Kim Rendfeld's Blog, page 20
February 6, 2015
���Wonders of the X-ray���
In this installment of the history of atom theory, physics professor (and my dad) Dean Zollman discusses how x-rays were discovered and later explained. ��� Kim
By Dean Zollman
 In the last decade of the 19th century, many researchers in addition to J.J. Thomson were using cathode ray tubes to conduct research. Before Thomson established that the cathode rays were electrons, several researchers had investigated their properties. Philip Lenard, a German physicist, had measured the range of the ���rays��� in air. In 1895, Wilhelm Conrad Roentgen was following up on that research by looking at what happened when the cathode rays struck some metals. In the process, he made an astonishing observation.
In the last decade of the 19th century, many researchers in addition to J.J. Thomson were using cathode ray tubes to conduct research. Before Thomson established that the cathode rays were electrons, several researchers had investigated their properties. Philip Lenard, a German physicist, had measured the range of the ���rays��� in air. In 1895, Wilhelm Conrad Roentgen was following up on that research by looking at what happened when the cathode rays struck some metals. In the process, he made an astonishing observation.

Wilhelm Conrad Roentgen by Nobel Foundation
Roentgen had recently moved in the University of W��rzburg in southern Germany. He was a professor of physics and had been appointed rector of the university, the equivalent to a president of an American university. However, in those days the university leader had time for research. So, Roentgen had a laboratory where he was studying the properties of cathode rays.
As we discussed last time, the location of cathode rays could be determined because light was emitted when the rays struck a fluorescent material. This process is the same as the way a pre-flat-screen TV creates a picture on it screen. So, Roentgen had some fluorescent material in his lab. A small amount of this material was placed a rather long distance from the cathode ray tube. Yet, Roentgen noticed that the fluorescent material glowed when the tube was operating and the cathode rays were hitting a metal. By this time, cathode rays were known to travel only a few centimeters in air, but the distance between the tube and the glowing material was much greater than that.
Roentgen was motivated to undertake a series of careful experiments. On November 8, 1895, he made sure that no extraneous light could reach the fluorescent material. The lab was dark; the cathode ray tube was covered with black cardboard. Even in this environment Roentgen saw a faint glow on the fluorescent material. Something was traveling through the cardboard and causing light to be emitted from material. Roentgen gave the name x-rays to these new type of radiation.

An early Crookes x-ray tube from a museum dedicated to Wilhelm Conrad Roentgen in W��rzburg, Germany (image used under the terms of GNU Free Documentation License
Why the Professor Didn���t Notice His Dinner
For the next six weeks, Roentgen neglected his students and his duties as university rector to investigate his discovery. (A luxury university administrators do not have today.) He kept his research to himself and talked to very few people about it. The intensity of his work even put some strain on his marriage. This situation was described by a story related on the 35th anniversary of the discovery.
���One of the few persons who knew about the discovery before the announcement was made [on December 28, 1895] was Roentgen���s wife, Bertha. One evening in November 1895, she became very angry with her absent-minded husband because he did not comment upon the excellent dinner she had prepared for him, and he did not even notice that she was angry until she asked him what was the matter. He finally took her downstairs to his laboratory, which was in the same building, and for the first time presented to her astonished eyes the wonders of the x-ray.���
This story, which sounds like it could be an outline for an episode of The Big Bang Theory, is contained in a paper by Otto Glasser and appeared in the American Journal of Roentgenology in 1931. It is verified by a letter which Frau Roentgen wrote to her husband���s cousin.
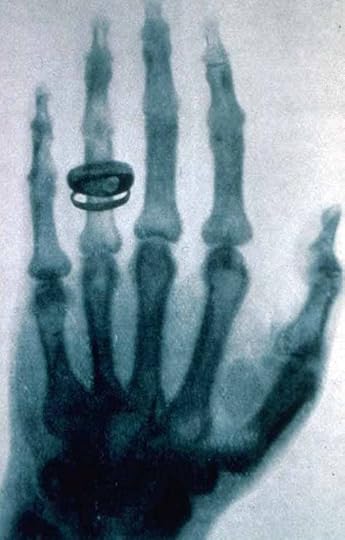
Bertha Roentgen���s hand x-rayed by Wilhelm Roentgen
In his six-week marathon research, Roentgen discovered that x-rays could penetrate materials such as wood, flesh, and a 2,000-page book. However, more dense materials such as metals and bone allowed much less, if any, penetration. He also discovered that photographic plates could be exposed by the x-rays. Three days before Christmas 1895, Roentgen took his wife into his lab and recorded the first (and perhaps most famous) x-ray of part of a person. The bones in Bertha Roentgen���s hand are clearly visible, as is the ring on her hand. Both of the Roentgens��� exposure to x-rays while this picture was being taken must have been tremendous. Today, x-ray machines carefully aim the rays so that only the area of interest is exposed. Over the years, special film and digital detectors have been developed so that only a very small amount of exposure produces the desired result. Roentgen had none of that. He had, at best, a very crude point and shoot x-ray device.
See Your Bones Everywhere, Even in Shoe Shops
On December 28, Roentgen submitted a paper, ���Ueber eine neue Art von Strahlung��� (On a New Type of Rays) to the Proceedings of the W��rzburg Physical-Medical Society. It included the picture of Frau Roentgen���s hand and quickly became a sensation worldwide. The medical implications were immediately recognized, and by May 1896, a handbook Practical Radiography had been published. However, it took a while for the value of x-rays to be fully understood. Tragically, it also took quite a while for the dangers of x-rays to be understood. Many people suffered damage and even death because their exposure to x-rays.

X-raying hands in the late 19th century (from William J. Morton and Edwin W. Hammer, 1896, The X-ray, or Photography of the Invisible and Its Value in Surgery)
The picture above from an early handbook on using x-rays shows the cavalier ways in which they were treated. Here, two images are being created. The sitting man has his hand on a photographic plate. He will obtain a picture much like the one of Frau Roentgen���s hand. The standing man is holding his hand in front of some fluorescent material. He will be able to see bones move as he changes the position of his hand. The x-rays for both of images are coming from one Crooke���s (cathode ray) tube which is the glass bulb above the sitting man���s hand and in front of the standing man���s hand. X-rays are being emitted in all directions, and neither person has any protection from them. A far cry from your dental hygienist leaving the room when he/she take an x-ray of your tooth (with a machine that focus the x-rays very narrowly on the tooth.)
To show how slowly some of the dangers were addressed, consider buying shoes in the 1950s. When I got new shoes both the salesperson and my parents wanted to be sure that the shoe fit correctly. I, of course, just wanted them to look cool. So, the shoe store had x-ray machines. I would stand on a platform with my feet in this machine. X-rays were directed up from below my feet. There were viewing places with fluorescent material for me, my mother, and the salesperson. We could see how well the shoes fit. Even better, I could wiggle my toes and watch the bones move. So, I had ���motivation��� to stay in the machine longer than necessary. This ���innovation��� did not last long, but it did expose us to unnecessary radiation. Maybe that explains why the 1960s were so weird.
So, What Causes X-rays?
Roentgen���s scientific contribution was quickly recognized. The first Nobel prizes were awarded in 1901. The first physics prize went to Wilhelm C. Roentgen ���in recognition of the extraordinary services he has rendered by the discovery of the remarkable rays subsequently named after him.” (Roentgen called his discovery x-rays. But in many parts of the world, x-rays are called Roentgen Rays.)
Explanations for what x-rays were and how they were produced took further research. Earlier in the 19th century, James Clerk Maxwell had shown that whenever an electrically charged particle was accelerated, it emitted or absorbed electromagnetic radiation. (For a physicist, acceleration means increasing or decreasing speed or changing direction. The change in direction will haunt us in a few blogs.) To create the x-rays, Roentgen had directed the cathode rays (electrons) into pieces of metal. As the electrically charged electrons slammed into the metal, they slowed and thus lost energy. This energy was emitted as x-rays.
Further research also showed that x-rays had the properties of light. They were much shorter wavelength and higher frequency than visible light. Maxwell had also concluded the light was a form of electromagnetic radiation. So the overall conclusion was that x-rays were the same physical phenomenon as visible light and radio waves. They were much higher energy and thus could pass through some materials that other forms of electromagnetic radiation could not.
Of course, the overall effect of x-rays on society has been much more positive than negative. And eventually they could be explained by models of the atom. In the meantime, other discoveries of the late 19th century were adding to the mystery of the structure of matter. We will look at a really big one ��� radioactivity ��� next time.
All images are from Wikimedia Commons. They are in the public domain unless otherwise noted.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards ��� the American Association of Physics Teachers��� Oersted Medal (2014), the National Science Foundation Director���s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo���s Trouble with the Inquisition?
Did Gifted Scientist���s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn���t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists

January 28, 2015
It’s Not Me; It’s My Characters: Why People Get Sick
Medieval people would look at you askance if you said the reason they were ill was that creatures too tiny to see had invaded their bodies.

Detail from 1910 illustration by Arthur Rackham (public domain, via Wikimedia Commons)
They knew to stay away from poisonous plants, rotten meat, and polluted water. Christian doctors attributed illness to an imbalance in the humors ��� blood, yellow bile, black bile, and phlegm. Beyond that, medieval folk turned to the supernatural for explanation.
And here is where Christian Franks and Continental Saxon pagans would argue.
To Christians, the cause for illness could be sorcery or punishment from God. A Saxon pagan might blame an evil, capricious dwarf. At least, I think the pagan would blame a dwarf, based on an Anglo-Saxon charm. So little is known about the Continental Saxons��� beliefs, I had to look for clues in other Germanic religions.
The tension over why people get sick comes into play in The Ashes of Heaven���s Pillar. My heroine, Leova, a Saxon peasant sold into slavery, accepts baptism, but she still holds on to many of her pagan beliefs, as you will see in the excerpt.
Sharing a pallet with Sunwynn, Leova caressed her sleeping daughter���s hair. Three weeks ago, the dwarves had sent fits of coughing throughout the house, and Leova had sung a charm to protect Deorlaf and Sunwynn. The dwarves��� magic had cracked the charm the way a spear cracked a shield. Leova and her children had coughing and a slight fever but were well after a few days.
But the charm had not worked for Ragenard. What did I do wrong? She had chanted the spell first in the left ear, then the right, then above the head. In the light of the night candle and glowing embers of the hearth, Leova stared at the bed, where Ragenard rested. Helewidis was kneeling on the floor, repeatedly murmuring, ���Ave Maria, gratia plena.���
But Ragenard, a Christian Frank, has a different explanation for why he was sick for months and what he must do when he has recovered.
���I almost died this spring. The Lord punished me for my sins but spared my life so I could atone.��� Ragenard leaned forward and pressed his fingers to his forehead. ���I cannot live with you as brother and sister. It is torture to see you every day and not touch you.���
Leova gasped and smiled. He desired her! He cared for her! ���So marry me. Then our lying together won���t be a sin. You said so.���
���I am supposed to do penance, not ignore my sins and enjoy pleasures of the flesh.���
Leova���s jaw dropped. ���Are you saying you cannot marry me because we would be happy? Why would the Church frown on your happiness with your wife?���
���All I know is that God healed me of this illness years ago, but He let me become sick again after I lay with you.���
Today, we would call Ragenard���s illness tuberculosis. Before antibiotics, this bacterial disease could go into remission for years but come back suddenly. Yes, those invasive creatures too small to be seen made him sick, and he didn���t know it.

January 21, 2015
When There Might Be More to a Martyrdom Story
Sometimes I am intrigued by what a story doesn���t say. Such is the case of the martyrdom of Saints Ewald the Fair and Ewald the Black in seventh-century Saxony.
It would be easy to see the pagan Saxon killers as my Frankish characters do ��� that they were nothing more than a bunch of brutes who hated Christians. Don���t get me wrong: nothing justifies the murder of the two priests. But when we put the story into the context of history and what the Saxons might have believed, it becomes more complex.
The story says the Saxons were convinced the Ewalds were trying to convert their lord and they feared the destruction of their temples. Such an act could offend the gods, the very beings who decided whether you had a bountiful harvest or famine, whether you had victory or defeat in battle.
To the pagan Saxons, the Ewalds might not have been two oddball priests but threats to their community. For more, see my post in English Historical Fiction Authors.

Stained glass window depicting the martydom of Saints Ewald the Fair and Ewald the Black (photo by Raymakers, public domain image, via Wikimedia Commons)

January 14, 2015
It���s Not Me, It���s My Characters: Human Sacrifice
At the beginning of The Ashes of Heaven���s Pillar, Leova, the heroine, belongs to a religion that practices human sacrifice. And that troubles me as a novelist.
I find such a thing revolting. Perhaps, I should���ve taken the easy way out and argued that we have no way of knowing for certain if the Continental Saxons killed people as part of their worship.
Just one problem. After my research, I get the sinking feeling they did sacrifice humans in extreme circumstances such as war or famine, and I owe it to the readers to be true to the characters, faults and all.
So Leova accepts human sacrifice as a way to call on the war god to save her community, as you can see in this excerpt:
Leova swallowed her tears and held her burden more tightly as she and Sunwynn stepped into the hot, dusty chaos of the road. Surrounded by screams and wails, Leova looked to her left and gazed down at the Irminsul, standing tall near the drought-shrunken Diemel River. As long as the oaken pillar stood with its treasure, the gods would favor the Saxon peoples. Leova made out the warriors kneeling before the Irminsul, vowing to sacrifice the first captives and booty they won in battle. Her gaze traveled up the pillar���s great length to the top, where it divided itself into two bent branches and the idol of Wodan stood.
For more about why the Continental Saxons��� religion might have included human sacrifice, see my post on Unusual Historicals.
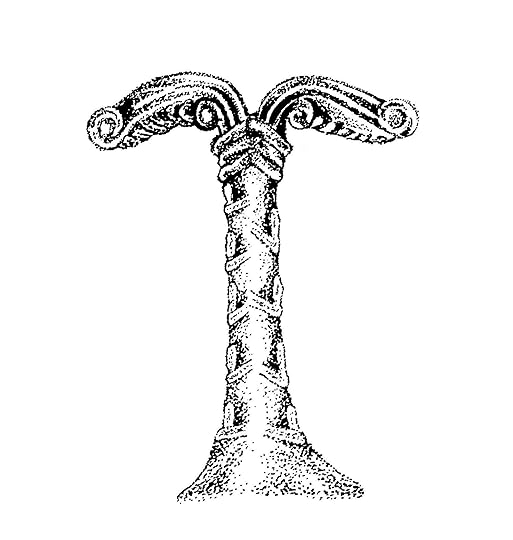
By Marianne Klement-Speckner (public domain, via Wikimedia Commons)

January 7, 2015
Pretzels in the Dark Ages?
Might an early medieval missionary have used pretzels as treats for children? Maybe.
The fifth century Codex 3867 in the Vatican Library has an illustration of something like a pretzel, whose twists resemble arms crossed across the chest, like someone in prayer. Although the first known use of pretzel dates to the 19th century, the word is derived from the German Brezel, which come from the Latin brachiatus, meaning having branches like arms.

A 12th century illustration of Esther and Ahashuerus at a banquet, with a pretzel (public domain, via Wikimedia Commons).
Because pretzels required only flour, water, and salt, they kept well, long enough for a missionary or a conquering army to take to a far-away land. Travel in those days was slow, 12 to 15 miles a day. That is, if no one broke a wheel, got sick, had to deal with ill or injured animals, or was attacked by bandits.
The pretzels��� shape gave them special significance during Lent, when the faithful abstained from meat, dairy, and eggs. Pretzels were among the acceptable foods and probably among the few available as peasants��� food stores dwindled in the early spring.
With this possibility, I used something like a pretzel in The Ashes of Heaven���s Pillar. Even though the events at the beginning of the book occur in the height of summer, it isn���t too much of a stretch to imagine missionaries would want to build a rapport with the youngest of their converts and remind them to pray, as you will see in this excerpt.
When prayers ended, one of the guards approached Leova and gestured for her to hold out her hands. Leova did so stiffly. The guard plucked at the bonds on Leova���s swollen, chafed wrists. Then he knelt by Leova���s feet as if he were going to untie the rope at her ankles. Instead, his hand slid under her skirt and up her calf.
Leova flushed. Oh, how she longed to kick him in the teeth, but it would only make matters worse. She drew in a sharp breath. If Deorlaf saw this, he would rush to defend her honor, just as his father would, and try to fight the guard. A vision of Deorlaf beaten and stabbed flashed before her. She glanced at her son standing a few paces away. A smiling Saxon priest from Britain bent over him and Sunwynn. Holding small strangely twisted rolls, he was telling them about a story about Jesus and children.
She had little time while her children were distracted. Gritting her teeth against the ache in her legs, she stepped back. ���Stop this nonsense before Count Pinabel sees you,��� she hissed. Although she doubted the guard understood her, she hoped invoking Pinabel���s name would stop him.
Sources
���Pretzels for God���

January 2, 2015
Discovery of the Electron Took Decades and Multiple Scientists
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses the discovery of the electron. Although one gifted scientist got the credit, he had help. – Kim
By Dean Zollman In the last decade of the 19th century, discoveries began new directions in our thinking about the composition of matter. Two of these discoveries – radioactivity and x-rays – were somewhat accidental. We will look at each of them in future posts. Another — the identification of the electron as a component of matter – was the result of careful research and the development of improved technologies. In this post, I will discuss the electron, how it was discovered, and some of the recent views about whether this research was really a discovery.
In the last decade of the 19th century, discoveries began new directions in our thinking about the composition of matter. Two of these discoveries – radioactivity and x-rays – were somewhat accidental. We will look at each of them in future posts. Another — the identification of the electron as a component of matter – was the result of careful research and the development of improved technologies. In this post, I will discuss the electron, how it was discovered, and some of the recent views about whether this research was really a discovery.
The generally accepted year for the “discovery” of the electron is 1897. However, this discovery had its roots in research and development that date to the first half of the 19th century. Because research such as this is always built on previous work, I have a difficult time knowing how far back to go. I have chosen to start the story with Heinrich Geissler (1814-1879).
Geissler was an instrument maker who, in 1857, created electric discharge tubes. These tubes were long, sealed glass cylinders and had metal electrodes at each end. Geissler connected a high voltage across the two electrodes and used another of his inventions, the vacuum pump, to decrease the pressure inside the tube. He found that the gas inside the tube would glow, with the color depending on what gas was trapped inside. The picture below shows a drawing, published in 1869, of several different Geissler tubes. These instruments might remind you of neon lights and they should. The tubes used in neon lights are modern variations on Geissler tubes.
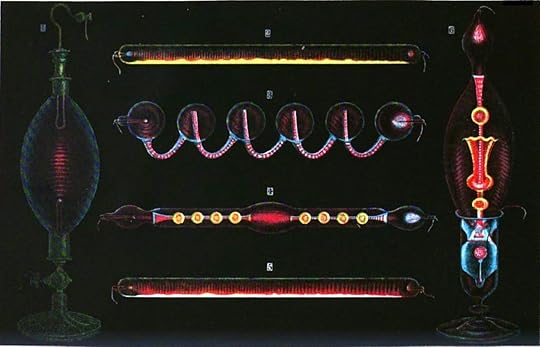
By M. Rapine (public domain)
Geissler conducted research to improve the tubes. He provided many tubes to other researchers and sold them to nonscientists for entertainment and decorative purposes.
The Cathode Ray

Sir William Crookes as drawn by Sir Leslie Ward in 1902 (public domain)
One of the researchers was William Crookes (1832-1919). Crookes improved on the tube and conducted many experiments. One of his conclusions was that something was being emitted from the negative electrode and was moving in a straight line to the positive end of the tube. Whatever was moving seemed to behave somewhat like rays of light. The negative end of an electrical device was called the cathode, so these “things” became known as cathode rays and the vessels were called cathode-ray tubes. (If you wish to see several photographs of Geissler and Crookes tubes, you should visit the Cathode Ray Tube Site.)
Just like light, the cathode rays could cast a shadow. In a famous experiment. Crookes inserted a Maltese cross in a tube. He saw that a shadow of the cross was cast on the end of the tube. However, the cathode rays in some ways acted differently from light. For example, they could be deflected by a magnetic field.

By D-Kuru, used under the terms of Creative Commons BY-SA 2.0 license
Two different views of cathode rays developed. Most British physicists concluded that the experiments indicated that the “rays” were some type of particle. Crookes proposed that they were negatively charged molecules. On the European continent, primarily in Germany, the light-like behavior led physicists to the conclusion that the rays were disturbances in the ether.
Each side had some experimental evidence to support its view. At the time, light was “known” to be a wave that traversed the ether, and all waves and other disturbances in the ether were assumed to travel at the same speed as light. However, cathode rays moved at much slower speeds. So this fact was an indication that the rays were not disturbances in the ether but particles. Also, on the particle side, the rays were deflected by a magnetic field, indicating that they had an electrical charge.
Atoms Have ‘Corpuscles’
Many of the prominent scientists who were involved in this debate also conducted work related to the model of the atom as a vortex in the ether that we discussed last month. One was John Joseph Thomson (1856-1940). Last month, I mentioned that he had written a theoretical paper on vortices in the ether. In 1884, he became the Cavendish Professor of Physics at Cambridge University where he undertook many experimental studies. In 1895, x-rays were discovered to be coming from a Crookes tube (more on that discovery next time). This result piqued Thomson’s interest in cathode rays. He set about to measure the ratio of the mass of a cathode ray to its electrical charge. A drawing and a picture of his apparatus are shown below.
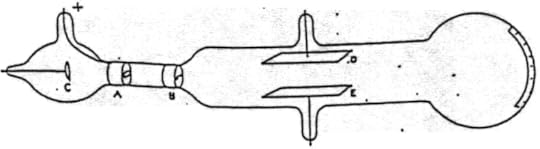
By J.J. Thomson (Philosophical Magazine, 44, 293 (1897, Public domain)

Photo by Science Museum London/ Science and Society Picture Library (used under the terms of Creative Commons BY-SA 2 license)
In the drawing the cathode is labeled C. That is where the cathode rays are emitted. The item marked A is the positive electrode (anode) so the cathode rays are attracted toward it. But there is a slit in the anode, so some of the cathode rays go through the slit and continue their journey. Object B narrows the beam of rays that pass into the next region. D and E are metal plates that can be connected to a battery. Not shown in the drawing but visible in the picture are two coils of wire that can be used to create a magnetic field. With all of this equipment Thomson and his assistants could deflect the cathode rays up or down. Connect the positive side of the battery to D and negative to E and the rays move up. Reverse it and they move down. The magnetic field is a little more complex, but up and down motion can be created by the direction of the electrical current in the coils.
Thomson’s plan was to balance the electrical and magnetic forces so that the cathode rays went straight through his apparatus even though they were subject to both electric and magnetic forces. From voltages and currents, he could determine the magnitude of these forces. Then, doing some algebra with equations that had been developed during the 19th century he could come up with a value for the ratio of the mass of a cathode ray to its charge. He could not determine either the charge by itself or the mass by itself. His measurements allowed only a determination of the ratio.
However, that ratio was enough to indicate that cathode rays were something quite different from any known object. First, they were particles. A disturbance in the ether could not have been deflected in this way. Second, the ratio that Thomson measured was about 1,000 times different than he would have expected if cathode rays were atoms. However, he could not with the experiment determine which was different. The mass could have been 1,000 times smaller or the electric charge could have been 1,000 times bigger. (Thomson measurements were not all that good. Today we know the electron is about 1,800 times less massive than the hydrogen nucleus. But nothing anywhere close to this small had ever been measured, so being off by a large amount did not matter.)
Thomson bet on the mass being smaller. On April 30, 1897, at a public lecture he announced the discovery of “corpuscles,” which he said were very small constituents of all atoms. Over the next few years, he completed several other experiments, including one that enabled him to determine the mass of the corpuscles. Eventually, he built a model of the atom which included the corpuscles. But, I will save that for a later post.
In an earlier post, I mentioned George Stoney (1826 –1911) who coined the word electron in 1891. Others began to use that label for the cathode ray corpuscles, but Thomson did not. In his Nobel Prize acceptance speech (1906), Thomson referred to “carriers of negative electricity” as “corpuscles.” Eventually however, “electron” became the commonly accepted name.
So, Who Deserves the Credit?
Historians and philosophers of science have lots of discussion about the discovery of the electron. Lots of experiments led up to Thomson’s. And others were doing similar experiments at about the same time. So, should Thomson deserve credit for the “discovery” when it was just one step in a many-step process? Further the impact of Thomson’s announcement was not immediate. It took a while to soak in.
Some philosophers will use this example to debate what it means to discover something new. I don’t want to go there. Clearly Thomson’s work was an important step in our understanding of the structure of matter. It built on other people’s work, and others built on it. Some people were doing similar work at the same time. That’s the way science happens.
Next time we will look at something which was clearly a discovery – x-rays.
Post Scripts
In addition to being an excellent scientist, Thomson was also a gifted mentor. Seven of his research assistants and his son received Noble Prizes.
The cathode-ray tube may seem like an esoteric device. However, until very recently almost all of us had at least one in our homes. Before flat screen televisions, the picture tube on our TVs was an advanced version of a cathode-ray tube. At the back was a device that accelerated electrons. It was quite similar to parts A and C in Thomson’s diagram. Then magnetic coils similar to the coils of wire in Thomson’s apparatus applied forces to steer the electrons to various locations on the front of the screen. Of course, doing this is such a way that we saw a picture required some technology that was not available until the 20th century. But the basic principles are much the same as they were when J.J. Thomson identified cathode rays as corpuscles that eventually came to be called electrons.
Images via Wikimedia Commons.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge

December 22, 2014
���Nine Herbs Charm���: A Fragment of a Lost Religion
All that���s left of the Continental Saxons��� pagan religion is fragments.
The Church, aided by Charlemagne, did everything it could to destroy what it saw as devil worship, and the eighth century Continental Saxons had no written language as we know it. So, when portraying people who practiced that religion in The Ashes of Heaven���s Pillar, I turned to clues ��� Charlemagne���s capitulary to the Saxons telling them what not to do or else, Jacob Grimm���s scholarship on Teutonic beliefs, folktales with deities and supernatural creatures, and poetry from the Anglo-Saxons.
One of those poems, ���Nine Herbs Charm,��� reveals how religion and medicine were intertwined. Whether is a Christian poem with pagan elements or vice versa, it provides a glimpse of Saxon culture before Christianity.
See my post at English Historical Fiction Authors for more on a poem that melds religion and medicine.

Mugwort, one of the nine herbs in the poem (by Christian Fischer, used under the terms of the GNU Free Documentation License, via Wikimedia Commons)

‘Nine Herbs Charm’: A Fragment of a Lost Religion
All that’s left of the Continental Saxons’ pagan religion is fragments.
The Church, aided by Charlemagne, did everything it could to destroy what it saw as devil worship, and the eighth century Continental Saxons had no written language as we know it. So, when portraying people who practiced that religion in The Ashes of Heaven’s Pillar, I turned to clues – Charlemagne’s capitulary to the Saxons telling them what not to do or else, Jacob Grimm’s scholarship on Teutonic beliefs, folktales with deities and supernatural creatures, and poetry from the Anglo-Saxons.
One of those poems, “Nine Herbs Charm,” reveals how religion and medicine were intertwined. Whether is a Christian poem with pagan elements or vice versa, it provides a glimpse of Saxon culture before Christianity.
See my post at English Historical Fiction Authors for more on a poem that melds religion and medicine.

Mugwort, one of the nine herbs in the poem (by Christian Fischer, used under the terms of the GNU Free Documentation License, via Wikimedia Commons)

December 17, 2014
Siegfried: Legend or History?
To us in the 21st century, Siegfried’s story is a legend. While it might incorporate a few historic events, it is mainly a fantastic tale with a dragon, sleeping beauty in armor, betrayal, and murder.
To my 8th century characters in Francia and elsewhere, he is as historic to them as George Washington is to us, and the fact that he is their hero reveals a lot about their culture and values. That’s why his story has a presence in both of my published novels and my work in progress.
The heroine of my first novel, The Cross and the Dragon, has grown up across the river from the high hill where Siegfried is said to have slain the dragon. I simply could not ignore his legend, as you will see in the following snippet:
During the evening meal in the great hall, Alda’s gaze fell on the tapestries recounting Siegfried’s deeds in reds, greens, and yellows, brilliant even by firelight. She realized how much she had missed Drachenhaus, built with stone from Drachenfels Mountain across the Rhine, where Siegfried had slain the dragon centuries ago and bathed in its blood for invulnerability. The mountain’s rock carried that magic, and Alda felt it envelop her.
See my post at Unusual Historicals for more about a hero whose story captivated the medieval imagination.

After slaying the dragon, Siegfried tastes his blood and understands the language of birds, by Arthur Rackham, 1911 (public domain image via Wikimedia Commons)

December 10, 2014
Medieval-Style Beef Stew
I got interested in stews for similar reasons as my medieval peasant characters: the need for an economical way to feed ourselves.
 In 2007, I had an eight-month non-vacation, the result of moving across the state of Indiana because of my husband’s job. As I searched for my own employment, I tried stretch the food budget, but what the heck do you with low-cost cuts of meat?
In 2007, I had an eight-month non-vacation, the result of moving across the state of Indiana because of my husband’s job. As I searched for my own employment, I tried stretch the food budget, but what the heck do you with low-cost cuts of meat?
Answer: stew. I first turned to The Joy of Cooking and then did my own experimentation. I found chuck roast to be the best cut for the job, rather than cubes labeled “stew meat” and rump roast.
Medieval peasants would have made a variation of this dish with whatever they had on hand, but a beef stew would have been a treat for them because they could not afford to eat meat every day. I wanted to create a recipe using ingredients similar to what was available in eighth century Francia and Saxony, the settings for my novels, The Cross and the Dragon and The Ashes of Heaven’s Pillar—and still be delicious to a modern palate.
Ingredients would also be determined by time of year, what was in season or in storage. For this recipe, I settled on this time of year, shortly after the livestock slaughter. Even in a good year, there was not enough fodder to feed all the animals through the winter. Meat could be preserved through salting, pickling, and smoking, and the temperatures tended to be cold already.
The reason I call this recipe medieval-style is that I cannot truly re-create what the folk would have eaten. Livestock has changed over the centuries, and the conditions they lived in were different from today’s cattle. Another compromise I’ve made is in the mushrooms. Peasants could have found mushrooms in the woods and perhaps dried them for future use, but I am sticking with the plain white button mushrooms I find in the grocery store. If you are at all tempted to use wild mushrooms, pretty please with sugar on top, get them only from a competent, experienced mushroom hunter, someone with lots of gray hair and wrinkles. If you eat the wrong mushroom, you could get seriously ill, as in needing a new liver.
 The medieval-style recipe worked at my house. Although some of the barley stuck to the bottom, my husband and I enjoyed the stew. I typically use little salt when cooking but add more at the table, which I did for this stew.
The medieval-style recipe worked at my house. Although some of the barley stuck to the bottom, my husband and I enjoyed the stew. I typically use little salt when cooking but add more at the table, which I did for this stew.
2-pound chuck roast
1/2 to 1 teaspoon dried herbs such as parsley, basil, and thyme
1 large onion
4 medium carrots
2 bottles of beer or ale (Something you would drink—I used Kolsch style ale. And yes, history police, I know medieval people didn’t keep their beer in bottles.)
2-3 stalks of celery with leaves (Medieval folk would have had celeriac in the stores, but celery is easier to get today.)
1 clove garlic
1 8-ounce package of mushrooms
1/4 cup dried split peas (OK, these are split by a machine, but it’s the closest I can get in my small Indiana town to what medieval folk might have had in late fall.)
1/2 cup pearled barely (Stews made by medieval peasants had grain such as rye or barley, and pearled barley is as close as I can get.)
6 radishes (They become mild with cooking.)
4 small turnips (It wouldn’t surprise me if medieval peasants grew these as large as they could, but smaller tastes better.)
If this recipe were truly written for the way I cook, it would include steps like “unload and reload dishwasher so you have space to work” and “if you want to take another step in this kitchen, feed the cats—now.” You definitely want to plan ahead for this. The meat cooks low and slow in moist heat. I typically use a Dutch oven, but some of this could be done in a crock pot.
Open the bottles of beer/ale.
Put a Dutch oven on the stove and let it heat on low
Chop the onion and celery and slice 2 of the carrots. Quarter the mushrooms. Set aside.
Rinse and sort the split peas and the barley. Set those aside, too.
Use the flat side of a chef knife to help peel the garlic, the mince and crush it. Set that aside. Yes, there is a purpose for all this.
Turn up the heat on the Dutch oven to medium low or medium.
On a separate cutting board, starting cutting the fat out of the chuck roast and throw it into the Dutch oven. You want to render a little fat, just enough to brown the meat. (You can also render bacon to get fat.)
While the fat is rendering, chop the meat into 2-inch cubes.
Sprinkle the dried herbs over the meat. For this recipe, I used basil and thyme. Sprinkle a little salt on it. Stir.
Remove the solid chunks of fat and brown the meat in batches, not letting the pieces touch. If the pot starts smoking, cheat and add a little vegetable oil. Don’t worry about the brown bits on the bottom. Those will flavor the stew.
Set meat aside and add the onion, celery, carrot, and mushroom mix. Sprinkle with a little salt and stir, letting the mix deglaze the pot. Cook the mix covered about five minutes, but stir frequently. You want the vegetables to soften and for the onions to start to become translucent.
Add the garlic, stir, let cook for 20 seconds. (If you’re using a crock pot, transfer the ingredients to it.)
Add the split peas and barley. Stir.
Add the meat, nestling it in if you can. And add the juices that accumulated.
Add the beer/ale to almost cover the mix.
Cover the pot and bring to a boil, then reduce to simmer. This is where you wait until the meat is fork tender, 1 1/2 to 2 hours in a Dutch oven, much longer in a crock pot.
While you’re waiting, halve the radishes, cut the remaining carrots into 1-inch pieces, and peel and dice the turnips. For now, set aside. (After I had cooked this recipe, someone suggested parsnips, which also were around in medieval Europe. If you like parsnips, you can cut 1 or 2 into 1-inch pieces and add them to the mix.)
When the meat is tender, add those veggies you chopped in Step 17. Then you wait a little bit longer, 30-40 minutes or until they are tender.
It’s done! Enjoy.




