Carl Zimmer's Blog, page 30
April 8, 2013
Bugs As Drugs
Let’s say you want to buy things with germs in them. There’s yogurt, of course, but there’s so much else.
You can buy pills for your gut, creams for your face, tablets for your breath. You can buy blueberry juice with germs, and pizza with germs. And a lot of these products make big promises about the benefits their germs will bring you. “Fungal Defense is specially formulated with ingredients that help maintain a balanced, healthy digestive environment,” for example. Natren Natasha’s Probiotic Face Cream “is enriched with DNA fragments of beneficial bacterial cells, which speed up the skins own natural renewal process.”
Did that last statement make not so much sense to you? Are you struggling to find the results of the clinical trials that demonstrated that Fungal Defense really will make you healthy? Relax! Somewhere on the labeling for the germ-laden product you just bought, you’ll probably find these words: “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.”
There’s ample evidence that the 100 trillion microbes that call us home–the microbiome–exert important influences on our biology. While some of them can make us ill, for the most part they help maintain our health–nurturing our immune system, moisturizing our skin, breaking down food and toxic compounds. (Here’s are a couple pieces I’ve written for the New York Times for a sampling of this field.) For the most part, though, the research has been fairly remote from the doctor’s office.
A huge amount of research has been carried out on mice rather than humans, for example. That’s because scientists can rear the rodents without any bacteria in their bodies and then observe what happens when they add in certain species to their microbiome. Scientists have also carried out a lot of research on humans, but it’s mostly been observations, not manipulation–what does someone’s microbiome look like before and after a gastric bypass, for example?
Actual experiments on people have been a lot rarer, not surprisingly. (Any parents willing to put their newborns in a plastic bubble to keep them germ free for the first five years of life? Hello? Anyone?) That’s not to say there are no such experiments. Scientists will sometimes test out bacteria on people to see if it can help their disorders. Some of these experiments are promising. The remarkable results with fecal transplants for people with dangerous gut infections have become a veritable poster-child for the microbiome’s application to medicine. But none of them have been the subject of exhaustive research that’s been given to an FDA-approved drug like, say, the cancer-fighting compound Gleevec.
There are many reasons for this shortfall. Scientists have only been able to study the microbiome with much clarity in the past couple decades, so they’ve got a late start. Another reason is that the microbiome is different from our own cells and organs. It’s an ecosystem made up of hundreds of species, with lots of diffuse, interlinked effects on our bodies.
Making matters worse (or a more exciting challenge, if you’re of a sunny disposition) is the fact that there isn’t any one microbiome. While the microbiomes of humans are similar to one another, each of us has a mix of species and strains that’s unique–a mix that also changes from day to day. That variability makes it hard to say that adding in one particular species is going to make a difference to anyone who’s sick with a particular disease. Even an exquisitely rare microbe might play a crucial part in the overall ecosystem.
None of these hurdles has blocked the growth of the business of the microbiome. But the $8.7 billion industry has thrived because the microbiome occupies a fuzzy middle ground in the regulatory landscape. Purveyors of germ-loaded products can vaguely hint that their wares will bring you medical benefits. But to the U.S. government, their products are not, officially speaking, medicine. They’re food or cosmetics.
It’s possible that the bottle of probiotics you buy in the drug store really will help your digestion, or your immune system, or your bad breath. But it’s also possible that the bacteria you’re buying will get annihilated in the ruthless jungle that is your body. A lot of species you’ll find in probiotic products do not actually belong to the dominant groups of species in the human microbiome. Stop eating them, and they’ll disappear from your body.
The fact that you feel better after taking probiotics might be due to entirely different reasons. Who knows–maybe the very notion that a pill can “restore your ecosystem” will have a powerful placebo effect. As long as companies don’t make any specific drug-like claims about their germs, they can say whatever they want. (Dannon got nailed in 2010 for claiming Activia yogurt can cure colds.) Such is no longer the case in Europe, however; at the end of 2012, companies were no longer able to tout health benefits of the germs in their products.
While these products may not harm you, they can’t stand in for medical treatment. If you’ve got a serious case of diabetes, you shouldn’t just randomly start popping probiotic pills because you’ve heard that your microbiome influences insulin signaling. That’s not to say that someday there might not be clinically proven microbiome treatments for diabetes. We’re just not there yet.
According to Nature Biotechnology, however, we’re getting there. The current issue has a special focus on the microbiome (behind a paywall, alas). As reporter Charles Schmidt notes in an article for the journal, biotech startups are setting out on the drug-approval road in order to prove that some bacteria are indeed safe and effective. Enterologics in Minnesota is testing out a strain of E. coli as a treatment for a type of gut inflammation called pouchitis. Bernat Olle, the co-founder of Vedanta Biosciences in Boston, also contributes an essay to Nature Biotechnology, in which he mentions that his company is testing out a cocktail of species that can restore gut ecosystems degraded by diseases.
All well and good, but the fact is that the bacteria that these companies are studying have been known for a long time. That’s because they were comfortable enough in laboratories to thrive. Most microbes in our bodies require much more exotic conditions. As a result, scientists are just starting to discover them by fishing out their DNA from our bodies. Some companies are trying to mine this newly discovered diversity for keystone species that could have important effects on our health. These new species could also serve as diagnostic tests for diseases. Certain microbes in the mouth are associated with a risk of heart disease, for example. Frederik Bäckhed of the University of Gothenburg in Sweden and his colleagues have patented a way to assess that risk by examining the bacteria in your spit.
Olle also notes that some companies are engineering bacteria that we could consume. This year marks the fortieth anniversary of the first insertion of an animal gene into E. coli. Today, E. coli and other engineered microbes pump out vast amounts of medicine and other products. But they do so in giant fermentation tanks, not in our guts. People with diabetes must wait for companies to harvest insulin from E. coli, purify the molecule, and sell it in vials which they can then inject into their bloodstream. (For more, see my book Microcosm.) Imagine, instead, that people could swallow bacteria that could take up residence in the gut and produce a life-saving drug right where it’s needed.
A company called Actogenix is trying to do that. They’ve carried our early human trials on a species called Lactococcus lactis they’ve engineered to carry a gene that encodes an anti-inflammatory protein. Another company, Osel, has engineered Lactobacillus jensenii to carry a gene for cyanovirin N, a protein that may help prevent HIV infection. They’re in preclinical trials.
This wave of efforts will not change medicine in the short-term. But in the medium-term it may. Nature Biotechnology surveyed experts on the microbiome about its future in medicine. ”The potential for gut microbiota manipulation is enormous, and so is the market,” declared Jeroen Raes of Vrije Universiteit in Brussels. “In 15 years, we will all be drinking specific, personalized probiotic cocktails. I suggest that every healthy person freezes a fecal sample now so they will be able to treat themselves in the future.”
April 4, 2013
Another Path For Evolving Bodies
This post is an unexpected sequel to a post I published last month about how single-celled microbes can evolve into multicellular bodies.
Here’s a quick recap of that story. Life became multicellular at least a couple dozen times over the past few billion years. To explore the factors that drove life through these transitions, scientists at the University of Minnesota ran experiments with single-celled yeast. They gave the yeast time to settle in a flask and then drew out some fluid from the bottom. Repeating this many times created conditions in which the yeast quickly evolved into snowflake-like clumps. Bigger clumps fell faster, providing a reproductive advantage over single-celled yeast, which drifted slowly to the bottom of the flask.
This week a team of scientists at Oxford published a study in the journal eLife on their own evolutionary experiments on yeast. They got to the same destination, but by a different route.
Yeast feed on sugar, but they start digesting their meal before it’s even inside them. Their cell walls are loaded with enzymes that break down sucrose into two smaller kinds of sugar, glucose and fructose. The yeast cell can then pump those small sugars into its interior. But a lot of that sugar diffuses away from the cell wall and away from the yeast.
This waste doesn’t matter much when yeast can gorge themselves on a dense soup of sugar. But when sucrose is scarce, losing so much sugar makes it very hard for yeast to grow. The Oxford scientists wondered what course evolution would take if they reared single-celled yeast on a such a meager diet.
They allowed yeast to grow in a flask supplied with only a little sucrose, and then they drew a little fluid to seed a fresh flask. The scientists repeated this for dozens of rounds, and ran a dozen separate trials on different populations of yeast. In eleven trials, the yeast evolved to form clumps.
The clumps typically took on the same snowflake-like structure seen in the University of Minnesota experiment I wrote about last month. And they developed in much the same way. When a cell budded off a new cell, its daughter remained attached rather than making a clean break.
To investigate these clumps, the Oxford scientists put them in a flask with their single-celled ancestors and let them compete for the sucrose. Every time the researchers ran the experiment, the multicellular clumps won, swiftly eliminating their ancestors. Their victory strongly suggests that natural selection was responsible for their evolution to clumps. But their transformation only gave them an advantage when they fed on sucrose. If the scientists added fructose or glucose to their diet, they lost their competitive edge.
The scientists then took a close look at the biochemistry of the evolved yeast. They gained an advantage partly from an improvement in how they fed. The evolved yeast produced more sucrose-digesting enzymes. They also made more proteins to transport the smaller sugars into their interior.
But multicellularity also helped them survive on scarce food. The reason a body provided an advantage has to do with how yeast eat. As yeast cells break down sucrose, they release a lot of sugar into their immediate neighborhood. If another yeast cell is nearby, it can enjoy this meal for free–in other words, without using energy to make the enzymes to prepare the sugar. And if a lot of yeast cells live next to each other, they can collectively create a bigger buffet of sugar that they can all enjoy.
So in the space of a month, we have two studies that see the origin of multicellularity in the same species–but for two separate reasons. In one experiment, the advantage of falling fast provided the push. In another, it was surviving on scarce food.
It stands to reason that bodies might have provided different advantages to animals and plants and other multicellular organisms. For the ancestors of animals, a body might have made them better predators. For the ancestors of plants, a body might have offered them protection from those predators. It’s hard to study those different factors in this major transformation of life, since they took place hundreds of millions of years ago. So it’s a delight to find that scientists can observe different forces at work a single species, right under their noses.
April 2, 2013
(Some) Extinction Is (Not Necessarily) Forever: Video of My TEDx talk
What would it take to bring back an extinct turtle, or a long-gone mammoth? Thanks to the folks at TED for posting the video of my talk about my story in the April issue of National Geographic, embedded below.
(TEDxDeExtinction will be posting the videos of the rest of the talks from the meeting over the course of the next few weeks.)
A New Push To Explore the Brain
This morning Barack Obama invited a small army of neuroscientists to hear him announce a new initiative to better understand the brain. Reports about the plan have been trickling out ever since John Markoff broke the story in February. In anticipation of the announcement, All Things Considered on National Public Radio talked to me over the weekend about the current state of our understanding of the brain, and what it will take to understand it better. I spoke in pretty hazy terms since the government hadn’t officially laid anything down. Now we can take a closer look at what is going to happen, at least in the near future. [Update: here is the official web site from NIH]
Markoff’s originally story generated a huge amount of buzz in science circles because of the bold scope of the idea it described. “The Obama administration is planning a decade-long scientific effort to examine the workings of the human brain and build a comprehensive map of its activity, seeking to do for the brain what the Human Genome Project did for genetics,” Markoff wrote. Like the Human Genome Project, the new project would dedicate billions to mapping the brain’s activity.
In his speech this morning, Obama sketched out all sorts of amazing possibilities: cures for Alzheimer’s disease, prosthetic limbs for the paralyzed, and lots of brain-related jobs. If that’s the ultimate goal of this plan, today’s announcement feels like a very soft launch. Obama announced $100 million in funding for 2014 for a project now dubbed the BRAIN Initiative.
BRAIN stands for Brain Research Through Advancing Innovative Neurotechnologies. There’s a heavy emphasis on the last word in that name. The scientists involved in the project I’ve spoken to emphasize that they aren’t pretending to be able to completely map the human brain any time soon. (Indeed, some skeptics I’ve spoken to wonder how you would know when you’re done.) Instead, the BRAIN Initiative and whatever it produces in the long run will have a strong focus on building new tools for mapping and recording neurons. And here the parallel between the Human Genome Project and the BRAIN Initiative feels apt.
When scientists embarked on sequencing the human genome, the tools they had for deciphering DNA were expensive and crude. Over the course of the project–and in the years afterwards–they came up with better and better tools. It used to be that a scientist might spent his or her entire career sequencing and studying a single human gene. Now sequencing human genomes is a fairly common part of scientific research, and it will probably become a fairly common part of medical diagnostics in years to come.
Today, it’s hard to listen to more than a thousand neurons at once. Scanners typically can only map the brain down to chunks the size of a poppy seed. With 86 billion neurons and about 100 trillion connections, there’s plenty of room to do better. Whether scientists can do for the brain what they’ve done for the genome we cannot predict.
I will have a lot more to say (and write) about exploring the brain before too long. But if you are eager to dig into the scientific rationale for the BRAIN Initiative, here are a series of papers to check out:
Nanotools for Neuroscience and Brain Activity Mapping
The Brain Activity Map Project and the Challenge of Functional Connectomics (Here’s a longer, open-access version.)
March 31, 2013
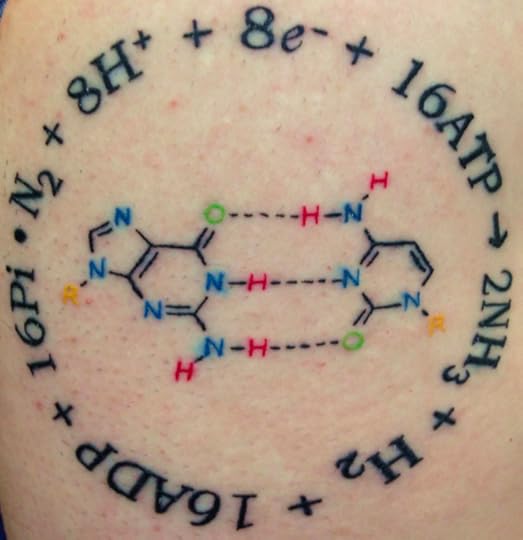
Pulling Life Out of Thin Air (Science Ink Sunday)
Jason Affourtit writes, “The encircling equation represents biological nitrogen fixation, which was at the core of my undergrad/graduate labwork. Working in that research lab (which was originally just part of requirements for med school!–my intended goal) totally changed my focus…So it’s an homage to that period of time, my wonderful advisor, and that lab. DNA has been central to my work life in genomics and has run through as a common theme. So to me, a G-C basepair seemed a natural symbol of that.”
You can see the rest of the Science Tattoo Emporium here and in my book, Science Ink: Tattoos of the Science Obsessed.
(Tattoo by Nick Bergin from Godspeed Tattoo in San Mateo, CA.)
March 28, 2013
Rewiring Life: Learning About Synthetic Biology In Debates, Videos, and Comic Books
Today scientists at Stanford University reported they had implanted transistor-like bundles of genes into E. coli, making it possible to transform cells into biological computers. At Download the Universe, a science ebook review where I’m an editor, I take a look at the history of synthetic biology that led up to this remarkable feat. I also reflect on how to help young people become both excited and wise about these new kinds of technology. Check it out!
March 25, 2013
Ducks Meet the Culture Wars
A few days ago, CNS News (“The right news. Right now.”) discovered that the National Science Foundation has been funding a study on the evolution of waterfowl genitalia.
When someone brought this item to my attention, I was puzzled. After all, this is not breaking news. I should know–I wrote about it for the New York Times almost six years ago.
This old news now seems to have gotten fresh currency thanks to the fact that some of the research was funded through the 2009 American Recover and Reinvestment Act, a k a the Stimulus Package. And so, CNS News believes, you can draw a direct line from the funds that went to this research to the new cuts in government services due to the sequester.
I kid you not. See this tweet from CNS News:
Please RT –> Gov spends $384K to study duck genitalia, cancels White House tours #sequester ow.ly/jfgKa
— CNSNews.com (@cnsnews) March 20, 2013
This “news” then got picked up by other outlets, such as Human Events (“Powerful conservative voices”). Politifact, the Pulitzer-Prize-winning political fact-checking site, even got into the act–confirming that, yes, the government supports research on duck sex.
Commenters on these posts left remarks like ”PLS SHOOT ME NOW AS I CAN TAKE NO MORE OF OBAMA AND HIS SPENDING.” (Of course, I wrote my article back in the Bush years, but, hey, who needs to get bogged down in reality?)
This is a tried-and-true tactic that politicians have trotted out for years–long before the sequester. Back in 2008, I wrote about how then-vice presidential nominee Sarah Palin sneered at spending that “goes to projects having little or nothing to do with the public good–things like fruit fly research in Paris, France.” In 2011, Republican Senator Tom Coburn groused about all the money NSF wasted on things like studying shrimp.
Now, I will not get into a debate about precisely how much money should go to the National Science Foundation, versus, say, subsidies for oil companies. I just want to address the question of why we fund basic research in the first place.
Scientists use the word “basic” to distinguish scientific research that’s not directed at some specific practical problem. Developing a vaccine for the latest strain of the flu is applied research. Learning how the body generates antibodies to flu viruses is basic research. Basic research can lead to applications, but we don’t know in advance what particular studies will or won’t. That’s because we have much left to understand about how the world works.
For some reason, people like Palin and Coburn are fond of animals when they’re looking for something to make fun of. Understanding the basics of how animals work seems to them like a joke. This just speaks to a misunderstanding of the research. Animals affect us directly in lots of ways. We eat them, they eat our food, they harbor diseases, and they produce interesting compounds that may lead to useful drugs. Scientists do a lot of applied research on animals to address these issues, and they also do basic research on animals, which sometimes leads to applications. If you actually take a look at the animal research Palin was mocking, she could not have picked a worse example to make her point. The research involved looking for parasitic wasps that can kill a fly that is devastating California’s olive orchards.
Studying animal biology is not just important for our own direct well-being. It’s also important if we want to be good stewards of the environment. To write my story about ducks, I went to the Livingston Ripley Waterfowl Conservancy in Litchfield, Connecticut, where some of the research is taking place. Obviously, if you want to bring back these birds with captive breeding, the birds have to breed. And if you don’t understand the equipment they use for breeding, then you’re doomed. Sniggering about duck penises won’t change that.
Studying animals is also a way for us to look in the evolutionary mirror. We share a common ancestor with other animals, and the same kinds of evolutionary processes play out in both us and them. Now, you may wonder what ducks–with gigantic cork-screw-shaped penises and a gigantic cork-screw-shaped reproductive tracts–could possibly have to do with us. The manifestation of sex evolution may be different in different species. But the process is similar.
As in many other species, the evolution of ducks has been driven in part by something call sexual conflict. The best reproductive strategy for a male duck is not the same as the one for a female. Females will have the most duckling if they can choose the best males to father their offspring. Males, on the other hand, try to mate with as many females as possible. This sexual conflict leads to an extravagant arms race, which has produced their extravagant sexual organs. (In addition to my story for the Times, I’ve blogged about this research at the Loom, and Ed Yong has at his blog.)
In other species, sexual conflict takes many other forms. Male flies, for example, will dose their mates with toxic chemicals to ensure that their sperm fertilize the female’s eggs and not the sperm of other males.
And guess what? Human biology is shaped by sexual conflict, too. Human sperm and seminal fluid shows signs of having evolved through the competition with other sperm. (The journal Reproduction–dedicated to research on fertility–recently published a review about sperm competition in humans and other animals.)
Sexual conflict may explain some of the disorders of pregnancy. Take preeclampsia, a mysterious condition in which pregnant women develop dangerously high blood pressure–so high that they risk death.
Some scientists have argued that the same sexual conflict that is manifest in other parts of the animal kingdom is the cause of preeclampsia. Male genes might drive mothers to provide extra resources to babies, while female genes might hold the flow of nutrients in check. Think of it as a tug of war that normally ends up as a stalemate–but every now and then gets out of control. If the placenta drives too much blood towards the baby, it can lead the mother to suffer high blood pressure.
A team of Danish scientists tested out this idea by reviewing 750,000 medical records of pregnancies and found that the data support this hypothesis. “Natural selection may be responsible for the maintenance of these disorders in modern humans,” they conclude in a paper they published last month.
These insights into sexual conflict’s effects on humans may prove important to our own health. They may guide us to new ways to treat preeclampsia and infertility. But they’ve arrived late in the study of sexual conflict. Other scientists first explored sexual conflict in many other species first–species including ducks. That’s just how science works, no matter what culture warriors may claim.
March 23, 2013
De-Extinction On CBS This Morning
Today I dropped by CBS This Morning to talk about my story in National Geographic on the quest to bring species back from extinction. I’ve embedded the video below; here’s the link.
March 20, 2013
Listen Closely To The Bats and You Can Hear the Viral Chatter
HCoV-EMC, by Beth Fischer. Source: NIAID/NIH
Last June, a sixty-year-old man in Saudi Arabia fell ill with pneumonia. His disease, it turned out, was caused by a virus no one had seen before. It was a coronavirus–in other words, it belonged to a lineage of viruses that includes ones that cause colds as well as ones that cause SARS. But this new virus was genetically distinct enough to be considered a species in its own right. Scientists now refer to it by the dreary, unpronounceable abbreviation HCoV-EMC. Eleven days after being admitted to a Jedda hospital, the man infected with this new virus died.
A single death from a new virus is hardly unheard of. But over the past few months, virus-watchers have gotten increasingly anxious about HCoV-EMC. So far, 15 people have been diagnosed with the virus, and nine have died. While some victims have turned up as far away as England, everyone with HCoV-EMC has had some connection to the Arabian Peninsula. Some victims belonged to the same family, suggesting that the virus can spread from one person to the next.
We can’t say for sure whether we’re at the beginning of a HCoV-EMC pandemic, or at the end of a minor outbreak, or experiencing something in between. But scientists are not waiting around until the virus has finished traveling down whatever path it will take. They’re working hard to figure out the biology of the virus, and they’re also trying to figure out its history. How it got into 15 people over the past 9 months might give us a hint as to what it may do in the future.
In other words, scientists have to probe the evolution of HCoV-EMC.
The closest relatives to HCoV-EMC are coronaviruses that live in European bats. That doesn’t mean HCoV-EMC came from Europe, however. Most of the diversity of coronaviruses is unmapped, especially the ones that live in animals. There could well be bats in the Near East with more closely related but undocumented cornaviruses. The fact that all the human victims were at some point in the Arabian Peninsula certainly raises the possibility that bats there spread the virus to people.
The link between bats, coronaviruses, and humans is a familiar one. SARS moved from bats to humans ten years ago, and since then scientists have found other coronaviruses that moved from bats to humans. The biology of HCoV-EMC itself offers more evidence for how this might have happened. Some viruses are very fussy about how they invade cells. They only infect one type of cell in one species. But HCoV-EMC is a lot less picky. It latches onto a receptor on cells lining the airway, and scientists have found that it can invade airway cells from not just humans, but pigs and bats. The ancestors of HCoV-EMC might have lived in bats and yet they may have already been prepared to infect humans.
Recently, a team of evolutionary biologists began to draw an evolutionary tree of HCoV-EMC based on a comparison of the viruses isolated from three victims–the first patient from June, and two later victims from England. They identified mutations that arose in each of the three lineages of viruses since they diverged from a common ancestor. Mutations accumulate at a roughly clock-like rate, which means that scientists can use them to estimate how long ago lineages split apart. In the case of the three HCoV-EMC viruses, their common ancestor dates back to 2009.
Three years is a long time for a virus to be circulating among people without anyone noticing. It’s conceivable that a lot of people passed it around and only got mildly sick. But the fact that nine out fifteen people identified so far with HCoV-EMC actually died suggests that this is a fairly deadly virus, making invisibility unlikely.
If that explanation fails, what happened? Virologists I’ve spoken to favor a phenomenon known as “viral chatter.” Viruses don’t just barge across the species barrier in one great rush. They tentatively make incursions–many of them spread across years. The SARS virus, for example, infected a few people before becoming a massive epidemic in 2003. During these incursions, animal viruses may acquire mutations that gradually prepare them to become good at spreading from person to person.
For now, thankfully, HCoV-EMC seems to be bad at that kind of transmission. Each sick person made contact with many others and almost none of the exposed people got sick as a result. Instead, people must be getting infected through contact with sick animals. They’re picking up viruses that diverged from a common ancestor a few years ago and are still circulating among animals.
It’s not likely that humans are getting sick from HCoV-EMC thanks to frequent contact with bats. When’s the last time you gave a bat a kiss? Instead, HC0V-EMC may be using a stepping-stone species to get from bats to humans.
Again, SARS offers some lessons. It appears that the SARS virus spread from Chinese horseshoe bats to civets, cat-like animals that live in East Asia, and then to humans.
A decade after the SARS epidemic, no one can say for sure how SARS got from bats to civets. But the answer must lie somewhere in their ecology. Matt Frieman, a virologist at the University of Maryland who studies bat coronaviruses, pointed me to an example of the interactions between bats and civets buried in a 2010 article about civets and coffee.
Civet cats eat wild coffee cherries and them poop them out. People collect their poop and clean off the beans they contain. The resulting coffee, the article claims, has a taste that’s “smooth, chocolaty and devoid of any bitter aftertaste.” Western appetite for this delicious coffee has led people to hunt for civet poop in forests, and to feed captive civets coffee beans on little farms.
One passage in the story should give you a jolt. A coffee supplier inspecting a batch of civet-processed beans knocks down his buying price because of some impurities: “inferior beans that the civet had spat out; beans chewed on, not by civets, but bats.”
So imagine SARS-infected bats in a rain forest chewing on the same coffee cherries as civets. It’s enough to start an epidemic, perhaps.
Ten years later, a continent away, bats with HCoV-EMC may be coming into contact with other animals as well. Goats drinking at watering holes might spend time near bats in neighboring fruit trees, for example. And the farmers of the goats might then pick up viruses from their livestock. At this point, it’s a notion–or, rather, a scientific hypothesis. The animals of the Near East will tell us whether it’s a good one or not.
(For more on the emergence of new viruses, see my book A Planet of Viruses.)
March 19, 2013
Your De-Extinction Questions Answered
On Friday, I was down in Washington to speak at (and mostly watch) TEDxExtinction, a day-long meeting dedicated to exploring the possibility (and advisability) of bringing extinct species back into existence. The meeting coincided with the publication my story in the new issue of National Geographic on the subject. I invited readers to ask questions raised by either the story or the meeting, and then on Monday, National Geographic hosted a tweet chat on Twitter, which became an hour-long rapid-fire volley. So I’m only now getting a chance to write this long-promised post. Here are some answers to a few of the questions posted on the Loom and on Twitter–first on the logistics of de-extinction, and then the ethics.
@natgeolive @carlzimmer how long extinct is OK?Dinosaur v. Dodo Are different issues, no?
— TimothyMJones (@MrJonesHistory) March 18, 2013
This is an interesting question, because dodos were dinosaurs. Not to mention robins and hawks and other living birds. If your idea of Jurassic Park is being surrounded by dinosaurs, you are living the dream. If, on the other hand, you desire (or fear) the lineages of dinosaurs that became extinct 65 million years ago, such as tyrannosaurs, then you are out of luck. No viable cells or nuclei can survive 65 million years. And while scientists have recovered lots of DNA from species that became extinct tens of thousands of years ago, they can’t reach back tens of millions of years.
____
Peteykins said, “Too bad the dodo died out too long ago for viable DNA to survive. Now THAT I’d love to see.”
Dodos only became extinct less than 400 years ago. While there are no intact dodo cells left today, scientists have retrieved bits of dodo DNA from a specimen stored at the University of Oxford. If scientists could find a lot more dodo DNA, they might be able to identify the genetic variations that turned the ancestors of dodos–small, flying pigeons–into big flightless birds. Then they might be able to reverse engineer the genome of a stem cell from a closely related pigeon species and then turn that cell into eggs and sperm, which could produce dodos.
Size would present a problem, if a small pigeon had to lay a massive dodo egg. But you could imagine gradually developing dodos over several generations, getting bigger and bigger. Mind you, all this is just speculation from a few facts–the fact that we now have a little dodo DNA and that scientists are doing amazing research on cloning based on stem cell engineering. Lots of practical obstacles stand in the way, some of which might simply be insurmountable. For example, the home of the dodo, Mauritius, is a tropical island where conditions are terrible for preserving DNA. Ironically, scientists have reconstructed much more of the mammoth genome, despite the fact that the last mammoths became extinct 3700 years ago. That’s because cold permafrost is pretty good at storing DNA fragments.
____
@carlzimmerAnd, could we avoid genetic drift-driven extintion for a small population of de-extinted animals? #NatGeoLive — Gustavo Rodríguez(@RodAG_) March 18, 2013
When a population gets tiny, its genetic variation gets tiny, too. Thanks to the random shuffle of heredity’s dice, gene variants can disappear, leaving the organisms more and more similar to each other. That can be dangerous, because it can leave populations unable to reproduce as quickly and may leave them less capable of adapting to new challenges. If scientists created a dozen genetically identical dodos from a single egg, they’d face some serious problems with genetic diversity. This is just one of many practical challenges scientists would face in trying to truly revive a species, rather than getting one animal alive again just long enough to be photographed. But we should not assume these challenges are insurmountable. It might be possible to find variants of genes in ancient DNA from fossils or museum specimens, for example.
____
@carlzimmer#NatGeoLive If 99% of all living things are extinct, why are we so consumed with playing God? Things come, thing go, that’s it. — John McMurray (@Iriesheik) March 18, 2013
I agree that it is important to think about Deep Time when we think about extinction. Perhaps 99.99% of all species that ever existed are gone from this planet. But what’s happening now is unusual for two reasons.
One is the rate at which species are going extinct. In the past few centuries, the rate of extinction for some groups of species has jumped by roughly a factor of a thousand. That jump is due to us–to our hunting, logging, and other actions that leave species struggling to hold on to existence. If those actions continue into the future, and if we continue pumping carbon dioxide into the atmosphere at a rising rate, we could jack that extinction rate to levels that life has achieved only five times in the past half billion years. So we’re not in a “things come, things go” situation. It’s more like, “Things go, and a lot more things go after them.”
The other reason that what’s happening now is unusual is us. In no previous pulse of mass extinction did a single species consciously drive a number of other species extinct. I’m not saying that a bird hunter shooting into a flock of passenger pigeons 200 years ago realized he was part of an exercise that would drive the entire species of passenger pigeons extinct within 100 years. But as a people, we know it now. And we know that other species are on the ropes, because of what we are doing. Hence we can decide if we want to let this extinction crisis continue to balloon.
The whole conservation movement is organized around the proposition that biodiversity is something worth saving. When a species goes extinct, it can leave a hole. Its ecosystem may suffer because the species can no longer carry out some important task, such as pollinating plants or filtering water. We lose the opportunity to investigate its biology and discover some fascinating piece of natural history or even find a valuable molecule for curing infections or sequencing DNA. And we end up living in a world without Great Auks and gastric brooding frogs. Is de-extinction a tool for slowing or reversing this trend? That’s a good question. But one thing’s for sure. We’re not playing God. We’re coming to terms with our own powers, as well as the unexpected results of our actions.
____
Aaron asks, “Should we bring any animal back from extinction which could threaten human life?”
What constitutes a threat? We have a habit of perceiving threats where the risks are tiny or non-existent. In fact, some species, such as the thylacine, were eradicated because they were considered a threat to human life–specifically, that they were killing off herds of sheep. That was untrue, but it didn’t stop people from driving the species extinct. Bringing them back would not pose a threat either.
I’m not saying that no revived species would pose a risk. But we do have to make sure we aren’t letting emotions ride roughshod over our decisions. Scientists have already revived a very dangerous life form: the flu virus that killed 50 million people in 1918. But no one has died from it, because precautions have been taken. And scientists have learned a great deal about how influenza evolves and kills–information that could help us in the future. This was a de-extinction of sorts that presented both risks and benefits.
____
@carlzimmer Wouldn’t resources be better spent preventing extinction before #deextinction were necessary?
— mitch merry (@mitchmerry) March 18, 2013
This is a point raised by many conservation biologists, both in my interviews for my article and at TEDx. “At this moment, brave conservationists are risking their lives to protect forest elephants from armed poachers,” David Ehrenfeld of Rutgers University said at the meeting. “And we’re talking in this safe auditorium about bringing back the woolly mammoth?”
If de-extinction really did make it harder to, say, pay guards to stop poaching, then I could definitely see a problem here. But where is the evidence of a zero-sum game at play? I don’t see it. No one at TEDx proposed cutting guard salaries to bring back a mammoth.
This concern could apply just as well to experimental research on animal reproduction–efforts to freeze cells of endangered species for research, assisted reproduction, and so on. They all cost money, they are not guaranteed success, and they all require people to do something other than guard against poaching. Yet some species have been introduced back to the wild, saved for now from extinction, thanks in large part to this kind of research.
These issues don’t just apply to extinct animals, but to the near extinct. There are four Red River giant softshell turtles left on Earth. They are not breeding with each other. We might be able to use stem cells to produce lots of new sperm and eggs and fertilize them to grow their population. Is this just a waste of resources, or will this end up saving the species? If we engineer frogs to resist chytrid fungus infections, is this just a simplistic technological fix, or the only way to keep them from going extinct?
My fellow Phenom blogger Brian Switek considers de-extinction little more than a slick marketing term. I disagree, if only because the issues that have emerged with its unveiling are going to stick around for a long time, even if no one tries to bring an extinct species back to life.
____
Do you think it would be ethical to reverse the extinction of Neanderthals? #NatGeoLive — Laurens Southgate (@laurens_s) March 18, 2013
If you want to go deep into everything that would be required in bringing back Neanderthals, check out this piece by fellow Phenom Virginia Hughes. Do not worry about meeting a Neanderthal on the street tomorrow, or next year.
If we could bring them back, should we? I think not, for many reasons. Neanderthals were humans, and research on humans requires informed consent, which is hard to get from someone who belongs to an extinct lineage. It would be unethical to bring people back without a place where they could live with dignity, and we have no idea what such a place would be for a Neanderthal in the twenty-first century.
____
@carlzimmer ethics and feasibility aside, if you *could* resurrect a species, which one would you go for? #NatGeoLive
— Pete Etchells (@DrPeteEtchells) March 18, 2013
I am quite taken with the idea of bringing back Steller’s sea cow. The first scientist to describe it was Georg Wilhelm Steller, who was on a voyage across the Bering Sea in 1741. He and his crewmates were shipwrecked on an island there, where they discovered herds of these amazing animals. They were relatives of manatees, reaching 25 feet long or more and weighing six tons. Here’s a wonderful image of them by the great illustrator Carl Buell, which is now on display at the Smithsonian.

Stelle’s sea cow. Copyright Carl Buell
Steller survived to write about the sea cows because his crew slaughtered some of the animals to eat on the voyage home. A single sea cow could feed a crew of 33 sailors for a month. Sailors on North Pacific ships killed so many sea cows that they vanished in 1768, just 27 years after Steller first described them.
Steller’s sea cow was part of the Pacific ecosystem for millions of years, and we are personally responsible for wiping it out. It would be quite something to figure out how put it back where it was just a couple centuries ago. But given the size of their potential surrogate mothers–not to mention many other obstacles–I’ll content myself with a daydream for now.