Carl Zimmer's Blog, page 31
March 15, 2013
De-Extinction: My story for National Geographic, an all-day exploration, and your questions
Advances in cloning, stem cell manipulations, and sequencing DNA raise a profound possibility: we might be able to bring some species back from extinction. That’s the subject of my cover story for the April issue of National Geographic, which comes out today, and which you can read here.
This morning I spoke on Morning Edition on National Public Radio. The interview will be archived here.
Later today, I will be giving an introductory talk to an all-day exploration of “De-Exinction” at a TEDx meeting at the National Geographic Society. The talks will come from a remarkable line-up of cloning experts, conservation biologists, bioethicists, artists, and others. You can watch the live stream here, and in a few weeks all the videos will be posted online.
This is a fascinatingly complex subject–there are all sorts of questions about how de-extinction would work and about whether it’s a good idea or not. I’d like to invite everyone to post any questions they have from the article or meeting in the comment thread for this post. When I get back home, I will answer as many questions as I can and publish them in a post on Monday
March 12, 2013
Watching Bodies Evolve
A lone yeast cell budding off a daughter cell. Photo by Carolyn Larabell. http://publications.nigms.nih.gov/bio...
Pretty much all the life you can see without the help of a microscope–toadstools, poplars, shortstops–is multicellular. Life began as single-celled microbes over 3.5 billion years ago. But at least 25 times over the course of the history of this planet, microbes have come together to form multicellular collectives–otherwise known as bodies.
These transitions are particularly intriguing to evolutionary biologists, because the nature of evolution itself changed along the way. If you’re a microbe, natural selection favors mutations that affect your nature as a single cell. If you stumble across a way to feed on a new nutrient, your descendants may grow faster than the competition and come to dominate your population. Single-celled microbes can evolve to be altruistic–even to commit suicide for the good of their fellow microbe–but they only do so to help their single-celled relatives.
Once microbes evolve a body, they’re playing a new evolutionary game. You have 10 trillion cells in your body, which develop into hundreds of types. Their evolutionary success depends not on their own single-celled reproduction, but the survival of your entire body. A neuron in your brain is not going to go hunting for food. It is going to depend on your body–the brain in which it’s embedded, your muscles, your bones–to find food, on your digestive system to break that food down, and on your circulatory system to deliver it up to the brain. If that whole system works well, it can reproduce. In other words, you have kids.
Scientists suspect that the first step towards a complex multicellular body like ours is for cells to evolve to live in primitive clumps. There may be a lot of advantages to living this way. It may be harder for a predator to eat you, for example. At the University of Minnesota, a team of scientists led by William Ratcliff and Michael Travisano figured out a way to create this kind of natural selection in a lab. As I reported last year in the New York Times, they were able to get yeast–which normally lives as single cells–to turn into simple multicellular clumps in a few weeks.
[image error]
A “proto-body” made up of dozens of yeast cells. Courtesy of William Ratcliff
The scientists reared genetically identical yeast from a single ancestor and then seeded ten flasks of broth with them. The yeast fed on the broth, grew, and budded off daughter cells. The scientists kept the flasks shaking for a day, so that the yeast swirled around. Then they stopped the shaking, and the yeast started to settle down towards the bottom of the flasks. Ratcliff and Travisano drew out a little of that settled yeast and put it in fresh flasks.
And then they repeated the procedure again and again and again. Any mutation that could speed up the yeast’s fall might be favored by natural selection, they reasoned, because fast-falling yeast would be more likely to get scooped up and survive to the next round of the experiment.
In a few weeks, a change did occur. In all ten flasks, the yeast fell faster, forming a cloudy layer at the bottom of the flasks. It was no longer growing as single cells, Ratcliff and Travisano found. In all ten flasks, the yeast was now forming snowflake-shaped clusters, made up of hundreds of cells. The snowflakes formed because daughter cells remained glued to their parents. And the clusters fell faster than their single-celled forerunners.
The cells inside these simple bodies even develop differences. After two months, some of the yeast cells started to commit suicide, while others continued to grow. The dead cells created weak points in the branches, causing them to break off. The broken branches then grew into snowflakes of their own–arguably, a simple form of reproduction.
Obviously, these yeasty snowflakes are a far cry from a human body (or a toadstool, to keep it among the fungi). But it turns out that the evolution of multicellularity didn’t stop with the emergence of these clumps. The initial experiment lasted two months. But then Ratcliff and Travisano extended it nearly five months more. In the journal Evolution, they report that the yeast are still evolving–and that some of the evolution is occurring on the level of their newly-evolved bodies.

Some cells, stained green in this image, commit suicide, allowing branches of yeast cells to break off. Courtesy of William Ratcliff
Over the course of the new experiment, the yeast continued to adapt to the environment the scientists created for them. By the end of the extra five months, they were falling 45% faster.
They sped up in three different ways. One was by increasing the cells in their bodies. Over the course of the experiment, the number of cells in each cluster nearly tripled, from 42 to 115. More cells made the clusters heavier and made them fall faster.
But after the fourth week of the experiment, the clusters also began to change in a second way: the cells themselves started to swell. By the ninth week of the experiment, the average mass of each cell had doubled. Bigger cells allowed the clusters to fall even faster.
While these changes made the yeasts better fallers, they also came at a cost. Large cells need more energy to reach their greater size. And living in a large cluster makes it hard to for yeast to grow, because the cells buried deep inside the core of a cluster have less food reaching them.
Ratcliff and Travisano found that after two months, the yeast evolution took a third turn, one they suspect was driven by this growing cost. The cells got bigger, but became less dense. Meanwhile, the clusters themselves changed their overall shape.
Originally, the clusters grew in snowflake-like clumps, with branches extended out on all sides. Ratcliff and Travisano found that after two months, the clumps grew more spherical. Analyzing these ball-shaped clumps, the scientists found that they fell faster than branched clumps with the same number of cells. That’s likely because the yeast could slip through the water more efficiently, without so many branches creating drag.
These changing shapes were not the result of identical adaptations occurring in each cell. They were changes to the overall anatomy of the clusters. Along with the emergence of the branch-snapping suicidal cells, this shape change demonstrates that evolution has shifted in Ratcliff’s experiments. It’s moved from the single cell to the proto-body. And as the proto-body grows and faces new constraints, it is evolving new solutions for many cells living together.
There may well be more strategies for getting bigger that the yeast have yet to evolve. I don’t expect man-sized yeast creatures walking out of Ratcliff and Travisano’s lab any time soon. But I am curious what another year or two of evolution will reveal.
(PS: When I wrote about this research last year, Ratcliff offered some responses to questions raised on the blog and on Twitter. For those curious for more details about this research, check them out.)
March 11, 2013
Resurrecting A Forest
For the cover story in the April 2013 issue of National Geographic, I explore an idea that sounds like pure science fiction: bringing extinct species back to life. What was once the purely the domain of Crichton and Spielberg is becoming a new field of research. Thanks to spectacular advances in cloning, reproductive technology, and DNA sequencing, scientists can now seriously explore the possibility of reviving some species from extinction. If not dinosaurs, then perhaps mammoths or passenger pigeons.
“De-extinction,” as its advocates sometimes call it, is part of a bigger trend these days in the world of conservation. Over the past five decades, conservation has usually taken the form of removing threats so that endangered species can recover–ban pollutants, protect habitats, stop hunting, and the like. Conservationists saved the brown pelican, for example, by protecting it from DDT and similar chemicals and by preserving the coastal wetlands where it lives. What they did not do, however, was tinker with brown pelican DNA to make the birds better able to survive. Indeed, the brown pelican gene pool–the product of millions of years of evolution before humans turned up–was ultimately what the scientists were trying to protect from oblivion.
Meanwhile, over those same five decades, molecular biologists have become adept at probing and manipulating genes. Sequencing genomes went from a dream to just another day’s work at the lab. In the 1970s, scientists began inserting genes from one species into another, and they can now build simple genetic circuits.
Conservation biologists have taken up many of these tools. They learned how to sequence DNA, for example, so that they could map populations of endangered species and track the flow of genes between them. They’ve used advanced reproductive technology to raise their success rate with captive breeding programs. The San Diego Zoo has frozen stem cells and tissues from thousands of species of animals to investigate for new ways to conserve them in the wild.
But conservation biologists have also seen some risks to biotechnology. If a synthetic organism could establish itself in the wild, for example, it could become an invasive species, putting native species at risk. (It’s important to point out that there’s no evidence that such an invasion has happened yet.) If we think of biodiversity as the world’s storehouse of genetic variation, then biotechnology has the potential to drive it down. Genetically engineered plants or animals might interbreed with wild relatives and spread their modified genes into the environment, reducing genetic variation in the wild.
Despite the potential risks, a number of conservation biologists are gingerly considering making even greater uses of biotechnology in order to protect biodiversity. Next month, for example, the Wildlife Conservation Society is hosting a meeting called “How Will Synthetic Biology and Conservation Shape the Future of Nature?”
Here’s a passage from the meeting’s framing statement:
“Critics have focused on the threats posed by novel life forms released into the environment, but little attention is paid to potential opportunities–to reconstruct extinct species or create customized ecological communities designed to produce ecosystem services. They may change the public perception of what is “natural” and certainly challenge the notion of evolution as a process beyond human construction.”

One of the few surviving American chestnuts, located in Maine. Photo courtesy of William Powell
To me, there’s no better example of the ambiguous future of conservation biology than the story of the American chestnut.
When Europeans arrived in North America, they found forests filled with American chestnut trees. These mighty plants, which could grow to be 100 feet tall, were the most abundant trees in the forests, making up 25 percent of the standing timber of the eastern United States. In the summer, the peaks of Appalachian mountains appeared to be capped with snow, thanks to the explosion of white chestnut flowers. Chestnut trees anchored the ecosystems of eastern American forests, providing food and shelter to bears, Carolina parakeets, and a vast number of other species. They were also a mainstay of loggers, who could fill an entire train car with boards cut from a single tree.
In 1904, a scientist observed that a chestnut tree at the Bronx Zoo was dying. It turned out to be infected with a fungus that came to be known as chestnut blight. No one is quite sure how it got to the United States, but all the evidence we have indicates it hitch-hiked its way in the 1870s on chestnut trees imported from Japan.
Chestnut blight, while harmless to Asian trees, proved devastating to the American ones. The fungi released a toxic substance called oxalic acid that killed off the tissue, allowing them to feed on it. An infected tree developed cankers on its trunk, and once they spread around the full circumference of a tree, it could no longer carry water and nutrients from its roots to its branches.

A stand of blight-infected chestnuts in New York, 1915. Courtesy of William Powell
Over the course of about eighty years, the chestnut blight spread across almost the entire range of the American chestnut, from Maine to Missippi. It conquered nine million acres and infected three billion trees. A few lone trees still survive unharmed here and there, but no one under the age of sixty has ever seen the forests of the eastern United States as they once were.
In the pantheon of extinction, American chestnuts are poised awkwardly at the door. Chestnut blight doesn’t kill the trees outright; as it spreads down to the roots, it encounters other microbes that outcompete it. As a result, infected trees become stumps. Sometimes they send up a new shoot, but once it reaches a few feet in height, the fungus attacks it again, and the shoot dies back.
“It’s basically functionally dead,” William Powell of SUNY College of Environmental Science and Forestry in Syracuse, New York, told me. “They sprout up, they get the blight again, and they are killed down to the ground. You know the story of Sisyphus? The guy who rolled the rock up the hill and it just kept rolling back down? Well, that’s kind of like what’s happening with the chestnut.”
It’s been a century since American foresters started trying to save the tree. They sprayed the trees with fungicial chemicals, to no avail. They infected the blight with fungus-invading viruses, but resistant strains continued to kill trees. They tried burning down chestnut trees to create a fungal firebreak, only to discover that the blight could silently infect oak trees, too.
They did what conservationists have always done–try to remove the threat–but nothing worked.
In the 1980s, a group of scientists embarked on a different approach, one that is now showing signs of success. If they couldn’t stop the blight, they would help the trees defend themselves.
The reason that chestnut blight was able to come to America in the first place was that Asian chestnuts can fight the fungus. They have genes that allow them to hold the cankers in check and scar them over. The trees can continue to grow and produce pollen and seeds. American chestnuts, evolving thousands of miles across the Pacific, never got the opportunity to evolve defenses against the blight. So the American Chestnut Foundation, a non-profit established to save the tree, decided to start breeding the two trees together, to see if they could provide the American chestnuts with Asian defenses.
When the foundation’s scientists interbred the American and Asian trees, the plants mixed together their genes in different combinations in their hybrid seeds. The scientists grew the seeds into saplings, and after a few years, it became clear that some of the hybird chestnuts had inherited some of the Asian defense genes. The cankers grew more slowly on them than on their American ancestors.
But the trees were no longer recognizable as American chestnuts, since half of their DNA came from Asian chestnuts. Asian chestnuts are small, orchard-like trees, and so the hybrids were far smaller than their towering American ancestors. These hybrids were not the solution to the chestnut blight, in other words. Their defenses were still weak, and they would not survive in American forests in the shadow of oaks and other big trees.
So the scientists kept breeding the trees. They used another tried-and-true method, known as backcrossing. They bred the American-Asian hybrids with American chestnuts, producing trees with only a quarter of their DNA coming from Asian chestnuts. Again, some of the new trees could resist the blight, while the others couldn’t. That was because the quarter of their DNA from the Asian trees contained the genes essential for fighting the disease. At the same time, the trees more closely resembled American chestnuts, because they inherited more of their DNA.
From this generation, the scientists picked the best-defended trees and back-crossed them again. They also mated hybrids with one another, shuffling the genes into new combinations, and selectively breeding the chestnuts that were both more resistant and bigger. They’ve now got thousands of trees that are 15 parts American and one part Asian growing on their experimental farm in Virginia.

A diagram of backcrossing experiments. American Chestnut Foundation
That one-sixteenth of Asian chestnut DNA may not sound like a lot, but it is. “There are thousands of genes in there,” says Powell. For all we know, some of those genes may impair the success of chestnuts in American forests. “It’s better to be precise about the genes you put in,” Powell argues. Working with the American Chestnut Foundation, he and his colleagues have developed a surgical approach to breeding resistant chestnut trees.
In 1990, Powell and some colleagues started investigating how to move single genes into American chestnuts. It took years to get the project off the ground. You can’t insert genes into a tree simply by sticking a needle into a trunk. Genes can only be inserted into individual cells. So Powell and his colleagues had to figure out how to rear chestnuts in their lab.
Some plants can survive as cells in a lab forever. But chestnuts are not one of those plants. Powell and his colleagues found that they had to combine pollen and ovules to produce embryos. With just the right concentration of hormones, the embryos bud off more embryos, which bud off embryos in turn. The scientists can then pick off individual embryonic cells, insert genes into the, and then grow the cells into full-blown chestnut trees.
After figuring all of this out, the scientists began to search for genes to insert into the chestnut cells. At the time, no one had mapped Chinese chestnut genes, so Powell and his colleagues turned to better studied plants. Plant scientists had figured out how wheat fights fungi, making enzymes that chop up the oxyalic acid into harmless byproducts. Powell and his colleagues inserted the wheat gene for the enzyme into chestnut cells and then grew the cells into trees.
At first the enzyme wasn’t much help, so the scientists fine-tuned the genes so that the chestnuts made more of it. The more oxalic acid they made, the better they fought the chestnut blight. The scientists eventually produced trees that could limit the cankers and heal them over.

William Powell inspects American chestnuts with blight-inhibting enzymes. Photo: Syracuse University
Last spring, the New York Botanic Gardens planted a few of the chestnuts for public display. (You can see the video of the ceremony here.) You can go to the gardens now and can see for yourself that the trees are growing and thriving, despite being exposed to chestnut blight spores wafting by. “We want to do everything transparently,” says Powell. “We don’t want people to think we’re hiding anything here.”
It may be five years or longer before these trees start growing in the wild. Powell and his colleagues need to spend a couple more years collecting data before submitting an application to the U.S. Department of Agriculture, and then the Environmental Protection Agency has to sign off on the project. Even the Food and Drug Administration will have to get in on the act, because the trees will produce nuts that people might eat.
But the trees growing in the Bronx are not the final version Powell hopes to see reviving America’s forests. It’s now finally possible for him and his colleagues to explore the Chinese chestnut tree genome, and so they’ve started hunting for blight resistance genes. One gene for chopping up oxalic acid won’t be enough to provide full resistance, Powell suspects. He’s pretty sure that Chinese chestnut trees have evolved a number of genes that together render the blight harmless.
Adding in extra genes is essential, Powell believes, because the chestnut blight is not a fixed target. It is evolving, and it will probably be easy for it to evolve its way around just one line of defense. Each tree will need to be equipped for many attacks from evolved pathogens over the course of its lifetime, which can be as long as a century. Powell suspects a few genes will provide a durable defense, but he can’t say for sure which genes those are. So far, he and his colleagues have identified a list of candidate genes in Chinese chestnuts. “We’ve narrowed it down to about 900 now,” Powell told me with a laugh.
If, a century from now, Powell’s chestnuts tower once again over the eastern United States, how will we think of those forests? Will we think of them as nature restored to its former glory, ecosystems thriving once more? Or will we think of them as unnatural, the product of human tinkering? Or both? Given the past century of struggle to save the chestnut, the choice here is not natural versus unnatural. It’s chestnuts versus no chestnuts. “It’s not going to fix itself,” says Powell.
____________
Powell and I will both be among the speakers at TEDxDeExtinction, taking place at the National Geographic Society in Washington DC this Friday. You can buy tickets to the all-day event here, or watch it livestreamed for free here. My story for National Geographic will be available online on Friday as well. For more information, visit National Geographic’s DeExtinction Hub.
March 8, 2013
The “Nightmare Bacteria”: An Explainer
Earlier this week, the Centers for Disease Control warned that we’re facing an onslaught of “nightmare bacteria”–a group of highly resistant, highly deadly microbes. Talk of the Nation, the National Public Radio Show, asked me to join them to talk about these bugs yesterday. You can listen to it here:
This is the sort of story that seems tailor-made for confusion, thanks to the squirrelly nature of microbiology. As my fellow guest on the show, NPR science correspondent Rob Stein, observed, we are not dealing with an out-of-control plague that will wipe out the planet. On the other hand, as I pointed out, these nightmare bacteria are killing people, and will probably kill more people in the future. I’m sure that listeners were left scratching their heads a bit. So I wanted to take a moment this snowy morning to explain the news at more length.
Not long after scientists invented antibiotics in the mid-1900s, they started observing bacteria that were becoming resistant to the drugs. Some of the microbes carried mutations that made them a little less susceptible, so that a few of them survived the onslaught and could reproduce. Their descendants mutated more, and some became more resistant.
At the same time, genes were slipping from one microbe to another–even leaping the species barrier–and that made things even worse. For one thing, the bacteria that make us sick could tap into the vast repository of resistance genes in the other bacteria dwelling in our bodies and in the soil. The bacteria could load several resistance genes into the same piece of DNA, becoming resistant not just to one drug, but to many.
For decades, microbiologists have been warning that resistance was rising, and that things were going to get worse. And worse they did become. Fearsome strains of bacteria emerged, such as methicillin-resistant Staphylococcus aureus, now familiar by its acronym MRSA. (For the full story of MRSA, read Maryn McKenna’s award-winning book Superbug. And then read all her other stuff too.)
This week the CDC raised a warning about a different kind of bacteria, called CRE for short. That stands for carbapenem-resistant Enterobacteriaceae. (I can hear you saying, “Um, is it okay if I just call it CRE?” I’m here to tell you yes.)
Enterobacteriaceae refers to a large taxonomic group of bacterial species. It includes some familiar bugs, like E. coli, as well as many you’ve never heard of. Only some of them live in the human body, and only some of those strains have the potential to make us sick. (You probably have a few billion E. coli in your gut right now. But if you get one of the nasty E. coli strains, you may get organ failure or die.)
Okay, that takes care of the E in CRE. CR refers to the ability of some of these strains to resist carbapenems, which are a class of potent antibiotics. When they were first developed, they were a godsend, because doctors could use them against bacteria that had become resistant to older drugs like penicillin. But in the 1990s and early 2000s, hospitals started seeing bacteria–members of the Enterobacteriaceae, spefically–that had evolved enzymes that they used to chop up the carbapenems.
One of the worst of these offenders is a strain of Klebsiella called KPC. I just wrote about an outbreak of KPC at a major research hospital for Wired, and I hope that my story gets across what a nightmare these outbreaks can be. These bacteria can ride silently on healthy people from room to room, from ward to ward, from hospital to hospital. And then they can strike vulnerable patients. Since these bacteria can resist carbapenems, doctors are left with few options. They can use a few truly nasty drugs that were abandoned decades ago because they were so toxic. And, as I write in my Wired feature, the bacteria can evolve resistance even to those drugs in the body of a single patient. And then it’s game over. As a result, CREs can be up to 50% fatal.
KPC is just one of the CRE bacteria doctors are worried about. In India, a new set of resistance genes has emerged that go by the name NDM-1. Several different species carry them, and they’re not limited to hospitals. Scientists have even found NDM-1 germs in ordinary tap water. Once these genes evolve, they don’t stay put. NDM-1 has made its way to the United States–possibly thanks in part to medical tourism. Meanwhile, KPC–which got its start on the east coast of the United States–has spread to many countries overseas.
Most of the trends for CRE are going in the wrong direction. New kinds of resistance genes are evolving and spreading to different species. Those resistant strains are showing up in more states and more countries. The percentage of these bacteria that are resistant is increasing. The CDC’s announcement this week was spurred by a recent survey they did. They found that 4.6% of the hospitals they surveyed had one CRE infection in the previous six months. And eighteen percent of long-term care facilities had one, too.
These are worrying figures, when you consider that previous generations of doctors simply didn’t have to contend with CRE. Strains like KPC just didn’t exist. On the other hand, the percentages are still fairly low. I asked a doctor who works at a hospital in a small Connecticut city what she thought about the report. She said, “Well, you hear about outbreaks at other hospitals, and you just hope it doesn’t come here.”
Unfortunately, there’s not much stopping it from coming here, and everywhere. That doesn’t mean that there’s nothing to be done. We just need to get our collective act together. Here are a few things that many experts are agreeing would help:
–Get the data. Right now, there are few states that demand that hospitals report the presence of CREs. If there was a nation-wide database, it would become possible to organize large-scale campaigns against the bacteria. If more hospitals get the means to sequence genes of the bacteria, they might even be able to track individual outbreaks from hospital to hospital.
–Get serious. Israel faced a KPC outbreak in 2007 and went a long way to reducing infection rates. They did so with a national campaign. When they saw hospitals getting hit by the bacteria, they required some tough measures be put in place. I describe some of those measures in my Wired story. Isolate the infected patients. Test other patients regularly with accurate tests. Dedicate nurses and doctors to the infected patients and don’t let them get near other patients. Bomb hospital rooms with bacteria-scrubbing chemicals. Wash hands. Wash them again. Put people in the wards whose job it is to go up to the chief of surgery and say, “You didn’t take two squirts of hand gel. That’s the rule.” Some experts are even suggesting that hospital doctors always wear gowns and gloves. It’s a pain in the neck, to be sure, but dentists already do it, so why not doctors?
–Become good stewards of antibiotics. It’s not surprising that NDM-1 took off in India, because you can walk into a drug store there and buy antibiotics without a prescription. That’s a fabulous way to breed resistant bacteria. Likewise, many scientists are warning that feeding antibiotics to farm animals breeds resistance and then make its way back to the bacteria that make us sick. The antibiotics free-for-all has to end.
Here’s one thing that’s especially scary about CREs: one of the biggest risk factors for acquiring them is having taken antibiotics to treat another infection in the previous few months. As I wrote here in December, antibiotics are not picky about who they kill. They can destabilize your microbial ecosystem, allowing invasive species to push their way in. Doctors have to become more careful with using antibiotics, and scientists have to explore alternatives, such as repopulating ecosystems with transplanted bacteria.
–Get the antibiotics pipeline flowing again. What makes the current crisis with CRE so scary is that we have just about nothing left in our arsenal, and we won’t be getting new antibiotics that are effective against CRE any time soon. The market incentives for developing antibiotics are dismal: these are very expensive drugs to develop and test, but the potential profits to be made from them are not enticing. In fact, if someone came out with a powerful new antibiotic against CRE today, doctors would say, “Thank you so much,” and then put it on their shelf, to save in case other antibiotics failed. There are bills being considered by Congress now to rejigger the incentives to get the antibiotics pipeline flowing again.
–Look beyond CRE. MRSA were once the organisms du jour. Now it’s CRE. Hospitals are being told to stay vigilant against each of these types of bugs in isolation. But that would be a bit like telling a country to prepare for a naval invasion, when the enemy has planes, missiles, and ground troops, too. There are several groups of dangerous bugs invading hospitals, and there will be new ones evolving in years to come. And, to completely shatter my military metaphor, the genes from one group may end up in another. Unfortunately, hospitals can’t test for a dozen species or genes at once. They simply lack the technological capacity. They need it.
Making matters worse, going after one group of bacteria at a time may potentially make things worse overall. For example, one way to fight MRSA is to use an antiseptic called chlorhexidine. This turns out to favor CRE. If you use alcohol hand rubs to fight MRSA and CRE, you may open the door to bacteria called Clostridium difficile.
Richard Wenzel and Michael Edmond of Virginia Commonwealth University have dubbed this one-at-a-time strategy a vertical interventional control program. They call instead for horizontal programs–in other words, recognize that we are fighting against lots of species at once and develop strategies for them all.
Up to ten percent of patients admitted to hospitals will acquire an infection. At least 90,000 people die in the United States alone of these hospital-acquired infections. It won’t be easy to fight these bacteria, but just sitting back is unacceptable. We know that more people will die.
March 4, 2013
The Brain-Chilling, Shrimp-Caressing, Lamppost-Sized, NSFW Organ Hiding In A Whale’s Mouth
This is a story about the discovery of an organ that measures twelve feet long and four inches wide. You might well assume that this is old news. After all, how could something the size of a lamppost go unnoticed by anatomists? And yet, in fact, it’s only just come to light.
The discovery emerged out of a blood-drenched confusion. Alexander Werth, an anatomist, was standing on an ice sheet miles off the coast of Alaska’s North Slope. He was watching Inupiat whale hunters dismember bowhead whales they had caught in the Bering Sea. This government-sanctioned hunt is one of the best opportunities for whale anatomists to get hold of fresh tissue from the animals.

Alex Werth in the middle of flensing a bowhead whale on the ice off of Alaska
To take apart the head of a whale, the hunters would slice off the lower jaws and the tongue, which could be as big as a minivan. They would then climb onto the roof of the whale’s mouth and cut away the baleen–the hair-like growths that the whale used in life to filter small animals from the water. On the roof of the mouths of bowhead whales, Werth and his colleagues noticed something strange: a peculiar rod-like organ stretching down the midline of the palate.
It had never been described in the bowhead before. What made the organ particularly peculiar was that, as the Inupiat cut the whales apart, it poured forth huge amounts of blood. Why, the scientists wondered, should a bowhead whale have an organ in the roof of their mouth? And why should it be so bloody?

The roof of a bowhead whale’s mouth. The fur-like growth is baleen. The pink strip is a newly discovered organ. Photo courtesy Alex Werth
One of Werth’s colleagues, Thomas Ford of Ocean Alliance, had noticed something similar in humpback whales twenty years ago. So Werth, Ford, and Craig George the Department of Wildlife Management at the North Slope Borough in Alaska decided to take a close look at the bowhead whales. They dissected some of the organs out of freshly killed whales, photographing them as they cut the tissue free. They brought one of the organs back to their lab, along with sections they chopped out of other organs, to examine under a microscope.
And this is where the story gets a little NSWF.
You see, the organ in the whale’s mouth turned out to be, biomechanically speaking, a twelve-foot-long penis.
Penises–in humans, whales, and other mammals–are made of a distinctively sponge-like tissue. When blood pours into the penis, the tissue stores it in a multitude of cavities, stretching out to hold the increased volume. As the penis swells, muscle fibers wrapped around the spongy tissue stretch and then tighten. Thus the penis becomes both enlarged and hardened. Unlike a bone, which is always hard, the penis can become soft again when its vessels pump out all the blood.
The organ in the bowhead whale mouth, Werth and his colleagues found, has the same distinctively spongy tissue, along with copious vessels supplying it with blood. Its anatomy strongly suggests that the whales can engorge it–hence the bloody mess it made when the whales were cut apart. Werth and his colleagues traced the blood vessels out of the organ and into the interior of the whale head. They found that they made close contact with a web of blood vessels at the base of the brain.
[image error]
A diagram showing the location of the brain-cooling, food-sensing organ (marked “palatal CCM organ”). The front of the whale head is at the top; the brain is at the bottom. From Ford et al 2013.
Based on these findings and others, Werth and his colleagues think they know what the organ–which they dubbed the Corpus Cavernosum Maxillaris–is for. It has two jobs, the first of which is to keep the whale’s brain cool.
Staying cool may seem like the last thing a bowhead whale needs to worry about. Water is very good at pulling heat out of a body, even at lukewarm temperatures. And bowhead whales lead extraordinarily frigid lives, spending much of the year in the Arctic Ocean. You’d think that bowheads would need special adaptations to keep their warmth in, not to get rid of it.
Indeed, bowheads, like other marine mammals, have a very good adaptation for that job: namely, blubber. Bowheads are blubber champions, growing layers that can get as thick as 40 centimeters. The shape of their bodies also helps keep them warm; Werth calls them “chubby, rotund zeppelins.” Their round shape gives them a low ratio of surface area to body volume. As a result, they can store more heat in their body and lose less of it through their skin than a thinner whale.
Unfortunately, solutions to biological problems have a way of causing problems of their own. As warm-blooded animals, bowhead whales generate heat, and when they’re foraging for food or migrating across an ocean, their muscles generate even more heat. Thanks to their anatomy, the whales are so well protected against the cold that this extra heat has nowhere to go. Too much heat can damage a mammal’s organs, with the brain being especially sensitive to even the slightest fluctuations of temperature.
Many marine mammals have adaptations to reduce this danger. A number of whale species, for example, have an intricate system of blood vessels that deliver hot blood from the core of their body into the dorsal fin on their back, where the heat can escape through the skin. The flukes of their tails and their fins can also dump heat. The whales can expand the vessels to release more heat when needed and constrict them to avoid losing too much.
Bowheads don’t have any dorsal fin at all, and the fins on their sides are small. So they swim very close to the thermoregulatory line. Werth thinks the Corpus Cavernosum Maxillaris keeps them from crossing that line.
The whales, Werth argues, fill the Corpus Cavernosum Maxillaris with some of the hot blood swirling around their heads. When they open their mouth, a colossal amount of chilly Arctic water pours in. The heat from the Corpus Cavernosum Maxillaris gets sucked away, cooling the blood. The cooled blood then travels back into the whale’s body. Because the Corpus Cavernosum Maxillaris contacts the base of the brain, it may be especially helpful for keeping the brain cool. The penis-like tissue it’s made of may allow the bowhead whales to switch off this heat dump by pinching off the blood vessels to the Corpus Cavernosum Maxillaris.

Heat escapes from the mouth of a dead bowhead whale. From Ford et al 2013
In a paper to be published in The Anatomical Record, Werth and his colleagues offer the details of their research that supports this theory. Here, for example, is a picture showing the mouth of a bowhead that was killed seven hours earlier. The bright colors show where it’s hot. The scientists found that the Corpus Cavernosum Maxillaris was still about twelve degrees hotter than the surrounding tissue. That’s the sort of intense heat you’d expect from a structure that had evolved to keep a whale cool.
This would be fascinating enough, but Werth and his colleagues suspect that the Corpus Cavernosum Maxillaris has a second job to fulfill. As they dissected the organ, they discovered that it is packed with nerve endings. What’s more, they have the shape and arrangement that makes them very sensitive to touch. In addition to dumping heat, the Corpus Cavernosum Maxillaris may be a sense organ. Werth proposes that these nerve endings in the Corpus Cavernosum Maxillaris help bowhead whales eat.
Baleen has enabled some whale species to become gigantic. The blue whale, in fact, is the largest animal to have ever existed. But using baleen to feed is no simple matter, and scientists are just starting to appreciate the complexity of the choreography it demands. Fin whales, for example, drop their jaws and let the skin balloon out like a parachute, engulfing a volume of water about equal to a school bus. They then swing their jaws shut and push their enormous tongue forward, squeezing the water through their baleen. Each gulp can yield a fin whale half a million calories. (See my pieces in the New York Times and the Loom, plus Ed Yong’s piece for more details.)
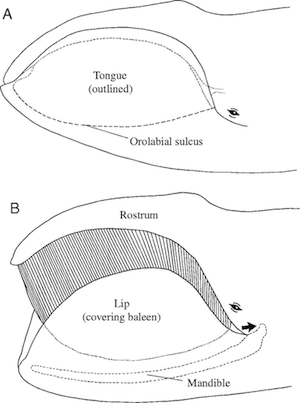
Bowhead mouth closed (top) and open for feeding. (Werth 2004 http://jeb.biologists.org/content/207... )
Bowhead whales use a different strategy that’s no less demanding. They open their mouths partway as they swim, ramming water through mouth and letting it spill out the corners. Animals get trapped in the baleen along the way. Bowhead whales can ram three cubic meters of water each second. While that’s a good way to capture a lot of food, it also demands a huge amount of energy. If a bowhead rams water with few animals in it, it ends up losing more calories then it gains.
It might be very useful to such an animal to know how much food is in the water it’s taking in. An exquisitely sensitive organ in the roof of their mouth might be just the thing a bowhead needs, telling it whether it can enjoy a banquet in its baleen or needs to shut its mouth and find better hunting grounds.
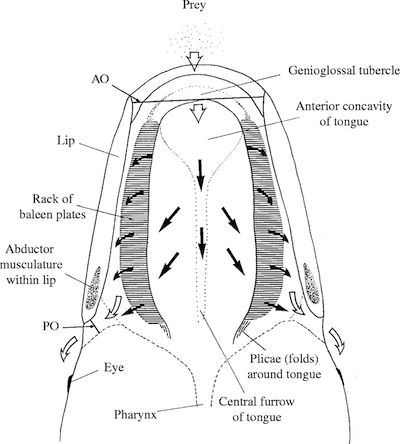
Bowhead ram feeding (Werth 2004 http://jeb.biologists.org/content/207... )
I asked Jeremy Goldbogen, an expert on whale feeding at the Cascadia Research Collective, for his opinion on the new paper. “What a fascinating and exciting study!” he wrote back in an email, endorsing Werth’s idea that the Corpus Cavernosum Maxillaris has two jobs to perform. This means a lot coming from Goldbogen. He was part of a team that also discovered a gigantic sensory organ in fin whale jaws; those whales probably use it to control their gulps. (See this post by Ed Yong for details.)
The work of scientists like Werth and Goldbogen makes clear that there are enormous mysteries left for anatomists to solve. And if Werth and his colleagues are right, scientists may rethink many aspects of bowhead life. Opening their mouths may not just be a way for the whales to catch food. It may also be a way to stay cool. And it may be no coincidence that on their long migrations between the Arctic Ocean to the Bering Sea, bowheads are sometimes seen with their mouths gaped open. Like a panting dog, they may be trying to stay cool among the icebergs.

Painting by Carl Buell
[Thanks to Carl Buell for his paintings. Visit his Facebook page for more natural history goodness.]
March 3, 2013
Marking An Environmental Success Story (Science Ink Sunday)
 Alyssa writes, “I’m a wildlife biology student at UC Davis with a particular obsession with ornithology, as well as a strong love for the rich, diverse ecosystems we have along the coast of northern California. Somehow seeing Brown Pelicans flying by, their bizarre combination of obvious goofiness with an odd elegance never fails to put a smile on my face. I also appreciate that (offshore oil drilling problems aside), their population growth after ESA listing is about as close to a success story as we have in conservation. My tattoo is based of a painting by one of my heroes, John James Audubon. I asked the tattoo artist to darken the hind-neck and redden the gular pouch to reflect the characteristic breeding coloration the pacific subspecies, Pelecanus occidentalis californicus. I also asked for the foliage to be removed to better reflect the roosting habitat in California. The tattoo is by Chris Arredondo at Royal Peacock Tattoo Parlor in Sacramento, CA.”
Alyssa writes, “I’m a wildlife biology student at UC Davis with a particular obsession with ornithology, as well as a strong love for the rich, diverse ecosystems we have along the coast of northern California. Somehow seeing Brown Pelicans flying by, their bizarre combination of obvious goofiness with an odd elegance never fails to put a smile on my face. I also appreciate that (offshore oil drilling problems aside), their population growth after ESA listing is about as close to a success story as we have in conservation. My tattoo is based of a painting by one of my heroes, John James Audubon. I asked the tattoo artist to darken the hind-neck and redden the gular pouch to reflect the characteristic breeding coloration the pacific subspecies, Pelecanus occidentalis californicus. I also asked for the foliage to be removed to better reflect the roosting habitat in California. The tattoo is by Chris Arredondo at Royal Peacock Tattoo Parlor in Sacramento, CA.”
You can see the rest of the Science Tattoo Emporium here and in Science Ink: Tattoos of the Science Obsessed.
And if you live in Connecticut, you’re invited to hear me speak at the Peabody Museum of Natural History at Yale University on Thursday at 5:30. Admission is free. (Poster here.)
February 28, 2013
Tongue-Eating Fish Parasites Never Cease to Amaze
NOVA put together a video, embedded below, about one of those animals that you have to keep persuading yourself is real, a parasitic crustacean that lives inside the mouths of fishes, eating–and then taking the place of–its host’s tongue.
I can vouch for these beasts, having written about them off and on since I first encountered them in my research for Parasite Rex–most recently on the Loom last year. But I was not aware that it’s the female that wins the Oscar for best performance as a fish tongue. The males just hang out around the gills of the fish and then–yep–mate with the pseudo-tongue.
This discovery led me to wonder about the latest research about tongue-eating isopods. I came across a 2012 master’s thesis by Colt William Cook of the University of Texas, which confirms what you see in the video–that the parasites are born as males, and then when they enter a fish, one turns female. This switch only occurs if there’s no female already installed in the host–otherwise, the males stay male. As this transformation takes place, Cook adds, the female’s body grows enormously. Its eyes shrink, since it no longer has to hunt for a home. Its legs stretch out, to help it anchor itself in the mouth.

Courtesy of Matthew Gilligan
After one of the males mates with the female, she gives birth to a brood of live male parasites. For their first few days, Cook found, they search madly for another host (each species of parasite seems to only live in a single species of fish). They sniff for the scent of their host, and if a shadow passes overhead when the odor is strong, they shoot upwards through the water. They burn through a lot of energy in the process; if they fail to find a host in the first few days, they settle down and hope they can ambush a fish that happens to be swimming by. It’s a hard way to start your life, and it may explain why several males will huddle inside a fish with only a single female in the offing. Looking for another fish with a single female parasite might be a less promising strategy than competing with the males you’re with.
Of course, these rules may only apply to the species that Cook studied, which infects Atlantic croakers off the coast of Florida. The full diversity of these tongue parasites is probably enormous. A 2012 study puts the total species at 280, but that’s just known species. A team of scientists from Annamalai University in India recently did a survey of the parasites in fishes off the coast of India. Before their study, scientists knew of 47 species of parasites in Indian waters. In just nine fishes, the scientists discovered ten new parasite species. I’d wager that some of the species waiting to be discovered will prove to be even more surreal.
February 27, 2013
The Virus That Learns
If you don’t have an immune system, you don’t last long in this parasite-riddled world. Your body receives a steady stream of invaders–viruses, bacteria, and other pathogens–which it has to recognize and fight. In many cases, it’s a brutal battle with an ultimate goal of eradication. In other cases, the immune system simply keeps strangers in check, preventing them from spreading. As many as a third of all humans have cysts in their brains containing a single-celled parasite called Toxoplasma. As long as the parasite stays in its cyst, the immune system lets it be. If Toxoplasma breaks out and starts to multiply, however, the immune system picks off the new cells. And if people lose their immune system–due to HIV infection, for example–Toxoplasma runs rampant and causes devastating brain damage.
The cells and molecules we use to recognize these invaders are unquestionably amazing. What’s perhaps most amazing is that the immune system can learn. When a new pathogen turns up, our immune cells undergo a kind of interior version of natural selection. Over the course of several cell divisions, new variants emerge that do a better and better job of recognizing the newcomer. Our bodies can then mount a powerful, focused attack on, say, a particular strain of the flu. And once the immune system learns how to recognize that new enemy, it can store that memory away, enabling it to attack the same pathogen years later.
This is the sort of thing that people often have in mind when they refer to us as a “higher” form of life, and bacteria and viruses as a “lower” form. Bacteria are just simple individual cells. They’re not multicellular organisms that can dedicate billions of cells to making antibodies, spewing poisons, and carrying out the many other tasks required for an immune system to work. Viruses–forget about it–they’re just protein shells that package a few genes, which they insert into a host cell.
But the higher/lower dichotomy is a blinkered way to look at life. If you can’t believe that bacteria can have an immune system, then you will miss the clues that they, in fact, have one. And the evidence is overwhelming.
Bacteria, after all, live in the same parasite-riddled world as we do. They may not get infected by the same pathogens that infect us, but they are continually hounded by viruses. A microbe that can defend itself against a virus will have a huge edge in the evolutionary race against its fellow microbes.
The threat of viruses has driven the evolution of some pretty impressive defenses. Bacteria make enzymes that lock onto certain, short sequences of DNA and slice them apart. When a virus injects its genes, these so-called restriction enzymes shred them into genetic confetti, so that they can’t take over the cell.
Our own immune system always runs the risk of turning against us and causing autoimmune disorders like arthritis and lupus. We have lots of safeguards in place to minimize that risk. Likewise, restriction enzymes are a dangerous defense, because they can chop up the distinctive stretches of DNA in a bacterium’s own genes. It avoids attacking itself by capping those sequences in its own DNA, so that the restriction enzymes can’t reach them.
The restriction enzyme defense is just one wing of the immune system in bacteria. Some species can muck up the production of new viruses, stealing their proteins before they can form shells. Others commit suicide upon infection, so as to avoid becoming an incubator for new viruses that would then kill their nearby relatives.
But the most impressive–dare I say it, most human–part of the bacterial immune system is its ability to learn. About forty percent of bacteria carry a set of genes known as CRISPR. When a virus invades these bacteria, they capture fragments of its DNA and insert them into their CRISPR genes. The bacteria then use those captured fragments as a guide for building weapons against the virus.
Here’s how this weaponizing works. In order to turn a virus’s genes into new virus proteins, a microbe must first make a copy of the gene in a molecule called RNA. CRISPR genes can produce RNA molecules with a matching sequence. They grab onto the virus’s RNA and prevent them from being turned into proteins. The virus factory grinds to a halt.
This defense helps bacteria withstand a virus infection, but it does more. The bacteria hold onto an invading virus’s DNA, so that they are now prepared for a fresh attack. And over time, bacteria can build up little libraries of these virus barcodes. A single bacterium may carry dozens of these viral barcodes. Last year, scientists at Indiana University surveyed the bacteria in people’s mouths and discovered 8,000 different viral barcodes–many of them corresponding to viruses scientists have yet to discover.
A single microbe can thus build up memories of its pathogens, in a manner reminiscent of the way we build up memories in our own immune system. But if you build up a healthy store of antibodies to various strains of flu, smallpox, and other diseases, all that knowledge dies with you. If you have children, they have to learn the same lessons all over again.
Not so for bacteria. When a microbe reproduces, it passes down its CRISPR genes and all of their viral barcodes to its descendants–including the ones it acquired in its own lifetime. Maybe Lamarck would have been better off as a microbiologist.
Now let’s swing around and consider the immune system from the pathogen’s point of view. If you can evolve a way to avoid the defenses of the immune system of your host, you will thrive where other pathogens are killed. This evolutionary pressure has led to all sorts of remarkable evasions carried out by the pathogens that make us sick. They camouflage themselves with human-like proteins; they attack key molecules, bringing our defenses to a standstill.
Viruses that infect bacteria have evolved their own set of tricks to evade the bacterial immune system. Last fall, for example, University of Cambridge scientists discovered viruses that carry an antidote for the suicide toxin made by their hosts. When the bacteria want to die, the virus forces them to live on. And just last month, University of Toronto scientists even discovered anti-CRISPR genes in viruses, which the viruses use to shut down the production of virus-killing molecules.
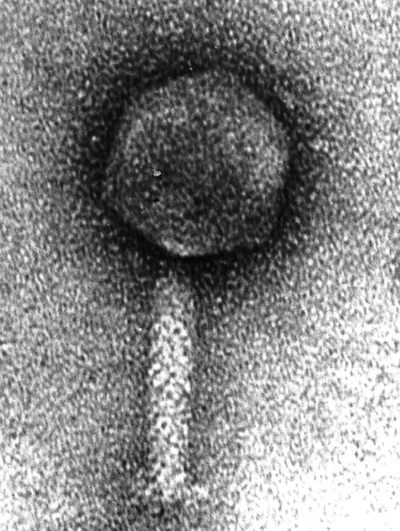
ICP1, a virus with an immune system. Source: Seed et al 2011 http://mbio.asm.org/content/2/1/e0033...
Now comes news of the most bizarre counterweapon I’ve ever heard of in a virus–and a serious challenge to fans of the higher-lower dichotomy. In Nature today, scientists at Tufts University describe their discovery of a virus with its own immune system.
The scientists, led by Andrew Camilli, stumbled across the virus while studying the bacteria that causes cholera, known as Vibrio cholerae. Scientists have long known that V. cholerae gets infected by viruses. In fact, there’s some evidence suggesting that these viruses can bring cholera outbreaks to a halt. As the V. cholerae hosts multiply, their viruses multiply even faster, until they send the bacteria’s population crashing down. Camilli and his colleagues set out to survey these viruses, to see how many species were making life hard for the bacteria.
They revisited a decade of cholera outbreaks by analyzing frozen stool that Bangladeshi doctors had stored from patients between 2001 and 2010. In those samples, they came across 15 different cholera-attacking viruses–12 of which were new to science. Fourteen of those 15 viruses came and went over the decade-long period, causing outbreaks among their bacteria hosts before disappearing.
But one virus–dubbed ICP1–was ominpresent.
Kimberly Seed, a postdoctoral fellow in Camilli’s lab, started sequencing the genes of ICP1 to look for the source its special strength. She found something none of them expected: a full-blown set of CRISPR genes.
Why would a virus carry a set of genes that bacteria use to destroy viruses? To use them against bacteria, it turns out. The ICP1 virus carries barcodes in its CRISPR genes that match pieces of its host’s own DNA. In particular, they match bits of DNA from a set of genes in V. cholerae that interfere with the production of new viruses. In a series of experiments, the scientists demonstrated that the ICP1 virus uses its CRISPR immune system to attack its host’s virus-attacking genes.
In one particularly cool experiment, the scientists engineered the V. cholerae hosts so that their DNA no longer match the virus’s attack molecules. The mutant bacteria managed to destroy most of the viruses. But over time, a few of the viruses somehow managed to acquire bits of DNA from the host and insert them into their CRISPR genes. The viruses regained the ability to shut down their host’s defenses and were able to invade successfully again.
In other words, the viruses had learned something about their enemy.
The ICP1 virus didn’t evolve its own CRISPR genes on its own, the scientists conclude. It stole them. Viruses sometimes pick up host genes and incorporate them into their own genome. The CRISPR genes in ICP1 most closely match those of the bacteria that cause bubonic plague. Long ago, it seems, the ancestors of ICP1 grabbed an immune system from that lineage of bacteria. Later, they turned this bacterial immune system against bacteria.
There are lots of very practical reasons to study immunity in the microbial world–reasons that sometimes only become clear in hindsight. As I wrote in my book Microcosm, the discovery of restriction enzymes in the 1960s made modern biotechnology possible. Scientists used the enzymes to cut and paste genes from one organism to another, creating microbial factories such as E. coli that makes human insulin. Last year, scientists reported that they had harnessed CRISPR genes to create a far more powerful way to edit DNA.
The discovery of a virus with an immune system could open up still other doors. It might be possible, for example, to use viruses to fight bacterial infections. In 2008, Camilli and his colleagues showed that viruses can prevent mice from getting sick with cholera, presumably by killing off the microbes. The mice were not harmed by the viruses, because they are adapted to infect bacteria, not animals.
With an adaptive immune system, these viruses might be able to learn new tricks to overcome any new defenses the bacteria evolve. And just because Camilli and his colleagues first discovered a virus with an immune system in V. cholerae doesn’t mean that there aren’t more of them out there. Indeed, some preliminary database searches hint that they are. Scientists might be able to harness CRISPR-equipped viruses to treat other diseases.
But these practical benefits will take time to emerge, if they ever do. Right now, we can enjoy the brain-stretching experience of looking out at the oceans, the forests, and even in our own mouths, and contemplate the existence of viruses that can learn something about their world.
Lower life indeed.
(For more about viruses, see my book A Planet of Viruses.)
February 25, 2013
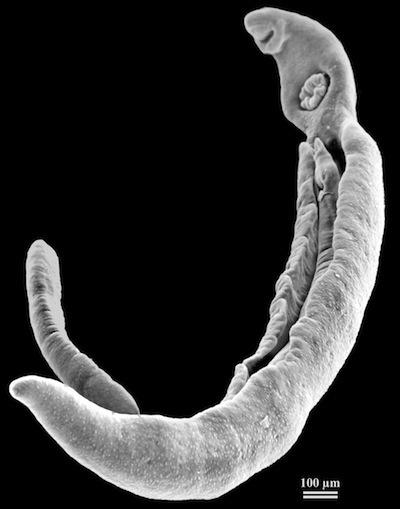
The Parasite’s Fountain of Youth
In 1980, a man walked into the Royal Perth Hospital in Australia, complaining that he was tired. He had been tired at that point for two years. The man’s medical history offered no good clues–at 44, his only indulgence was a glass of white wine at dinner each night. His doctors pushed and poked until they discovered his liver was swollen. Yet he showed none of the symptoms you’d expect from cirrhosis or liver cancer. The cause of the man’s trouble only became clear when the doctors got the report on his stool. It contained eggs from an animal known as Schistosoma mansoni–otherwise known as the blood fluke.
Blood flukes are parasitic flatworms. They get their start living in snails, which shed the parasites into the surrounding water. If you go wading into a blood fluke-infested pond, the missile-shaped flukes will sniff their way to your skin and drill in. Once they reach a blood vessel they surf the sanguine tide until they reach your intestines. They take up residence in the blood vessels there, producing eggs that they nudge into the intestinal walls. The eggs get washed out of the body with their host’s stool, perhaps to infect a freshwater snail. Sometimes, however, the eggs get swept off in the wrong direction and wind up in the liver, where they cause chronic inflammation.
Getting blood flukes (the disease is known as schistosomiasis or bilharzia) is, sadly, nothing special. Two hundred million people suffer from infection with Schistosoma mansoni or a related species of blood fluke, Schistosoma haematobium. But the case of the man at the Royal Perth Hospital was singular in one respect. In order to get infected with blood flukes, you have to go to the places where its snail hosts live. And Australia is not one of those places. The man had, in fact, traveled to East Africa, where the blood flukes are common. But he hadn’t been there in 31 years.
This was remarkable, and more remarkable than you might think. It didn’t mean that the blood flukes had taken up residence in the man’s body and had produced 31 years of new generations. Remember, the eggs cannot develop inside a human body. They have to reach fresh water to hatch, and if they can’t find a snail to invade, they die. The tired man in Australia had been carrying the same blood flukes with him that he had picked up in Africa. These parasites were themselves at least 31 years old.
Male and female blood flukes. Source: PLoS ONE 3(10): e3328. doi:10.1371/journal.pone.0003328
The many years that a blood fluke can spend in a human host are striking, and all the more striking the more you contemplate how they spend those decades. Most species of flatworms are hermaphrodites, with both eggs and sperm. Blood flukes are either male or female. The females are thin and small. The males are larger, shaped like a canoe. At one end of their body, they had a mouth for drinking blood and a giant sucker. In their human host, female blood flukes select their mates and fit themselves into the trough of the male’s body. There they will remain, getting nourishment from their mate, along with the sperm necessary to produce their eggs. Blood flukes will spend years in this monogamous union, although sometimes they will get divorced and seek a new mate.
In all that time, the blood flukes manage to survive inside enemy territory. These are not microscopic creatures; they can get to be a centimeter long. Yet the immune system of their host typically ignores adult blood flukes. How they manage to escape notice–instead of getting rejected like a transplanted organ–isn’t yet clear. The parasites probably evade destruction by producing camouflage in the form of human-like proteins.
But blood flukes may have another source of longevity. It turns out that inside their bodies is a kind of fountain of youth.

Free-living flatworm. Source: http://web.mit.edu/neuro/planaria.html
This discovery is the work of Philip Newmark of the University of Illinois and the Howard Hughes Medical Institute and his colleagues. Newmark–like many other scientists–has spent years studying free-living cousins of blood flukes called planarians. Planarians can reproduce in a manner that seems to defy the rules of zoology. They simply split in half, and each half then regrows the rest of its body.
Scientists can regrow new planarians from tiny cuttings, complete with muscles, intestines, nerves, and the many other organs that are necessary for a full-blown flatworm. We’ve known about this power of regeneration for centuries, but it always bears appreciating anew. Imagine that someone cut off your ear and tossed it on the ground, where it promptly grew into a complete copy of yourself–complete with a new brain.

Lobo et al 2011 PLoS Comput Biol 8(4): e1002481. doi:10.1371/journal.pcbi.1002481
This power makes planarians an object of fascination for cell biologists. They’ve searched these flatworms for the secrets of their renewal. It appears that their bodies are sprinkled with unusually flexible stem cells called neoblasts. These cells can travel the planarian body to wherever they’re needed. Once they arrive, they start dividing quickly and differentiate into any tissue the planarian requires.
Our bodies can also regenerate themselves, albeit on a far more modest scale. As adults, we carry small collections of stem cells that can produce new tissues. They heal a gash by producing new skin cells. In the intestines, they produce new lining to replace sloughed-off cells. Stem cells in bone marrow rejuvenate our blood supply. Stem cells may also be important to the workings of the brain. But in these cases, the stem cells have only a narrow scope. They can only develop into a few cell types. We can regenerate skin and even a lobe of our liver, but we can’t regrow an eye or a hand. Studying planarians allows scientists to discover some of the signaling molecules that might be able to trick our own stem cells into more dramatic rebirths than they can manage on their own.
Newmark and his colleagues asked themselves one of those questions that seems so obvious in hindsight that you have to wonder why no one asked it before. Given that planarians and blood flukes are cousins, do blood flukes have this same power of regeneration? It’s an easy question to ask, but a vexingly hard one to answer. Planarians live cheerfully in a lab tank, but blood flukes require snails and mammals to complete their life cycle. Newmark and his colleagues have helped make it easier to study blood flukes in recent years; for example, they’ve developed a toolkit of molecules they can apply to blood flukes to probe their cells.
The neoblasts in planarians are constantly dividing. So Newmark and his colleagues started their search for similar cells in blood flukes by looking for signs of proliferation. As a cell multiplies, it makes DNA, and chemists have found that a compound called EdU can get taken up in the structure of new DNA. Newmark and his colleagues doused blood flukes with EdU and then inspected the parasites under a microscope to see if any cells had taken it up. They found proliferating cells sprinkled throughout the blood fluke body.
Newmark and his colleagues teased out some of the multiplying cells and gave them a closer look. They bore a striking resemblance to neoblasts, not just in their structure, but in the activity of their genes. All these clues pointed in the same direction: the proliferating cells in the blood flukes seemed a lot like neoblasts from planarians.
To watch these cells in action, the scientists infected mice with blood flukes. Then they injected the mice with EdU, which the parasites took up in their proliferating cells. Those cells then migrated through the body of the blood flukes. Some headed to the intestines of the parasite, while others headed to the muscle. There the cells divided into new cells, rejuvenating both kinds of tissue.
Newmark and his colleagues conclude that blood flukes have flexible stem cells akin to the neoblasts of planarians. It’s possible, they suggest, that these stem cells help blood flukes live so long. They may rejuvenate the parasites after they’ve been attacked by the immune system, or allow them to recover from a dose of anti-schistosome medicine. And as the blood flukes get old, the cells can be a fountain of youth, rejuvenating their tissues.
If these cells are indeed important to blood flukes, then we would do well to learn how to manipulate them. Newmark and his colleagues noticed that one of the genes switched on in the neoblast-like cells belongs to a family of signaling genes that are important to keep stem cells multiplying. They figured out how to make a drug that blocks that gene in blood flukes. In the drugged parasites, the neoblast-like cells slowed down.
If these flexible stem cells really are essential to the longevity of blood flukes, then this drug could be a powerful weapon against them. To fight against some parasites, we may have to take away their fountain of youth.
(For more information on blood flukes and other marvels of the parasitic world, see my book Parasite Rex.)
February 24, 2013
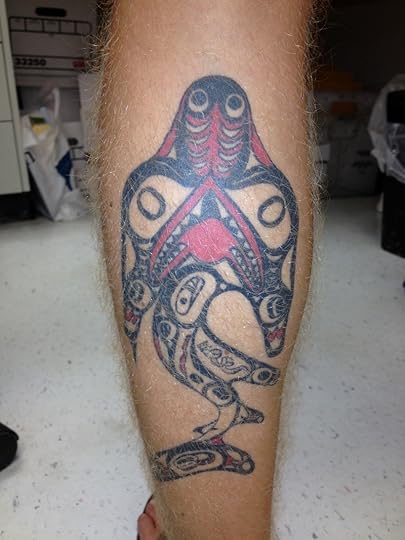
A Shark and Its Little Friend (Science Ink Sunday)
A reader writes, “I’m James Bernot, a graduate student studying shark and tapeworm coevolution at the University of Connecticut. Here is a tattoo I have on my calf of a Northwestern Pacific tribal shark, complete with a tetraphyllidean tapeworm near the shark’s pelvic fin.”
It’s hard for me to resist a tattoo of a shark tapeworm.
You can see the rest of the Science Tattoo Emporium here and in Science Ink: Tattoos of the Science Obsessed.
Close-up of tapeworm: