. The worm-fashioned quantum dots played nicely with living cells, the scientists found. They could use the dots to make cancer cells shine amidst a background of ordinary tissue.
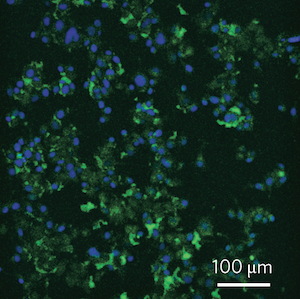
Cancer cells glow green with quantum dots made by earthworms. (The blue is from a dye that stains the nuclei of cells.)
There are many things to marvel at in such a study. We can imagine engineers harvesting quantum dots from giant earthworm farms and not be considered mad. But what I marvel at most of all is the fact that the earthworms were naturally so well prepared for the challenge. They have evolved to be underground alchemists. After all, when you make a living eating dirt, you have to be prepared for all sorts of unexpected nastiness.
Earthworms can sense metals in their meals. They immediately respond by making special enzymes. Exposing a worm to cadmium, for example, causes it to produce enzymes called metallothionein in its gut. The metallothionein grabs hold of the cadmium and stores it away in special cavities inside the cells, where it undergoes chemical reactions to make it less dangerous to the worm. Then immune cells attack the cells and engulf them. The worm eventually excretes them safely out of its body.
When scientists began decipering the chemistry that the worms use, their first idea was to enlist worms to clean up heavy metal pollution. That turned out to be a failure of the imagination. It may be that in the realm of nanotechnology, earthworm may truly shine.
[Worm engraving: Florida Center for Instructional Technology]
December 22, 2012
The Return of the Science Tattoo Emporium
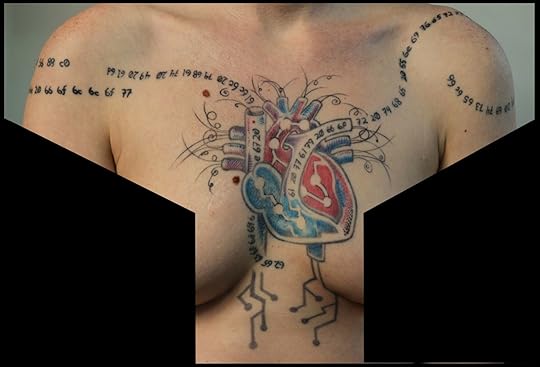 A few years back, I noticed a DNA tattoo on the arm of a neuroscientist. He informed me that it spelled out his wife’s initials according to the genetic code. And that enchanting discovery turned me, much to my own surprise, into an amateur anthropologist of scientist body art and the author of Science Ink: Tattoos of the Science Obsessed. (Reviews)
A few years back, I noticed a DNA tattoo on the arm of a neuroscientist. He informed me that it spelled out his wife’s initials according to the genetic code. And that enchanting discovery turned me, much to my own surprise, into an amateur anthropologist of scientist body art and the author of Science Ink: Tattoos of the Science Obsessed. (Reviews)
I’ve archived the ~300 tattoos here at National Geographic so that future generations of researchers can analyze them for clues to the folkways of scientists and the scientifically-minded in the early twenty-first century. And I’m still getting fresh ink via email. I will continue to post them here on Saturdays. (The tattoo-averse can thus safely continue reading the Loom while avoiding a scorch of their retinas.)
The tattoo shown here is from Willow Brugh. She writes:
I had this deep fascination with transhumanism in college (still do, to a degree), and geared up to go to law school in anticipation of the coming legal war over prosthetics and implants. Who controls the information going through your cochlear implant? If you would gain more dexterity through an artificial limb, is it legal for a surgeon to remove a fully operational biological limb? But I’ve seen law school brainwash a few people, so I started getting quotes tattoo’d on me as reminders of what I hold dear. It’s ASCII hex because the abstraction of language is fascinating to me, and because while my relationship to the quotes might change, our relationship to the networks we build (and how we speak to/with them) will only become more complex. Three lines of code in, I found out about the EFF and figured I should go into helping make a world where those cases happen. So I co-founded a maker space (Jigsaw Renaissance) and a way to link hacker and maker spaces together (Space Federation), and then got into engaging those communities with humanitarian response. That’s still my focus, with Geeks Without Bounds, we’re an accelerator for humanitarian projects.
The heart and its surroundings are because the quotes just keep coming up, and I didn’t want to look like the matrix. Many of my mental battles have been about accepting organic nature and seeing how it relates to the digital, and wanted to express that by having body art which embraced form. So I was sending all these pictures of images and tattoos I liked to my tattoo artist. A month before I went in for two more lines of code, all the conversations I was having started revolving around how I needed to pay more attention to my heart (I’m a bit of a robot, and learning to interact with emotion for the sake of people I care about has been a long and arduous journey). I walked into Action Tattoo, and Aaron had drawn this thing up – transit lines for the structures we build reflecting the organic world we come from, and the particle trails, and circuits, and of course the heart. So we got it done.
More details are at Willow’s blog. (Thanks to Vaughan Bell for noticing Willow on a train and pointing her in my direction.)
You can zoom in on the details below, in this Gigapan by Rich Gibson:
December 21, 2012
The Evolution of Cavities

Teosinte (left), corn (right), and a teosinte-corn hybrid (middle). Photo by John Doebley/Wikipedia
We’ve been tinkering with the DNA of other species for thousands of years. We just didn’t know what we were doing.
Starting about ten thousand years ago, humans began to steer the evolution of animals and plants. Our ancestors collected certain seeds instead of others, started to plant them in gardens, and gradually produced domesticated crops. They didn’t know which genetic variants they were choosing, or how those genes helped build new kinds of plants. All they knew was that some plants were better than others. Over thousands of years, for example, an innocuous bush called teosinte turned into tall stalks with gargantuan seeds–otherwise known as corn.
Our ancestors could see the outward changes they were bringing to crops like corn, as well as livestock like cows and pigs. But hidden from view, other species were evolving in response to the dawn of agriculture. Early dairy farmers had no idea that they needed certain bacteria to turn their milk to yogurt or cheese. And they didn’t realize that the bacteria were adapting to this strange new environment that had never existed before.
And it wasn’t just the bacteria in pots of yogurt that were evolving. So were the microbes in our mouths.
The human mouth is home to hundreds of species of bacteria. While some of them keep our mouths healthy, others can cause us trouble. One of the worst offenders is Streptococcus mutans. It lives in the nooks of our teeth, feeding on carbohydrates. It excretes lactic acid as waste, and the acid eats away at the enamel on which it rests. Over time, Streptococcus mutans can dig a hole in a tooth–otherwise known as a cavity.
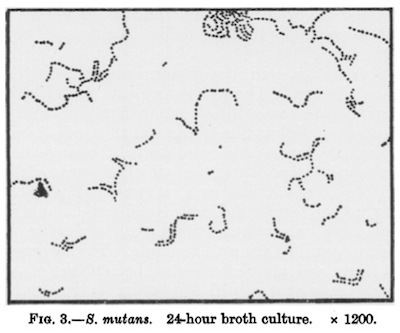
A drawing by J. Kilian Clarke's 1924 paper on the discovery of S. mutans as the cause of cavities
Streptococcus mutants is not some jack-of-all trades microbe that happens to drop onto our teeth once in a while. For them, human teeth are the world. They are passed down from mothers to their children, and colonize those children for life. Other mammals have closely related Streptococcus strains on their teeth as well, suggesting that these bacteria have been tracking their hosts–and dwelling on their teeth–for tens of millions of years.
Recently Michael Stanhope, a biologist at Cornell, and his colleagues carried out a large-scale study of Streptococcus mutans. They looked at 57 colonies that had been gathered from the mouths of people in Brazil, Britain, Iceland, Hong Kong, South Africa, Turkey, and the United States. The scientists tallied up the genes that the bacteria had in common, as well as the ones that were only present in some people. (Bacteria sometimes pick up genes from other species.) Then the scientists compared all the human strains of the bacteria to Streptococcus living in rats, hamsters, and monkeys to pinpoint the DNA that is unique to our own passengers. And when they analyzed all these genes, they discovered something remarkable: these bacteria appear to have evolved with resounding success in response to the rise of civilization.
The Streptococcus mutans the scientists found in people’s mouths shared 1490 genes in common–a core genome, as it’s known. These shared genes varied from mouth to mouth, Stanhope found, with minor mutations sprinkled among them. It’s possible to tally up these mutations and use them to reconstruct some of the history of their ancestors. Stanhope and his colleagues found that Streptococcus mutans underwent a population explosion. And that explosion started ten thousand years ago.
It may well be no coincidence that this was also when our ancestors began to farm. Those early farmers shifted to diets dominated by corn and other grains–which turned the human mouth into an endless banquet of carbohydrates.
Stanhope and his colleagues were also able to pinpoint some of the genes that were important for Streptococcus mutans’s adaptation to civilization. Fourteen genes, for example, show signs of having experienced strong natural selection. Some of those evolved genes are essential for breaking down sugar. Others help the bacteria survive in the acidic conditions that arise in the mouth when we eat starches.
Perhaps most intriguing of all the genes in Streptococcus mutans are the 148 that Stanhope and his colleagues found in every human strain, but in none of the related bacteria in the mouths of other animals. The best explanation for this is that Streptococcus mutans picked these 148 genes up from other species in our bodies. Once Streptococcus mutans grabbed these genes, it didn’t let them go.
The function of the genes hints at their value. Some provide additional help at breaking down sugar. Others create more defenses against low pH. Others produce toxins that can kill off other species of bacteria that are competing with Streptococcus mutans for the spoils of civilization.
These adaptations have made Streptococcus mutans spectacularly successful, and they’ve also provided us with a lot of misery. The cavities would be bad enough. Archaeological evidence indicates that cavities went from rare to common with the advent of agriculture. Making matters, worse, however, is the fact that when Streptococcus mutans gets into the bloodstream through the gums, it can make its way to the heart and cause problems there, too.
Appreciating how Streptococcus mutans evolved into such a successful burden might offer a way to fight it. Stanhope’s study provides a veritable catalog of adaptations that the germ relies on to transform the agricultural revolution into trillions of new bacteria. It might be possible to target one of those adaptations and attack Streptococcus mutans with pinpoint accuracy, leaving the rest of the residents of our mouths unharmed. We may have unwittingly made Streptococcus mutans what it is today, but we can wittingly do something about it now.
December 20, 2012
Redrawing the Tree of Life
 In 1837, Charles Darwin scribbled a simple tree in a notebook and scrawled above it, “I think.”
In 1837, Charles Darwin scribbled a simple tree in a notebook and scrawled above it, “I think.”
That little doodle represented a big idea: that species were descended from common ancestors. They looked different from each other today thanks to the differences that evolved after their lineages split.
It wasn’t until 1859 that Darwin presented this idea–buttressed by hundreds of pages of argument and evidence–to the public, in his book On the Origin of Species. He included a tree-like diagram in the book to illustrate his concept of how species evolved over time.
In neither of these two pictures did Darwin actually use the names of real species. But as biologist Theodore Pietsch explains in his wonderful new book, Trees of Life: A Visual History of Evolution, Darwin did try to map the kinship of some real species. In 1868, for example, he sketched a tree with humans on one branch and other primates on the others.
Generations of evolutionary biologists have continued to draw more extensive ones–trees that encompass not just primates, not just mammals, not just animals, but all living things.
A tree of life is a visual hypothesis. It’s a statement about how a scientist thinks species are related to one another, an arrangement that best explains the data the scientist can analyze. Those data grow over the years, as scientists find new species, as they find new methods for comparing more species at once, and as they find new things in those species to compare. And along the way, new hypotheses replace old ones.
Darwin could only compare humans to other primates based on their anatomy. In the mid-1900s, scientists began opening up a new lode of information to mine: DNA. There’s not much anatomy you can use to compare E. coli to a mountain lion, but both species share a number of genes, each with its own modified version.
In the 1970s, Carl Woese of the University of Illinois and his colleagues started comparing bits of genetic material across a vast span of species, and drew an entire tree of life. It displayed a stunning large-scale structure. All life, they found, belonged to three great branches. And the next couple decades only strengthened the hypothesis that life belonged to three great domains. This tree, from 1997, is the work of Norman Pace of the University of Colorado.
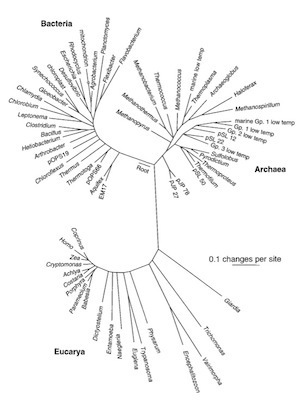 One branch was our own, the eukaryotes. This branch includes animals, plants, fungi, and protozoans. Eukaryotes share a lot in common. For example, our DNA is packed in a nucleus, where it’s coiled up into packages called chromatin.
One branch was our own, the eukaryotes. This branch includes animals, plants, fungi, and protozoans. Eukaryotes share a lot in common. For example, our DNA is packed in a nucleus, where it’s coiled up into packages called chromatin.
Another branch were the bacteria. This familiar lineage includes E. coli and lots of microbes you haven’t heard of. They have no nucleus, and they copy their genes with enzymes not found in eukaryotes.
And the third branch came out of the blue: the archaea. The species that Woese put on this branch were thought to be no different from bacteria, except that they were odd methane-producing microbes that turned up in swamp bottoms and other in nasty places. When Woese and others compared these species, they discovered some unique traits, such as certain types of molecules in their membranes. (In later years, scientists have found archaea in lots of environments, from the open oceans to our bodies, so they don’t deserve their initial reputation for extreme living.)
The three-domain tree continued to gain support as the years passed, and as more species came to light. But things, as they often do, got complicated.
For one thing, it became clear that some genes were not staying on their branches. DNA from one species can sometimes be delivered to the genome of another. That’s how antibiotic resistance spreads from microbe to microbe in your gut, for example. Huge portions of the genomes of species like E. coli were inherited from other lineages.
Some scientists have argued that this transfer of genes obliterates the tree-like structure of evolution. But a lot of the scientists I’ve talked to don’t go so far. They think of those shuttling genes as cars taking side roads to move from one superhighway to another. The flow of traffic still runs recognizably down the interstates.
Meanwhile, a second complication emerged. Maybe there were not three big branches, but just two. James Lake of UCLA first proposed this idea in 1984. He examined the protein-making factories of cells, called ribosomes. The ribosomes of eukaryotes were more similar to some kinds of archaea than others. That suggested to Lake a close kinship. In other words, we are just another branch of the archaea.
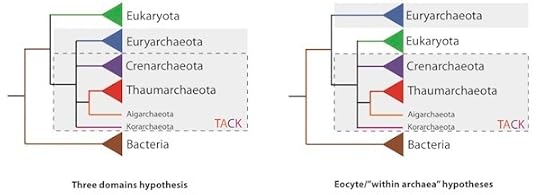
The latest test of this idea appears in the December issue of the Proceedings of the Royal Society (open access). It was carried out by Martin Embley of the University of Newcastle and his colleagues. They included some newly discovered archaea that are quite different from previously known species. They compared 41 proteins sequences from all the species, as well as 64 genes among the archaaea and the eukaryotes. Instead of just building a single tree, they constructed many trees from the genes and proteins and then compared them to each other to find the best fit to the data. They consistently found that eukaryotes fit best within the archaea, not on a separate branch.
Tom Williams, the lead author on the new paper, kindly made this figure to show the two alternatives they tested. We are on the green branch, the eukaryotes. The blue rectangle indicates the major branches of the archaea. “TACK” refers to four of those lineages. In the three-domain hypothesis, on the left, our ancestors split off from the ancestors of archaea. Williams, Embley, and their colleagues rejected that hypothesis and put eukaryotes within the Archaea, most closely related to the TACK microbes. It’s not clear which of them is our closest relatives, but the scientists are emphatic about one thing: there are not three domains of life.
 Why does this matter? For many reasons. Our ancestry runs through the ancestry of archaea. And by looking at archaea, we may be able to discern some key steps on the path to our eukaryote cells. Our cells have skeletons, for example: molecular scaffolding that give them structure and which they can reassemble to travel from one place to another. Recently, scientists have discovered archaea with two components of our skeleton: actin and tubulin. And in the new journal eLife, Corey Nislow and his colleagues report the discovery of the same coiled-up chromatin in archaea.
Why does this matter? For many reasons. Our ancestry runs through the ancestry of archaea. And by looking at archaea, we may be able to discern some key steps on the path to our eukaryote cells. Our cells have skeletons, for example: molecular scaffolding that give them structure and which they can reassemble to travel from one place to another. Recently, scientists have discovered archaea with two components of our skeleton: actin and tubulin. And in the new journal eLife, Corey Nislow and his colleagues report the discovery of the same coiled-up chromatin in archaea.
In other words, a lot of what we think of as the eukaryote cell may have already evolved over 2 billion years ago in our archaea ancestors–features that stil survive in some archaea today. This proto-eukaryote cell may have been like a chassis, onto which genes from bacteria were plugged in. And at some point, an entire bacterium snuck into our ancestors. Today, we use it to generate energy with oxygen.
****
Postscript: In working on this piece, I contacted some experts on the tree of life. Most favored the two-domain tree Embley argues for. “I’m pretty sure that the three domain hypothesis has been falsified,” James McInerney of the National University of Ireland told me.
I also contacted Norman Pace, who published the three-domain tree I reprinted above, to get his thoughts. But he didn’t get back in touch until after I had published this post.
Pace is not convinced by Embley’s new work. Every scientist who uses molecular data to build a tree of life has to rely on a model for how DNA evolves–whether some sites are more prone to mutation than others, for example. Pace suspects that the models of scientists like Embley and Lake are using are joining together branches in misleading ways. He argues that the molecules found in the membranes of all archaea are so different from those found in all eukaryotes that they must be separate domains.
“In short, my faith in the three domain tree is not fazed,” says Pace.
Image: A detail of a tree of birds by Max Furbringer, 1888. Universitätsbibliothek Heidelberg, via Creative Commons
[Update 12/20 3 pm: Fixed date of Darwin's sketch]
December 19, 2012
The Angelina Jolie Project

Lake Apoyeque, Nicaragua. Courtesy of Henrik Kusche
About 1800 years ago, a volcano in northern Nicaragua exploded. The crater formed by the eruption slowly filled like a rain barrel. Eventually the water rose high enough to warrant the title of lake. Today it is called Lake Apoyeque. Although Lake Apoyeque is over 300 feet deep, the rains have a long way to before they reach its brim. Lake Apoyeque remains ringed by volcanic cliffs towering as high as 1200 feet. And yet, despite its young age and its remote location, it is filled with fish.
For thirty years, Axel Meyer, an evolutionary biologist now at the University of Konstanz in Germany, has journeyed to Lake Apoyeque and other lakes of Nicaragua to study the evolution of their fish. He and his colleagues have caught cichlids and sequenced their DNA. By comparing their genes, the scientists can work out how the fish spread across the country. At Lake Apoyeque, for example, they found that the cichlids shared a number of mutations with the cichlids of Lake Managua nearby. The fish of Lake Apoyeque have accumulated relatively few mutations of their own. Meyer and his colleagues studied those mutations to estimate how long it took for their genetic diversity to evolve. They concluded that the cichlids came from Lake Managua to Lake Apoyeque only about a century ago.
There are forests and cliffs dividing Lake Managua and Lake Apoyeque, and no one can say exactly how the fish leaped over them around 1900. One likely route is a water spout. Water spouts are known to pass over Nicaragua, and it’s possible that every few thousand years one of them happens to suck up fish from one lake along the way and drops them in another.
But this aerial journey is not the most remarkable thing about the fish of Lake Apoyeque. What is most remarkable is obvious if you pick them up and take a close look at them.
It’s their lips.

Thin- and thick-lipped cichlids from Lake Apoyeque. From Elmer et al BMC Biology 2010
Most of the cichlids in Lake Apoyeque, like the one on the top, have thin, pursed lips. But some have plump, full lips, with the sort of curves that Upper East Side plastic surgeons charge dearly to provide to human beings.
You can find the same two types of fish mouths in Lake Managua. In lake after lake in Nicaragua, you can find more thin-lipped cichlids living alongside thick-lipped ones. You can find them thousands of miles away, on the cichlids in African lakes. In many cases, cichlids that have colonized a new lake have rapidly evolved into two distinct populations: one with big lips, and one without.

A thick-lipped cichlid from Lake Tanganyika in Africa. Photo by Ad Konings
Meyer and his colleagues study the cichlids of Africa and Central America to explore many of the most profound questions about how life evolves. They investigate how these fishes are diverging into new species, how they are evolving preferences about their choice of a mate, how they are evolving different ways of making a living. One of the most interesting things about cichlids is that once they become isolated in lakes, they end up evolving many of the same traits found in other isolated lakes.

Photo by Stefan Servos/Wikipedia/Creative Commons
To understand this parallel evolution, Meyer and his colleagues are taking a close look at those lips, investigating how the cichlids use them and how their genes produce them. In their lab back in Germany, the scientists like to call it “the Angelina Jolie project.”
If you watch thick-lipped cichlids in action, you can see how natural selection could favor the mutations that plumped up their mouths. They typically push their mouths into crevices of rocks on the bottoms of lakes. They then use their ample lips to form a seal around their prey–they often choose crabs or other animals lurking in the crevices–which they then suck into their mouths. The volcanic rocks are sharp-edged, and the cichlid’s padded lips provide them with protection. Thin-lipped cichlids, on the other hand, search for other kinds of food in the open water, where they don’t need to vaccum up their prey.
These are both perfectly suitable ways of making a living in a volcanic lake. But cichlids that are better adapted to one way or the other may have better luck than a cichlid-of-all-trades. Natural selection will then favor cichlids at the two anatomical extremes instead of those in the middle.
Until recently, it was impossible to probe the genes of the cichlids to see what mutations natural selection was acting on. Scientists like Meyer didn’t know what to look for. But in recent years a large consortium of scientists (including Meyer) have sequenced the genomes of cichlids. Now they have a map to guide them as the zoom in on the molecular details of cichlid evolution.
To investigate cichlid lips, Meyer and his colleagues caught cichlids from four lakes. Two of the lakes were young (Apoyeque, 1800 years old; Mayasa, 6000 years old), while the other two were big, old lakes from which the young lake cichlids traveled. (Lake Managua and Lake Nicaragua are both about 500,000 years old.)
As you can see from this figure from their new paper, all four lakes have their own pairs of thin- and thick-lipped cichlids. (Click on the image to enlarge)

From Manousaki et al, Molecular Ecology 2012
Meyer and his colleagues clipped off a sample from the lip of each of their cichlids. They then looked for signs of active genes in the lip cells. They wanted to see if thick-lipped cichlids made a bigger (or smaller) supply of certain proteins than thin-lipped ones. Changing the levels of proteins has long been known to be a powerful engine for evolutionary change.
The scientists found many such genes. In Lake Apoyeque, for example, 149 genes had significantly different levels of activity in the thin- and thick-lipped cichlids. In Lake Managua, there were 512 genes that were different.
Across all four lakes, however, six genes consistently behaved differently in the two kinds of cichlids. Meyer argues that these genes are crucial for evolving big lips. Some of them are known to control the growth of cartilage and muscle. Changing the levels of the proteins made by these genes might allow cichlids to bulk up their lips. Some of the other genes Meyer and his researchers identified help build the nervous system. This discovery raises the intriguing possibility that the thick-lipped cichlids use their lips not just to form a seal over their prey. Their lips may be studded with odor-sensing receptors that help them sniff out their prey.
Meyer’s new study suggests that evolution repeats itself, up to a point. In four different lakes, it appears that the same kinds of adaptations have evolved in cichlids through changes to the same six genes. If you want thick lips, there are not an infinite number of ways to get them. Meyer and his colleagues have also found that thick-lipped cichlids also evolve other traits in all four lakes. As you can see in the figure above, their heads have a conical shape, whereas thin-lipped cichlids have a blunter face. All those parallel traits may be part of the same suction-feeding package. To feed among rocks, it helps not just to have luscious lips, but also to have a narrow head you can push into crevices.

Carmelo Fruciano, Gonzalo Machado Schiaffino, and Axel Meyer check out cichlids for sale at a market in Granada, on the banks of Lake Nicaragua
At the same time, however, evolution is playing out differently in the lakes. Most of the genes with different activities in Lake Apoyeque are not the same as the ones in Lake Nicaragua, for example. There are differences in anatomy, too. Thick-lipped fishes in the some lakes have narrow teeth, while thick-lipped fish in other lakes have broader ones.
There are several possible explanations for these differences. The lakes are not identical, for one thing. Some lakes have a bigger variety of habitats for cichlids to adapt to; one lake may have a different collection of animals for the fish to hunt. And some of the differences Meyer and his colleagues have found may be little more than the result of a genetic roll of the dice. When a water spout picked up a few cichlids in Lake Managua, they may have had some peculiar mutations that were unusual in the population as a whole. But when they fell into Lake Apoyeque, those peculiar fish now became the entire population of cichlids in their new home.
Other scientists have also found a mix of similarities and differences in parallel evolution. They’re finding this complex pattern in the legs of lizards on Carribean islands, in bacteria adapting to a starvation diet, and echolocation in bats and whales. The thick lips of Lake Apoyeque are not an odd reflection of a Hollywood star, it turns out. They are a clue to one of the most important patterns in the history of life.
[Unless otherwise noted, all images are courtesy of Axel Meyer. Jolie on Wikipedia]
December 18, 2012
When You Swallow A Grenade
 In 1941, a rose killed a policeman.
In 1941, a rose killed a policeman.
Albert Alexander, a 43-year-old policeman in Oxford, England, was pruning his roses one fall day when a thorn scratched him at the corner of his mouth. The slight crevice it opened allowed harmless skin bacteria to slip into his body. At first, the scratch grew pink and tender. Over the course of several weeks, it slowly swelled. The bacteria turned from harmless to vicious, proliferating through his flesh. Alexander eventually had to be admitted to Radcliffe Hospital, the bacteria spreading across his face and into his lungs.
Alexander’s doctors tried treating him with sulfa drugs, the only treatment available at the time. The medicine failed, and as the infection worsened, they had to cut out one of his eyes. The bacteria started to infiltrate his bones. Death seemed inevitable.
But then, on February 12, 1941, Alexander was injected with an experimental drug: a molecule produced by mold.
The molecule was, of course, penicillin. It had been discovered thirteen years earlier but soon abandoned because there didn’t seem to be any way to turn it into an effective drug. In the late 1930s, Howard Florey and his colleagues at the University of Oxford revived the drug and began testing it on mice. They found the penicillin could cure them of infections by killing their bacteria. Florey then gave a dose of penicillin to a woman dying of cancer and found that it wasn’t toxic to her.
Now Florey and his colleagues wanted to see if it could stop an infection in a human being. Alexander, with nothing left between him and death, was their first subject.
“Striking improvement” was how Florey described what happened next. Within a day, Alexander’s infections were subsiding. After a few more days, his fever broke and much of his face cleared up.

An early apparatus for collecting penicillin
Florey could have saved Alexander’s life, if he hadn’t run out of penicillin after a few days. Nobody but Florey knew how to make the stuff, and his recipe only yielded a tiny amount at a time. To stretch out their supply of penicillin, a member of Florey’s lab would visit the hospital each morning to collect Alexander’s urine. He would carry it back by bicycle to the lab, where the scientists extracted the penicillin that Alexander’s body hadn’t absorbed. Alexander’s doctors then injected the recycled antibotic into Alexander’s arm.
But the salvaging operation didn’t recover enough penicillin to keep the bacteria from growing again. The infection returned and grew worse than before. On March 15, Alexander died. In his final report, Florey called Alexander’s death “a forlorn case.”
It is hard to imagine a time when a scratch could so easily lead to death. Albert Alexander died precisely at the dawn of the Antibiotic Era. Shortly after failing to save Alexander’s life, Florey harvested more penicillin and gave it to another patient at the hospital, a 15-year-old boy who had developed an infection during surgery. They cured him in a few days. Within three years of Alexander’s death, Pfizer was manufacturing penicillin on an industrial scale, packing 7500-gallon tanks with mold, fed on corn steep liquor. In that same year, Selman Waksman, a Rutgers microbiologist, and his colleagues discovered antibiotics made by soil bacteria, such as streptomycin and neomycin.
What made antibiotics so wildly successful was the way they attacked bacteria while sparing us. Penicillin, for example, stops many types of bacteria from building their cell walls. Our own cells are built in a fundamentally different way, and so the drug has no effect. While antibiotics can discriminate between us and them, however, they can’t discriminate between them and them–between the bacteria that are making us sick and then ones we carry when we’re healthy. When we take a pill of vancomycin, it’s like swallowing a grenade. It may kill our enemy, but it kills a lot of bystanders, too.
It’s understandable that few scientists gave this fact much thought in the 1940s, when the lives of people like Albert Alexander hung in the balance. Even if they did wonder about the 100 trillion microbes that live in our healthy bodies–known as the microbiome–they were poorly equipped to investigate them. They could only study bacteria that they could rear in their labs. E. coli thrived outside of the body, sucking in oxygen and feeding on just about any sugar on offer. That’s why scientists now understand E. coli better than any other species on Earth.
But in its natural habitat–the human gut– E. coli is a rare bird. Only about one microbe in a thousand in the gut belongs to the species. The rest of the microbes are far too fussy to survive in just any Petri dish. They need a special balance of gases, acidity, and nutrients. In many cases, they can’t survive unless they’re living alongside other species. Their fussiness has slowed down scientists trying to explore the microbiome. But now that they can fish out the DNA of the microbiome, scientists are beginning to get a sense of the staggering diversity of microbes we harbor.
Each of us is home to several thousand species. (I’m only talking about bacteria, by the way–viruses, fungi, and protozoans stack an even higher level of diversity on top of the bacterial biodiversity.) My own belly button, I’ve been reliably informed, contains at least 53 species. Many of the species I harbor are different than the ones you harbor. But if you look at the kinds of genes carried by those species, our microbiomes look very similar. That’s partly because surviving on a human body requires certain skills, so any species that is going to last long in your lungs, say, will need many of the same genes.
Three vital organs
But the similarity speaks to something else. The microbiome keeps us healthy. It breaks down some of our food into digestible molecules, it detoxifies poisons, it serves as a shield on our skin and internal linings to keep out pathogens, and it nurtures our immune systems, instructing them in the proper balance between vigilance and tolerance. It’s a dependence we’ve been evolving for 700 million years, ever since our early animal ancestors evolved bodies that bacteria could colonize. (Even jellyfish and spongeshave microbiomes.) If you think of the human genome as all the genes it takes to run a human body, the 20,000 protein-coding genes found in our own DNA are not enough. We are a superorganism that deploys as many as 20 million genes.
It’s not easy to track what happens to this complex organ of ours when we take antibiotics. Monitoring the microbiome of a single person demands a lot of medical, microbiological, and genomic expertise. And it’s hard to generalize, since each case has its own quirks. What happens to the microbiome depends on the particular kind of bacteria infecting people, the kind of antibiotics people take, the state of their microbiome beforehand, their own health, and even their own genes (well, the human genes, at least). And then there’s the question of how long these effects last. If there’s a change to the microbiome for a few weeks, does that change vanish within a few months? Or are there effects only emerge years later?
Scientists are only now beginning to get answers to those questions. In a paper just published online in the journal Gut, Andres Moya of the University of Valencia and his colleagues took an unprecedented look at a microbiome weathering a storm of antibiotics. The microbiome belonged to a 68-year-old man who had developed an infection in his pacemaker. A two-week course of antbiotics cleared it up nicely. Over the course of his treatment, Moya and his colleagues collected stool samples from the man every few days, and then six weeks afterwards. They identified the species in the stool, as well as the genes that the bacteria switched on and off.
What’s most striking about Moya’s study is how the entire microbiome responded to the antibiotics as if it was under a biochemical mortar attack. The bacteria started producing defenses to keep the deadly molecules from getting inside them. To get rid of the drugs that did get inside them, they produced pumps to blast them back out. Meanwhile, the entire microbiome powered down its metabolism. This is probably a good strategy for enduring antibiotics, which typically attack the molecules that bacteria use to grow. As the bacteria shut down, they had a direct effect on their host: they stopped making vitamins and carrying out other metabolic tasks.
In another intriguing response, the microbes dimmed their immune systems. To defend against invading viruses, bacteria deploy a collection of enzymes that recognize foreign genes and chop them up. As the bacteria dialed these enzymes down, they may have allowed viruses to infect them more easily. In some cases, the invasion led to their death. But in other cases, the viruses may have delivered them useful genes, including genes that let them resist the antibiotics.
Moya and his colleagues found that some types of bacteria were able to survive the onslaught of antibiotics, while others failed. As a result, the overall diversity of bacteria in the man’s gut changed from day to day over the course of his treatment. Before he started taking antibiotics, the scientists identified 41 species in a stool sample. By day 11, they only found 13. Six weeks after the antibiotics, the man was back up to 38 species. But the species he carried six weeks after the antibiotics did not represent that same kind of diversity he had before he took them. A number of major groups of bacteria were still missing.
This long-term disturbance was not unusual. Other scientists have tracked the diversity of the microbiome for many months after people get antibiotics. Even after all that time, the microbiome may not return to its original state. By disturbing our inner ecosystem, antibiotics can affect our own health.
In some cases, for example, antibiotics can make it easier for pathogens to invade. Eric Pamer of Memorial Sloan Kettering Cancer Center and his collegues recently provided a striking demonstration of this effect. They gave mice a single dose of the antibiotic clindamycin. Ninety percent of the diversity in the gut of the mice disappeared and was still gone four weeks after the treatment. The scientists then inoculated the mice with the spores of Clostridium difficile, a particularly nasty pathogen that can cause lethal cases of diarrhea. They invariably got an overwhelming infection, and half of them died within a few days. Pamer could wait as long as ten days after giving the mice antibiotics, and they were still felled by C. difficile. Healthy mice, on the other hand, easily kept the invasion in check.
Antibiotics may also exert subtler, longer-term effects on our health. Matthew Kronman of Seattle Children’s Hospital and his colleagues, for example, recently reviewed the medical records of over a million people. They found that children who took antibiotics were at greater risk of developing inflammatory bowel disease later in life. The more antibiotics they took, the greater the risk. Similar studies have found a potential link to asthma as well.
A study carried out by Dennis Kasper at Harvard hints at how antibiotics can send the immune system off the rails. They reared mice in isolated containers so that they never developed a microbiome. The germ-free rodents developed unusually high levels of an aggressive type of immune cell called an invariant natural killer T cell. If Kasper inoculated baby germ-free mice with a normal microbiome, the T cells remained rare. Antibiotics, the scientists propose, allow the T cells to explode and to run amok.
It’s even possible that long-term antibiotic use may influence how people put on fat. Martin Blaser of New York University and his colleagues carried out an experiment on mice in which they fed the animals antibiotics and then tracked their metabolism. The scientists found that the mice fed with antibiotics developed a higher percentage of body fat than mice that didn’t.*
Antibiotics cause this rise in fat, Blaser and his colleagues argue, by creating long-term changes in the microbiome. The species fostered in the mice produce enzymes that change not just how they break down our food, but also send signals to our own hormones to change the way we store energy from our food.
None of these results would ever lead a doctor to give up on antibiotics altogether. Seventy years after Albert Alexander died, they remain the best tool we have to fight off deadly infections. But we shouldn’t be blasé about them. Doctors often prescribe antibiotics to patients simply on the hunch that they have a bacterial infection. It often turns out that viruses are causing the trouble instead. Many parents are all too familiar with the endless cycle of ear infections and antibiotics. That cycle may take a toll.
There are changes that would help fight against that toll–some that we could make right away, and others that will demand a lot more research before becoming practical. We could become less casual about asking doctors for antibiotics. If DNA-sequencing becomes cheap enough, doctors might become able to diagnose bacteria infections quickly and accurately, so as not to prescribe antibiotics when they can’t help. And when it turns out we are infected, there are other ways to fight bacteria. For a century, some scientists have explored using bacteria-infecting viruses as a weapon against infections, for example.
It might even be possible to fight bacteria with bacteria. Instead of blasting both pathogens and harmless microbes alike, we might tend the microbial garden better and keep down the weeds. The most dramatic example of this gardening is the fecal transplant. Half a million people get C. difficile infections a year, many of which can’t be stopped by antibiotics. Doctors have found that a little stool from a healthy donor can crush these invasions. Fecal transplants may also help against inflammatory bowel disease, by restoring the immune system’s essential partners. Transplants might treat infections elsewhere in the body, from cavities in the mouth to rashes on the skin.
These treatments would do more than tamp down the harmful effects of antibiotics. They’d also help keep antibiotics themselves useful. When Florey first tried out penicillin against Alexander and other patients, he worried that the bacteria might adapt to the drug. It eventually did; for many pathogens, penicillin is now useless because they’ve evolved strong resistance against it. C. difficile and many other pathogens have become resistant against many other antibiotics, too. Developing new antibiotics is essential for stopping this decline, but we will need to use them just as sparingly to slow down evolution’s relentless push.
Otherwise, we may return to a time when roses killed policemen.
[This post emerged from the research I did for a talk I gave last week at Rutgers]
[Images: Grenade, Wikipedia; thorn, macrophile on Flickr via Creative Commons; bacteria, Health Research Board on Flickr via Creative Commons; Penicillin, NIH ]
[Update 12/18 11 am: Fixed Howard Florey's name.]
[*Upate 12/20 7:20 am: In the original version, I incorrectly stated that the mice's overall body mass increased. This was only true among female juveniles. By seven weeks, however, there was no increase in body mass--only its composition of fat and lean mass. Thanks to zmil for pointing out my error.]
Moving Day
Welcome to the Loom. I’d like to use my first post here to introduce myself and my blog, as I set up camp at National Geographic.
My relationship with National Geographic goes way back–back to a prehistoric era when I didn’t even know what blogs were. My first story for the magazine appeared in 2001. It was an exploration of the ways scientists figure out how old things are–the universe, the Earth, the animal kingdom, our own species. It was the first article I wrote as a freelance writer, having just left Discover, where I had been on staff for ten years. I’m forever grateful to National Geographic for welcoming me into the uncertain world of freelance journalism with journeys to mountaintop observatories and to the oldest patches of Earth still exposed on the planet’s surface.
Since then, I’ve written more stories for National Geographic, on topics ranging from Venus fly traps to feathers to brains. (I’m working on a couple now that I’m particularly excited about, but more on those in good time.) I also started regularly contributing pieces to the New York Times, where I still write frequently. (I’ve won some awards for my stories.) And since 1998, I’ve written 13 books. Here is a web site with more information if you’re interested.
In 2003, I noticed that some scientists and science writers had discovered a new way to write. They had software that allowed them to update their web sites on a daily basis. I was so fascinated that I had to join the experiment, and launched a blog I called the Loom (named after my favorite passage in Moby Dick).
Over the past nine years, the Loom has served as a lab where I can experiment with topics and styles that interest me but might not work as articles for newspapers or magazines. And I can try out different formats. The first time I embedded a Youtube video, I may have actually drooled.
When I was recently invited to join National Geographic’s salon, I immediately said yes. If I had to sum up what I write about, it would be natural history–the unfolding of life on Earth (and maybe elsewhere). No magazine in the world does a better job than National Geographic at exploring the majesty of natural history, both in text and in images. I’m delighted that they now want to make blogs a part of that mission.
On this blog, I will be exploring the many forms life has taken, and is now taking. I will also ponder the big science stories that will grab the headlines. In some cases, what really matters are the headlines themselves–in other words, the fate of science in the public square. I am a sworn enemy of sensationalistic distortions of science, along with science denialism in its many forms. I use the Loom to call them out. I also use the Loom to explore the utterly unexpected–such as the astonishing prevalance of science tattoos on scientists. (In 2011 I turned that particular line of blog posts into a book.)
If you use RSS to keep up with new blog posts, here’s my feed. Along with my new posts, National Geographic has kindly archived the nine-year archive of the Loom. If you are new to this blog, let me direct you to a few representative posts.
As you’ll see from these posts, the comment threads on the Loom can sometimes get pretty intense. This is a conscious decision on my part. Writing about science can mean triggering intense reactions in readers–whether the subject is evolution, the biology of the brain, global warming, or genetic engineering. I think readers should feel free to express those reactions–within reason–on my blog.
I actually find many of these comments useful to my own work. Sometimes people fact-check me and show me where I’ve made a mistake. Other times, they point me to a line of research that’s new to me. I’ve actually ended up writing magazine articles based on those helpful suggestions. (There is no copy-editor looking at this blog, so I appreciate notes about typos, which I will remedy quickly.)
This policy sometimes causes distress to some readers. I’ve gotten messages from people who think I shouldn’t allow any creationists to comment, for example. I completely disagree. If people want to leave posts disputing that the Earth is 4.5 billion years old, I see no harm in letting them do so. I will, however, respond by explaning why they’re wrong. I often find that the outcome of these exchanges is useful, if only as an opportunity to explore what science actually tells us, and what it doesn’t.
Besides, you know who won’t let you leave comments on their posts? Creationists. That should tell you something.
While the comments can get rough and tumble, I also expect people to behave. Even if you recognize that the Earth is 4.5 billion years old, that’s no excuse to use an obscenity on this blog against someone who doesn’t. If you make your point once, you don’t get to make it a hundred times, in the hopes of getting in the last word. If I write about global warming, you don’t get to make fun of Al Gore’s weight. If you accuse commenters or me of being a Nazi, or brain-damaged, or what have you, you are not welcome here. (National Geographic has additional rules about comments which you can find here.)
I can attest from personal experience that moving a blog always involves a lot of little technical glitches that take a while to eliminate. The folks at National Geographic and I are addressing them right now and will continue to do so for the next few weeks. Please bear with us during the transition, and please leave a comment on this post to let me know of any problem you encounter.