Carl Zimmer's Blog, page 32
February 22, 2013
On the Possible Shapes of the Brain
The Star Trek fans among you are no doubt familiar with “The Menagerie,” a two-episode story from the first season in 1966. The crew of the Enterprise gets trapped on a planet occupied by aliens called Talosions who look like humans, except for their massive, vein-encircled brains.*
That story was produced the year I was born, and when I saw it about a decade later, it made a big impression on me. Could brains get that big? Would they wind up with that cantaloupe shape? Would they provide Talosians with super-intelligence?
My ten-year-old brain was too underdeveloped to process the paradox that these were aliens living on another planet–and therefore would have likely followed an unimaginably different route of evolution. Chances are, the Talosians wouldn’t look like short middle-aged men wearing smocks, with foam padding pasted to their scalps. But as a grown man, I can still picture those Talosians in full Technicolor. They embody an interesting question: what are the possible shapes of the brain?
All of this is to explain the delight I experienced when I stumbled today across a recent paper entitled, “On the Possible Shapes of the Brain.”
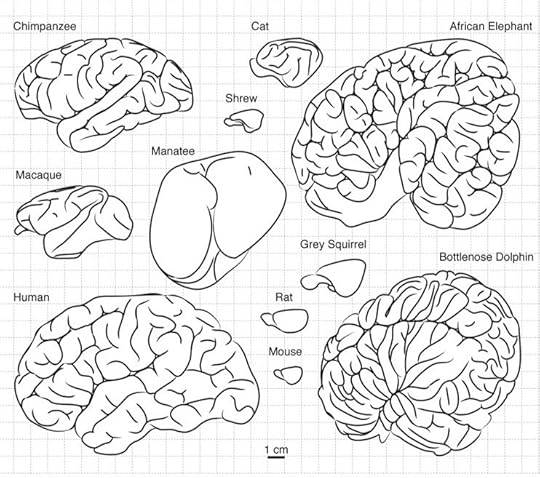
The paper is the work of Roberto Toro of the Insitut Pasteur in Paris. Toro investigates a couple key features of the brain: its size and the folds on its surface. Some mammals have smooth surfaces, while others have crumpled ones. This difference is important, because the outer layer of the mammal brain does most of the cognitive heavy lifting. The neocortex, as it’s known, is packed with neurons that have dense interconnections, enabling it to do sophisticated information processing. The interior of the mammal brain is taken up, in large part, with fibers that link different parts of the neocortex to one another, enabling them to share their information with one another.
The more wrinkled a brain gets, the bigger the surface of the cortext becomes. The human brain is especially wrinkled. If you look at a human brain, you only see about a third of its surface–the other two-thirds are hidden in its folds. If you could spread it out flat on a table, it would be 2500 square centimeters (a small tablecloth). A shrew’s brain surface would be .8 square centimeters.
The human brain isn’t just wrinkly, of course. It’s also big–three times bigger than the brains of chimpanzees, our closest relatives. And the relationship between the the size of brains and their surface area is what interests Toro in particular. If our brains were like balloons, increasing their volume would increase their surface area proportionally.
But our brains, it turns out, are not just like balloons. Bigger brains have a much bigger surface than you’d predict from just scaling up their volume like a balloon. Big-brained mammals have much more gray matter (the stuff of the neocortex) than small-brained mammals, in proportion to the connecting fibers.
This extra surface comes to use courtesy of our especially wrinkled brains. But there’s another intriguing thing about those wrinkles: they are not spread uniformly across our heads. The front of the neocortex is more wrinkly than the back. This is intriguing, because the front of the cortex handles much of the most abstract sorts of thinking. Our brains pack extra real estate there with additional folds. And if you look at mammals in general, they tend to have more wrinkles at the front than the back.
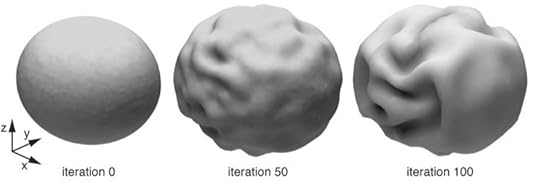 Given the importance of this architecture of the human brain, a question naturally arises: is the shape of the human brain precisely encoded by genes, or does it emerge from its enormous size? Toro and his colleagues have created mathematical models of the growing brain, which suggest that size does indeed matter. As a brain grows, the different regions of the cortex get further apart from each other. The white matter fibers that link them start to stretch. To avoid snapping these fibers, the brain needs to start wrinkling, so that neighboring regions of the cortext can stay close to each other. Wrinkles, in other words, are a natural result of a bigger brain. Wrinkles may develop more at the front of the brain because the back of the brain finishes developing earlier, when the brain is still small.
Given the importance of this architecture of the human brain, a question naturally arises: is the shape of the human brain precisely encoded by genes, or does it emerge from its enormous size? Toro and his colleagues have created mathematical models of the growing brain, which suggest that size does indeed matter. As a brain grows, the different regions of the cortex get further apart from each other. The white matter fibers that link them start to stretch. To avoid snapping these fibers, the brain needs to start wrinkling, so that neighboring regions of the cortext can stay close to each other. Wrinkles, in other words, are a natural result of a bigger brain. Wrinkles may develop more at the front of the brain because the back of the brain finishes developing earlier, when the brain is still small.
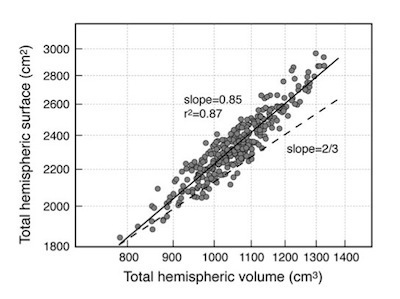 You can see the link between the size of brains and their wrinkles not just by comparing different species, but by comparing people. Toro and his colleagues have compared the brains of 300 Canadian teenagers. As the graph here shows, there’s a remarkable range in the sizes of their brains, from 800 to 1400 cubic centimeters. The total surface of the brain is proportional to the volume of the brain. But if our brains were mere balloons, people’s brains should fall along the dotted line. Instead, the surface of the brain increases faster as its volume expands. This expansion is the result of extra wrinkles–with more wrinkles towards the front than the back.
You can see the link between the size of brains and their wrinkles not just by comparing different species, but by comparing people. Toro and his colleagues have compared the brains of 300 Canadian teenagers. As the graph here shows, there’s a remarkable range in the sizes of their brains, from 800 to 1400 cubic centimeters. The total surface of the brain is proportional to the volume of the brain. But if our brains were mere balloons, people’s brains should fall along the dotted line. Instead, the surface of the brain increases faster as its volume expands. This expansion is the result of extra wrinkles–with more wrinkles towards the front than the back.
One of the most striking patterns in human evolution is the expansion of our brains, especially over the past two million years. If Toros is right, this expansion led to its reorganization. Simply making a mammal brain bigger forces it to take on other features, such as extra wrinkles on its front end, increasing its surface allowing it to get parcelled up into specialized areas.
These patterns are not the whole story of why our brains are shaped the way they are. A Talosian brain might be impossible to evolve for other reasons, such as the strain it would put on mothers in childbirth, or the huge amount of fuel it would demand. But if natural selection could favor even bigger brains than we have today, perhaps we’d end up as Talosians. But if we did, our brains would become fiercely wrinkled.
*If any Star Trek fans are tempted to correct me and point out that “The Menagerie” was originally a pilot for Star Trek called “The Cage,”, I’ve just done your work for you. You can thank me later.
[All images Toro 2012]
February 20, 2013
An Infinity of Viruses
When I talk about viruses, I have to struggle with big numbers.
If you get sick with the flu, for example, every infected cell in your airway produces about 10,000 new viruses. The total number of flu viruses in your body can rise to 100 trillion within a few days. That’s over 10,000 times more viruses than people on Earth.
If there can be so many viruses in a single person, how many viruses are there in total on our planet? I’ve hunted around for a number, and the one I’ve seen most often is 1031. As in, 10000000000000000000000000000000. As in over 10 million times more viruses than there are stars in the universe. As in, if you were to stack one virus on top of another, you’d create a tower that would stretch beyond the moon, beyond the sun, beyond Alpha Centauri, out past the edge of the Milky Way, past neighboring galaxies, to reach a height of 200 million light years.
Now it seems that I may have been lowballing that number.
Scientists don’t come up with a number like 1031 by counting every single virus on Earth. They take surveys in different environments–the soil, the water, the ocean floor–and then extrapolate. As they take more samples, they can adjust their estimate up or down.
It used to be that scientists could only conduct these surveys by squinting down a microscope and actually spotting the individual viruses. That turns out to be a bad way to count viruses, in part because their hosts–such as bacteria and protozoans–often don’t grow in the artificial confines of a laboratory. Without viable hosts, the viruses can’t reproduce, and so they go unnoticed.
Things got a lot better once scientists found a new way to count. Instead of looking for full-fledged viruses, they just looked for pieces of their DNA. The ocean, once considered a desert for viruses, turned out to be a broth of viral DNA. Given the sheer volume of the oceans, most of the world’s viruses reside there. And most of the viruses that scientists discovered in the ocean turned out to be parasites of bacteria, known as bacteriophages.
But scientists have long known that there’s another kind of virus out there, one that uses a somewhat different molecule for its genes.
DNA is a double helix, which encodes genes along both strands. When our cells make a protein from a gene, they make a copy on a single-stranded molecule called RNA. DNA-encoded genes can also make RNA molecules that do other jobs, such as sensing the concentration of various elements inside a cell.
In some viruses, RNA also takes on DNA’s job, and encodes genes. Influenza is just one example of the many RNA viruses out there (or in you). Unlike bacteriophages, RNA viruses almost never infect bacteria. Instead, they infect us, plants, fungi, protozoans–all members of the same great lineage of life known as eukaryotes.
The methods that scientists use to tally up DNA viruses often miss RNA viruses, so it’s been hard for scientists to estimate how many RNA viruses are on Earth. In the current issue of the ISME Journal, scientists at the University of Hawaii describe a new method they developed to fill that hole.
The scientists first scooped up water from off a pier on Oahu (about 115 liters all told). They then passed the water through fine filters to exclude bacteria and larger organisms. Through a series of additional steps, they concentrated the viruses down further and then extracted all the DNA and RNA from their samples. The scientists then measured how much of each kind of molecule was present in the sea water. Based on the mass of RNA in an average virus, they could then estimate how many RNA viruses were in the sample.
Their conclusion was remarkable: RNA viruses made up between 38 and 63% of the viruses in the sea water. In other words, about half of the viruses in the ocean are RNA viruses.
This can be surprising when you consider the number of hosts that RNA and DNA viruses can infect. In the ocean, bacteria (hosts to DNA-based bacteriophages) far outnumber eukaryotes (hosts to RNA viruses).
But it’s important to bear in mind that eukaryotes are better as virus incubators. A single eukaryote cell can spew out far more viruses than a single bacterium. Bacteria can’t churn out viruses the way you can, my dear eukaryotic reader.
This new study is just a first cut at estimating the number of RNA viruses on Earth. It’s possible that for some reason the waters off the coast of Hawaii are weirdly good places to find them. The scientists themselves acknowledge that they may be overestimating the number of RNA viruses through some flaw in their methods. But when I contacted an expert on global virus surveys, Curtis Suttle of the University of British Columbia, for his opinion, he gave a thumbs-up. “I think it is an interesting and provocative paper,” he told me. “We will have to see if it holds up.”
If it does, I’ll have to find some new metaphors to describe 10000000000000000000000000000000 + 10000000000000000000000000000000.
[For more on viruses, here's a recent talk I gave, and here's my book, A Planet of Viruses.]
_________________________
*Some people say viruses are truly alive, and some say they aren’t. But if they aren’t, then we need some name that can encompass the things that are “really” alive, along with the things that carry genes, can evolve, and carry out all the other life-ish tasks that viruses do.
February 18, 2013
The Weird Youth of the Animal Kingdom (Slide Show)
Paleontologists have found traces of animal life dating back at least 635 million years. Those earliest animals may have been like today’s sponges, rooted to the sea floor and filtering food particles from the water. Over the next 100 million years or so, new kinds of animals emerged. Some were recognizable members of living groups of animals, while others were so bizarre that paleontologists suspect they belonged to long-extinct lineages. And then, around 520 million years ago, the fossil record of animals starts to roar like a firehose switched from a trickle to full blast. Many of the oldest known members of living animal groups–including our own–appear during the Cambrian Period. But the Cambrian fossil record is also rife with forms only distantly related to animals on Earth today, some of which were so weird that the sight of a reconstruction of the creatures made scientists burst out laughing.
Many people have become familiar with this period of evolution through Steven Jay Gould’s 1989 influential book, Wonderful Life. In the 24 years since then, scientists have learned a lot more about the Cambrian. Two of the leading experts on the period, Doug Erwin of the Smithsonian Institution and James Valentine of Berkeley have collaborated on a new book, The Cambrian Explosion: The Construction of Animal Biodiversity, in which they synthesize evidence, both old and new, about this exceptional chapter in animal evolution.
The so-called Cambrian Explosion probably had many fuses. Erwin and Valentine explain how the Earth was undergoing drastic changes in the millions of years leading up to the flowering of the animal kingdom, with global ice ages and a burst of oxygen flooding the oceans. The stage was set for big, active creatures to evolve. As predators emerged, their prey became better defended with spikes and shields; the predators in turn became even deadlier. The animals changed their environment–burrowing animals, for example, pierced the sea floor with countless tunnels. As the environment changed, new kinds of animals evolved that could occupy new niches. The animal kingdom became both physically and ecologically complex.
But the diversity of the Cambrian had another source: the DNA of the animals themselves. Animals evolved genetic programs for turning a single egg into a complex body. These programs turned out to be supremely evolvable–with relatively minor mutations, they could give rise to new forms.
The book is also accompanied by some remarkable paintings by Quade Paul. I’ve reproduced some of them here as a slide show, and below.

Myllokunmingia may be the oldest known vertebrate, with a skull made of cartilage and other hallmarks of vertebrates (like us). Copyright Quade Paul

Herpetogaster may be related to living starfish and acorn worms
Banffia is baffling; it’s not clear yet what its closest living relatives are. Copyright Quade Paul

Diana had spiked, segmented legs that show some similarities to those of insects and other arthropods. Some scientists see that as a sign of close kinship. Copyright Quade Paul

Hurdia was a primitive cousin of insects and other arthropods. Copyright Quade Paul

Anomalocaris was a giant of the Cambrian, reaching over a meter long. Copyright Quade Paul

Fuxianhuia is a close relative of living arthropods such as insects. Copyright Quade Paul

Odontogriphus had a circular mouth ringed with teeth. Copyright Quade Paul

Wiwaxia may have been related to today’s mollusks. Copyright Quade Paul

Maotianoascus and Ctenorhabdotus were early relatives of today’s jellyfish. Copyright Quade Paul
Orthozanclus, a relative of mollusks. Copyright Quade Paul

Pikaia was a relative of vertebrates. Copyright Quade Paul

Opabinia had five eyes and a single appendage extending from its head. Copyright Quade Paul
All the pictures in this post are copyright 2013 Quade Paul.
(Full disclosure: The publisher of this book, Roberts & Company, also publishes my textbooks, which include some illustrations by Paul.)
February 17, 2013
Today’s Microbiology Class Will Be At the Tattoo Parlor (Science Ink Sunday)
 Jonathan Kurtz writes, “I started graduate school four years ago, studying the immune responses to chronic Salmonella infection in mice, similar to typhoid fever in humans.
Jonathan Kurtz writes, “I started graduate school four years ago, studying the immune responses to chronic Salmonella infection in mice, similar to typhoid fever in humans.
“My project developed into defining how infections are combated in different anatomical locations and the host/microbial factors dictate these responses. I am scheduled to do my post-graduate studies with a collaborator of ours, so that I may stay in the Salmonella field, studying what is now a lifetime love/interest/career.
“There are few things in my life that have had greater impact in my life than Salmonella. Therefore, I thought it’d only be appropriate to have a permanent reminder. I quickly decided I wanted it placed on my inside ankle. The problem was finding the right person for the job. Once I found an artist willing to tattoo it (Scott Barbier at Electric Ladyland in New Orleans), we went back and forth about an accurate versus an artistic representation of Salmonella. Scott decided to freehand the tattoo on my ankle.
“While nervous at first, I was very pleased with his conturing of the peritrichous flagella around the natural curves of my ankle. As any good artist, Scott wanted to add more details and begun tattooing what to him were dots, but to me were outer membrane porins or pili. He also decided to shade the cell wall core.
“I could not have been happier with the results. Throughout the process, I discovered that this tattoo was the oddest one that he–or the store, for that matter–had ever tattooed before. This of course led to many artists popping their heads in the room with eager eyes to witness my developing tattoo. I certainly felt like I was a unique spectacle, so I shared a few different fun facts about Salmonella with them as they watched. The whole experience added to my personal love of Salmonella and my tattoo. Now what to get tattooed next!”
You can see the rest of the Science Tattoo Emporium here and in Science Ink: Tattoos of the Science Obsessed.
February 13, 2013
A Flurry of Frog Legs
In the mid-1990s, people in the United States and Canada began to notice something grotesque. The frogs in their local ponds were sprouting extra legs.
As news of the deformed frogs spread, the Minnesota state government set up a hot line for sightings, and soon they got hundreds of calls from 54 out of 87 counties. “I’ve seen a lot of frogs over the years, and I’ve never seen anything like that,” a University of Minnesota herpetologist told the New York Times in 1996.
Citizens and scientists alike feared that whatever was altering the frogs–pesticides perhaps–was also having an effect on humans. But researchers didn’t find any compelling link between frog deformities and humans diseases such as cancer. In fact, within a few years it looked as if the frogs were getting their legs naturally–through the manipulations of a parasite.
The parasite in question is a flatworm called Ribeiroia. It starts out life in snails. It grows and reproduces inside the snails, which it castrates so that they don’t waste time on making eggs or looking for a mate. In its castrated host, the parasite produces a new generation of flatworms that can escape the snail and swim in search of a vertebrate host. They typically infect fish or tadpoles. When they invade tadpoles, the parasites bury themselves in the tiny buds that will eventually grow into legs.
As the frogs develop their legs, the parasites wreak havoc. In some frogs they will stunt the growth of a leg, leaving it a stump. In other frogs, a developing leg forks in two. A single frog may even sprout a dozen legs. It’s not clear yet how the parasites manage this feat, but one recent experiment offers a clue.
In order for a limb bud to develop properly, its cells have to produce certain molecules. The molecules spread out across the limb bud, causing other cells to make other molecules, to grow faster, to die off, and to do all the other things required to make a limb. (See my article in the New York Times for more on this process.)
One of the crucial molecules for building legs is a version of Vitamin A, known as retinoic acid. Dorina Szuroczki and Nicholas Vesprini of Brock University and their colleagues found that before the swimming parasites find a tadpole, they are producing retinoic acid. Once they’re buried in the frog’s limb bud, their level of retinoic acid drops. Meanwhile, the level of retinoic acid in the limb bud shoots up 70 percent. All of these findings are consistent with the idea that the parasite is injecting limb-deforming drugs into their host.
The deformities of the frogs are not merely a side effect of their getting sick, in other words. They’re part of a strategy that the parasite uses to advance its life cycle. To understand why this would be so, you have to bear in mind that the frog is just a way station for the parasites. They cannot mate or reproduce in frogs. Instead, they have to wait to get into a bird, where they take up residence in the gut and produce eggs that are shed by their host. And they can only make that trip if the frog they inhabit is caught by a bird. Some frogs get eaten, and some don’t. A parasite in a frog that escapes death by bird will die without reproducing.
Brett Goodman and Pieter Johnson of the University of Colorado ran an experiment in 2011 to see what effect the limb deformities have on the frogs. They infected frogs in their lab and then compared their performance to healthy animals. The scientists found that the jumps of malformed frogs were 41 percent shorter than those of healthy frogs. They swam 37% slower and had 66% less endurance. They tried just as often to catch crickets, but they caught 55% fewer insects.
Goodman and Johnson then studied the frogs in the wild, observing how well they survived in a pond in California. Surprisingly, extra legs had no significant effect on the survival of the frogs–as long as Goodman and Johnson kept their ponds free of predators. The deformed frogs could still get around well enough to find enough food to stay alive. But in ordinary ponds, the parasitized frogs were at grave risk. Over a fifth of them died every two weeks, and when a given generation of frogs became adults, there were almost no deformed frogs left among them. Instead, they had delivered their parasites to their next home.
The discovery of this parasite manipulation was not the end of the story, though. Even though Ribeiroia has probably been infecting frogs long before people showed up in the United States, the level of infection might be influenced by a number of factors. Johnson and his colleagues have found, for example, that frogs that live in water contaminated with high levels of fertilizers were more likely to be infected with Ribeiroia. Pesticides can kill off the parasites, some studies show, but they also lower the defenses of the frogs, which may lead to higher infections.
In this week’s issue of Nature, Johnson and his colleagues now offer evidence for another factor in the success of leg-deforming parasites: biodiversity.

Credit: D. Herasimtschuk, Freshwaters Illustrated
For some years now, a number of ecologists and parasitologists have developed the idea that biodiversity protects against disease. The notion is this: parasites in search of a new host sometimes end up in the wrong species. A bird flu virus that gets into a bird, for example, can make billions of new viruses that are shed in the bird’s droppings, which can then infect other birds. But bird flu sometimes gets into a human and cannot spread any further. The more species in an ecosystem, the argument goes, the more likely parasites are going to hit a dead end and get diluted. In low-diversity ecosystems, parasites will be more likely to hit the right host, make more copies of themselves, and cause more disease.
It’s a very influential idea, but, like many ideas in ecology, very hard to test. Johnson and his colleagues realized that Ribeiroia offers a very good opportunity to do so. The parasite only manages to trigger limb deformities in some of its hosts, and it has more success in some amphibian species than others. What’s more, each pond occupied by the parasite and its hosts is like a test tube in which the experiment is replicated. Johnson and his colleagues have visited 345 sites in California wetlands to examine Ribeiroia and its hosts. All told, they studied 24,215 amphibians and dissected 17,516 snails.
In these wetlands, the parasite is most successful when it infects Pacific tree frogs. But it can infect other frog species and even the salamanders that live alongside the frogs. Johnson and his colleagues found that in ponds with high biodiversity–up to six species of amphibians–the parasites did much worse at getting transmitted than in low diversity ponds. This was no minor difference: there was a 78.5% decline in deformed frogs in high-diversity sites. To test this pattern, Johnson and his colleagues put groups of amphibians into tanks along with infected snails. The frogs in high-diversity tanks had half the parasites as the ones in low-diversity tanks.
It seems, then, that we can add low biodiversity to the list of factors that can produce a flurry of frog legs, along with pesticides and fertilizer. Johnson also suspects that the snails are important too. As the parasite population grows, it castrates more and more snails, until their population crashes. Once the snails become scarce, the parasites become scarce, too, giving the frogs a break. Unfortunately, no one has yet conclusively shown that any of these factors has driven a long-term change in frog deformities of the sort that made headlines in the 199os.
Nevertheless, this study is important for a few reasons. For one thing, frogs are in big trouble these days. Species are winking out around the world, and diseases appear to play a big part in their demise. Biodiversity itself may defend the frogs against dangerous outbreaks.
It also tells us something about our own well-being. We don’t have to worry about frog flatworms getting into our bodies and causing us to sprout extra legs, thank goodness. But many other pathogens that do make us sick also lurk in other species, such as West Nile virus and hantavirus. And the more species that can dilute those pathogens, the healthier we’ll be.
[For more information on the link between biodiversity and human health, see David Quammen's new book, Spillover. I write about parasite manipulations in Parasite Rex.]
February 12, 2013
Lifting Brain Fog
T cell. Wellcome Institute. http://www.flickr.com/photos/wellcome...
Ten years ago, Jonathan Kipnis decided to run an experiment to see how well mice can learn new things. He suspected that the immune system was important to cognition, and so he wanted to compare mice with normal immune systems to mice with deficient ones. Kipnis engineered mice that lacked T cells, a type of white blood cell that fights pathogens. At the time, Kipnis was getting his Ph.D. at the Weizman Institute in Israel, and the lab he was working in didn’t have the equipment necessary for the test. So he shipped off his mice–a group of normal ones and a group lacking T cells–to Ben-Gurion University. There, his colleague Hagit Cohen put the mice through their paces.
Cohen gave the mice a task known as the Morris water maze. She put them in a pool of water, where they started to swim frantically. Just under the surface of the water there was a hidden stand. If the mice could find the stand, they could climb onto it and stop their desperate swimming. Over several rounds, the mice learned where the stand was hidden and swam straight for it.
Once she had tested the animals, Cohen gave Kipnis a call. “She said, ‘One of the groups of the mice you sent me are real idiots. I’ve never seen such idiotic mice,’” Kipnis told me when I talked to him about his research.
The idiot mice were the ones without a T cell between them.
Kipnis, who is now at the University of Virginia, has spent the past decade following up on his discovery, exploring the potential influence of the immune system on the brain. It may help to explain all sorts of puzzles, such as why getting sick can put our minds in a fog. His work is the subject of my column in the latest issue of Discover. This piece is my final one as a Discover columnist, and I’m particularly happy to end with such intriguing research, which encourages us to understand the brain by looking beyond it. Check it out.
February 11, 2013
To Stop Rabies, Stop Helping It
If you get rabies and don’t get help in time, you will almost certainly die. While there are a few tantalizing reports of people surviving a case of rabies without medical attention, there are all too many cases of people dying after getting bitten by a rabid animal. The virus sneaks from the bite wound into a nearby nerve and then makes its way through the nervous system. Along the way, it can make people mad and send them into comas before killing them. Officially, over 50,000 people die of rabies each year, but that number is likely a gross underestimate. A lot of rabies cases occur in places where the causes of deaths go unrecorded. In one study in Tanzania, doctors determined that the actual number of deaths from rabies was over 100 times higher than the official count.
The rabies virus is hugely dangerous not only because of its deadliness, but because doctors are so ill-equipped to fight it. The standard way to treat people with rabies is to give them a massive dose of rabies vaccine. The vaccine primes the immune system to fight the rabies virus. On top of the vaccine itself, patients also need to get shots of immunoglobin, a molecule that stimulates the immune system. The treatment leaves a lot to be desired. For one thing, it takes a while for the immune system to rise up against the rabies virus. By the time it has swung into action, the virus may have made too many copies of itself to be wiped out. As a result, getting a rabies vaccine only works if you get to a doctor soon after getting bitten. Making matters worse, the vaccine is expensive and needs to be kept refrigerated. What the world needs is a penicillin for rabies: a pill that’s cheap to make, can sit on a shelf for years, and kill viruses quickly.
In Wired last year, I wrote about one of the teams of scientists who are looking for a rabies antiviral–a group based at a small San Francisco start-up called Prosetta. I paid a visit to Prosetta while researching the story. On the day of my visit, Vishwanath Lingappa, the company’s co-CEO, had a batch of rabies virus stewing in a flask on his lab bench. I sat in on a meeting where he proudly showed off some of the results of the experiments to his co-workers. Lingappa is an American-born Indian with a deep radio-talk-show-host voice. As he explained the results of his experiment, pointing eagerly to details of his slides, he got more and more excited. Finally, in triumph, he invoked his favorite philosopher, John Dewey, in a bellow: “The givens of experience are not given. They are taken! With great difficulty.”
It would take many more months to get the results ready for publication. Now, finally, Lingappa and his colleagues (including his sister Usha, a virologist at the University of Washington) are publishing the study this week in the Proceedings of the Academy of Sciences. They don’t have a cure for rabies yet, but they have found drugs that show a lot of promise. And what makes their study even more interesting is that they took a very unusual path to find those drugs.
Most scientists who look for antivirals hunt for molecules that interfere with a virus’s own molecules. Viruses often make enzymes called proteases, for example, that help prepare new virus proteins. So-called protease inhibitors lock onto the proteases and prevent them from doing their job. These inhibitors have proven effective against HIV and extended the lives of millions of people.
But viruses can’t make new viruses entirely on their own. They need an enormous amount of help from their host cell. The Lingappas have done a number of experiments over the years to figure out the nature of that assistance. They’ve found that many of our own proteins join forces to assemble the pieces of viruses into their final shape.
This figure shows how the Lingappas and their colleagues think the process works. The host cell makes proteins for the virus (A), which begin to stick together (B). But then a swarm of host proteins have to come together around the virus proteins (C ) in order to organize them into their proper shape. This work takes energy, in the form of a molecule called ATP (D). Once the host proteins have finished their work, the new virus is ready to leave the cell (E).
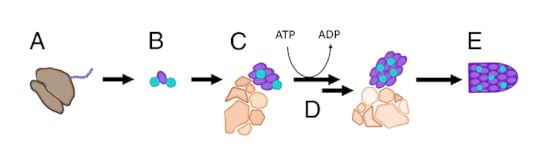
A model for virus assembly. From Lingappa et al 2013 PNAS
This idea is controversial, because it challenges a long-held view about how viruses form. Many scientists argue that once a host cell makes the parts for a new virus, the parts can assemble themselves without any more help. If you were to illustrate what they do with the figure above, the viruses should jump straight from B to E.
If the Lingappas were right, they reasoned, they should be able to block new viruses by interfering with the host proteins that build them. The experiment wouldn’t just lend support to their model. It would also lead them to potential drugs against the viruses. They could kill two viral birds with one stone.
To test their idea, the Prosetta team launched a series of experiments on a lot of viruses at once. But rabies is the first one they’re reporting on.
Their first step in search of an anti-rabies molecule was to create a kind of cellular stew. They mixed together proteins and other molecules from cells in a broth known as a cell-free system. They then added rabies viruses to the broth. In a matter of hours, the viruses were using the molecules in the cell-free system to make new viruses.
The scientists then turned to a catalog of candidates for a drug. Chemists have built up a list of tens of thousands of molecules that have promising medical properties, such as being small enough to slip easily into cells. They added some of the molecules to their rabies stew to see if any of them would grab onto the host proteins as they were assembling the rabies viruses.
They found a few. When these molecules were added to the cellular stew, the rabies viruses reproduced more slowly. Presumably, the molecules made it harder for the viruses to organize the host proteins to build new viruses.
The Lingappas and their colleagues fine-tuned these molecules. After some adjustments, the molecules would only latch onto to the proteins when they came together to make rabies. That way, the drug wouldn’t cause side effects by interfering with proteins that were doing what they were supposed to be doing for our own health.
One drug, which they dubbed PAV-866, proved to be both safe to host cells and potent against rabies. In a dish of cells infected with rabies, PAV-866 wiped the virus out altogether.
To see if PAV-866 was indeed blocking the host proteins, the scientists ran an experiment. They infected cells with rabies and then waited for different periods of time before adding PAV-866. They found that the drug PAV knocked down the virus if it was added up to six hours after the experiment started. Afterwards, it didn’t do much good.
You wouldn’t expect this result if the drug was interfering directly with the virus itself. If that were the case, then the drug would have been able to attack the viruses throughout the experiment. But if it interfered with the initial assembly of the viruses, then it would need to be present in cells as new viruses were being assembled.
The scientists then probed their cellular stew for proteins that were sticking together and found a couple proteins that PAV-866 consistently latched onto. These may be a couple of the proteins that rabies viruses depend on to be built. It will take more work to figure out exactly how these proteins assemble rabies and how PAV-866 stops them. But the Prosetta researchers already know enough about the drug to move forward with more experiments, in the hopes of getting it someday to the marketplace.
While researching my Wired story, I spoke to one of the Lingappas’ co-authors, Charles Rupprecht, a virologist who heads the rabies program at the Centers for Disease Control. I asked what he thought about this new approach to antivirals. “It’s provocative, it’s intriguing,” he told me. But he immediately listed all of the tasks that lie before him and his colleagues to see if PAV-866 is indeed a cure for rabies.
First off, the cells they infected for the new study were from the kidneys of monkeys. They chose the cells because they’re easy to work with. But now they have to run the same experiment all over again with neurons. If PAV-866 stops rabies in neurons, they will then move to experiments on animals. But to do so, they’ll have to figure out the right dose to give them, and then they’ll have to figure out how to keep infected animals alive long enough to study the effects of the drug.
“How are you going to intubate a mouse? How are you going to keep it alive as it goes into cardiac arrest?” Rupprecht asked me.
Still, he’s ready to keep on working with the Lingappas to see where the research goes. “We have nothing else to fall back on, so of course we’re going to be optimistic,” Rupprecht said. “You’ve got to find a coping mechanism.”
Prosetta is not the only place where scientists are trying to fight viruses by targeting the host. Other researchers are investigating the possibility of stimulating the virus-killing defenses inside all of our cells. Others are developing drugs to rewire the networks of genes in cells to cause them to commit suicide as soon as they are infected. What makes these studies especially exciting is that they might lead to a single antiviral drug that can treat a wide range of viruses–or perhaps even all viruses. Curing rabies would be remarkable enough. But it might be the start of something much bigger.
(For more information on rabies, see Rabid, written by my editor at Wired, Bill Wasik and his wife, Monica Murphy. For more on viruses in general, see my own book, A Planet of Viruses. I also spoke on Fresh Air about research on host-targeted antivirals.)
[Update 4 pm: Corrected timing for vaccine. It has to be given shortly after exposure, not symptoms.]
February 8, 2013
Your Inner Lions: Get to Know Your Virome
T4 phage, via Purdue University and Seyet LLC Source: https://news.uns.purdue.edu/x/2008b/0...
I’ve been blogging a lot recently about dangerous viruses, like the flu and norovirus. But, truth be told, a lot of viruses we harbor don’t make us sick. They may even make us healthy. You’d think they’d be worth getting to know. But they’re mostly a mystery to us.
The collection of viruses that are dwelling inside you right now is known collectively as the virome. You might think these viruses were the sort that infect your cells. Some indeed are. There’s a good chance you harbor papillomaviruses in some of your skin cells, for example. And chances are they won’t cause you any harm. Rather than blasting apart your cells, papillomaviruses merely accelerate the rate at which your cells divide. Since you slough off your skin cells, you’re rid of the viruses soon enough anyway. No harm, no foul. The only trouble these viruses can cause is when they end up accelerating your cells until they spin out of control. They can cause cervical cancer and warts when this happens.
And yet your own cells are not the only targets for viruses. The bacteria, fungi, and other residents of your body can serve as hosts as well. Indeed, of the several million viruses in you right now, most probably infect bacteria. As Sarah Williams writes in the Proceedings of the National Academy of Sciences (open access), virologists who have charted the huge diversity of viruses in the outside world are now going inside. There’s not that much difference between us and a coral reef when you look at us as an ecosystem.
The virus survey is in its very early stages, partly because it’s trickier to identify viruses than bacteria. At this stage of the virome saga, most of the viruses that researchers dredge out of our bodies are entirely new to science. Each of us has a unique virome as well, with a unique balance of species that can change quickly. But someday they may understand the virome well enough to manipulate it for our well being. Some scientists suspect that viruses may influence our health in many ways, some obvious and some subtle. As Williams notes, a person’s virome could influence how severe unrelated diseases might be, such as asthma and cystic fibrosis.
Over at Environmental Health Perspectives, Carol Portera takes a look at another way we might harness the virome: to kill bad bacteria (also open access). The idea of using viruses to cure infectious diseases is about a century old now, but the method–known as phage therapy–has never managed to go mainstream in the West. That’s just starting to change. The FDA has approved viruses to kill infectious bacteria on food, for example. Research is fairly far along in using viruses to disinfect wounds, cure acne, and clean water supplies. If you’re worried about putting viruses in your body, relax–there are swarms of bacteria-infecting viruses in many foods you eat, such as yogurt.
Someday, once we get to know our virome much better, a new generation of scientists may study our bodies as ecologists today study lions on the savanna. Predators play a huge role in food webs, influencing the populations of a vast number of species (I wrote about this in detail in Scientific American last year). Viruses probably play a similar role in our bodies, influencing who wins and who loses in the fierce competition among thousands of species for survival inside us. Ecologists are exploring how to restore dysfunctional ecosystems by returning their predators. Perhaps we may manage our inner lions in the future.
For more information on viruses, see my book, A Planet of Viruses. And, in closing…here’s a cool video of a virus invading a bacteria. It’s really a virus, not a lunar lander.
Michael Rossman, Purdue – T4 Bacteriophage Infection from Seyet LLC on Vimeo.
February 7, 2013
The Future Evolution of Bird Flu
It’s been a rough flu season this winter in the United States and Europe, but it could be worse. A lot worse. The flu viruses that are making us sick go by names like H1N1 and H3N2, referring to the kinds of proteins that stud their surface. There’s another sort of flu lurking in other parts of the world, like Egypt, India, and Cambodia, known as H5N1. Since 2003, 615 people have come down with H5N1, and, as of Feburary 1, 364 of them had died. In January alone, 5 people in Cambodia were diagnosed with H5N1. Four of them died.
There’s a lot of debate about precisely how bad H5N1 is. It’s possible that a lot of people are getting sick with H5N1 without making it onto the official records. They’re crawling off into bed for a week, recuperating, and then getting on with their lives. So the 59 percent death rate you get from the official numbers (what’s known as the case-fatality rate) may be a serious overestimate. Still, even if the true rate was only half as high, H5N1 would not be a virus you’d want to cross paths with. The most famous flu outbreak of all, the so-called Spanish flu of 1918, is estimated to have killed 50 to 100 million people worldwide. But it infected billions, with a death rate of roughly 2 percent. If H5N1 could somehow take off and become a global pandemic, it would become an unparalleled catastrophe even if its official 59 percent death rate was chopped down by a factor of ten.
Right now, H5N1 does not have what it takes to race around the world. It might someday, although nobody can say for sure what the odds are. And, as a team of scientists now report in the Philosophical Transactions of the Royal Society, H5N1 would continue to evolve as it spread. Its ability to spread would evolve, along with its ability to kill. The scientists can’t look into their crystal ball and say for sure how many people would die. But they can say this: what we do when and if we face an H5N1 pandemic could alter the evolution of the virus itself. And thousands of lives could be saved or lost as a result.
All of the flu strains that make us humans sick get their start in birds. Our feathered friends harbor a huge diversity of flu viruses in their guts, which they shed in their droppings. Most bird flu strains are fairly harmless to the birds themselves–they don’t overhwhelm their immune system, in other words. Most of them can’t infect animals other than birds. But on rare occasion, a strain of bird flu evolves that can also latch onto cells in another species, such as a seal or a human.
That’s what H5N1 has done. In at least some of the people who are exposed to it–from their chickens or from wild waterfowl–H5N1 can latch onto cells in the airway and invade. What H5N1 can’t do is spread from one person to another. There may be a number of reasons for this. A successful human flu virus needs to find a way to enter human cells quickly, it needs to hijack human biology to make new viruses, it needs to escape human immune attacks, and it needs to be able to spread efficiently from one person to the next.
Exactly what it would take for H5N1 to become a true human flu got two teams of scientists into hot water for back in 2011. They introduced mutations into H5N1 and then infected ferrets with it. (Ferrets get sick from the flu a lot like we do, so they’re pretty good models to study.) They then swabbed viruses from the noses of the ferrets and then infected healthy ferrets. Within several rounds of this transfer, the viruses evolved the ability to spread on their own between ferrets through the air. Intriguingly, the H5N1 viruses became much less dangerous along the way. In its mammal-adapted form, H5N1 didn’t kill any of the ferrets.
The prospect of a mammal-adapted H5N1 was so alarming that the researchers agreed to a moratorium while the scientific community debated the risks and benefits of the research. At the end of January, the moratorium came to an end, and research will continue in countries that have established clear guidelines for studying the virus.
Their experiments revealed a fairly short list of mutations that were sufficient to make H5N1 a mammal flu. That was a sobering discovery, because H5N1 doesn’t depend on scientists to acquire new mutations. It’s mutating all the time in the wild.
In the February issue of EMBO Reports, three H5N1 experts, Yohei Watanabe, Madiha S. Ibrahim, and Kazuyoshi Ikuta, describe how H5N1 has been evolving as it has spread to new countries. In Egypt, for example, the virus first emerged in 2006, and doctors there have documented 169 infections of humans so far. Since then, Egyptian viruses have evolved that are better adapted to infecting humans. Their new adaptations include proteins that let them latch more tightly to human cells than their ancestor could. Watanabe and his colleagues suggest that this evolution may explain why Egypt has had 50 percent of all the human case of H5N1 since 2009. They warn that Egypt may now have the highest potential for an H5N1 pandemic.
Let us, then, imagine the worst. A chicken pecking in the dirt on the outskirts of Alexandria picks up the flu. Inside its gut, the viruses replicate, picking up some crucial mutations for infecting humans, and are then shed in the chicken’s droppings and blown around in the dust.
An unlucky farmer breathes in the virus, which infects his airway and leaves him deathly ill. Inside his body, the H5N1 further mutates, and one of viruses now has the wherewithal to fly out of his body and infect his wife as she changes his bedding. It starts spreading from village to village, from Egypt to other countries, until we are in the grip of a global H5N1 epidemic.
A group of flu experts led by Maciej Boni of the Oxford University Clinical Research Unit in Vietnam have now tried to make some rough predictions about that scenario. They didn’t simulate viruses infecting millions of people. Instead, they created mathematical models based on the observations doctors have made on H5N1 infections. Unlike most epidemiological models, theirs takes into account the fact that viruses never stop evolving.
As with other pathogens, natural selection sculpts the genes of flu viruses in many different ways. On the one hand, natural selection should favor mutations that speed up the replication of viruses in their host. But over the long term, flu viruses also have to get from one host to another. Those two demands can sometimes come into conflict–if faster replication leads to less spreading from host to host, for example. This tradeoff can arise if, for example, fast-replicating viruses also tend to be deadlier. Kill of your hosts too soon, and you ultimately kill off yourself.
A flu virus like H5N1 experiences a special form of this tradeoff. In their current form, H5N1 grabs more tightly to receptors in the lower respiratory tract than the upper respiratory tract. A number of flu experts suspect that this preference is what makes the flu so deadly, because it’s so far down the airway that it can wreak more havoc with the lungs. Because the virus is so deadly, it has less time to spread to a new host. And being nestled so deep in the respiratory tract also means that relatively few of the viruses can leap up the airway and escape their host.
Human flu viruses have this tradeoff flipped. They tend to latch onto cells in the upper respiratory tract. They’re less deadly than H5N1, but they also have an easier time getting sneezed or coughed into the air. Instead of dying in a few days, people with human flu are far more likely to remain alive and infective. They can even walk around, transporting their virus to stores, schools, and subways.
Boni and his colleagues created a model of an H5N1 outbreak spreading through a population of 10 million people. In their model, the virus could evolve a stronger or weaker preference for the lower or upper airway. That preference would influence how deadly it was and how easily it could spread.
They could also include the human factor as well. When flu outbreaks occur, one of the most effective strategies is to keep people away from each other. No school, no movies, no parades. Boni and his colleagues analyzed the course of the outbreak if this isolation did or didn’t happen, or occurred soon or a long time after the outbreak began.
First the scientists looked at what it would take for a human-adapted H5N1 virus to trigger a full-blown pandemic. If it had the 60 percent case-fatality rate that doctors have seen in bird flu, it would fail. People would die so fast that the virus wouldn’t have enough time to replicate and spread to other people.
Sounds great, right? Not so fast. If the mortality rate dropped to 15 percent, the scientists found, the flu would take off. So we’d be facing a fast-spreading virus that was far deadlier than the 1918 pandemic. Boni and his colleagues point out that in Egypt, the fatality rate of H5N1 is dropping to about 36 percent. They call the drop “worrying.” Normally, you’d think a milder pathogen is a better one. But thanks to the quirks of the flu, it might actually be entering its pandemic sweet spot.
In the early part of an epidemic, natural selection will favor viruses that can spread quickly. They can do so if they evolve the ability to colonize the upper airway and get coughed onto new hosts. But this preference is not an all-or-nothing choice. As the flu shifts to the upper airway, it could still continue to infect the lower airway, albeit at a lower rate. So the virus could continue to cause a lot of deaths even as it began spreading faster through the human population.
As the outbreak progressed, evolution would shift in a new direction. What started out as one huge population of vulnerable people, would become more complex: a population in which many people had already been exposed and fought the virus off, some people had died, and some were in hospitals. Now it would take longer for the virus to successfully jump from host to host. Instead of transmitting fast, natural selection would favor viruses that were less deadly, but also able to cause longer-lasting infections.
Public health officials like to isolate people from each other in order to drive a virus to extinction. If it gets too hard for viruses to find new hosts, they eventually die out like a sputtering fire. But they usually don’t consider the fact that this isolation can also prompt the virus to evolve in important ways.
If people are suddenly isolated from each other, many of the viruses will die out. Selection will become intense for viruses that can survive longer. Instead of killing their hosts, they will make them ill for many days. The viruses may be able to survive the isolation and trigger a second epidemic–albeit a milder one.
It’s not easy to get people to stay home for very long, so public health workers may try to establisher an isolation plan that’s less strict but lasts longer. Under these conditions, the virus may not die out quickly, but selection may produce tamer forms. As a result, most people who do get sick won’t face as grave a risk as before.
This study is too preliminary to serve as a guide for dealing with an outbreak. But it does help to sharpen our senses to potential H5N1 has to evolve into something new. Whether the surprise is pleasant or horrifying may be, in part, up to us.
February 6, 2013
Radiolab and Point of Inquiry: Of Speed and Viruses
Just a quick note to let you know about a couple new radio/podcast programs I’m on at the moment.
1. Radiolab. The merry band of Jad Abumrad, Robert Krulwich, and company have put out a new episode all about speed. They got in touch with me after reading a column I wrote about the speed of thought, and I took them on a journey through our not-exactly-light-speed nervous system. They also explore other aspects of speed, such as the agonizingly slow drip of pitch and the superfast world of high-speed stock trading. I’ve embedded the show here.
2. Point of Inquiry: At Science Online this weekend, I sat down for a wide-ranging conversation with Indre Viskontas. We talked about whether viruses are alive, how to do a better job of fighting the flu, and much more. Check it out.