Kim Rendfeld's Blog, page 16
December 9, 2015
Help Wanted: Where Was Cumeoberg?
Cumeoberg was an Avarian fortress, or an old name for a mountain, or a mountain-top fortress, and the site of a nonbattle in Charlemagne’s 791 war against the Avars. And it is a setting for a scene in my work still in progress, Queen of the Darkest Hour.
The problem: I have multiple options for where it might have been. I know it was somewhere near the Austrian city now named Tulln, called Comagena in Roman times, and in today’s Wienerwald, a huge forested area near Vienna.
So, was in on a rise just south of Tulln? Was it on a mountain near today’s Königstetten? Or does it overlook today’s Klosterneuburg?
I’ve flipped though books without arriving at any certain answer. The reason for all this labor is that even after nine years away from my days at a daily newspaper, I still live in dread of The Call.
If the caller was polite, they would start with, “Thank you for the nice article.” And on the other end, I would be waiting for the But, as in “but you spelled my name wrong.” Callers who were less than polite would start with, “Don’t you people have proofreaders?”
Odd to think that in fiction—where we confess from the get-go that we’re making things up—that we novelists want to be accurate. But that’s why we often get caught in research rabbit holes, even for one scene.
For now, my best guess is that Cumeoberg was on a mountain near Königstetten, but dear reader, if you’ve heard otherwise, let me know in the comments or by email at kim [at] kimrendfeld [dot] com.
Sources (somewhat contradictory)
Charlemagne: Translated Sources, P.D. King
A History of Charles the Great (Charlemagne) by Jacob Isidor Mombert
The History of France: From the earliest period to the present time by Thomas Wright

Albrecht Altdorfer’s 1518 The Victory of Charlemagne over the Avars near Regensburg (public domain via Wikimedia Commons)


December 4, 2015
How Bohr’s Famous Model of the Atom Was Created
In this installment in the history of atom theory, physics professor (and my dad) Dean Zollman explains how Niels Bohr built on the work of others to craft his model of the atom – Kim
By Dean Zollman
 When asked to visualize or draw an atom, most of us would probably draw a small central nucleus with electrons orbiting in circles or ellipses. Some of the electrons would be close to the nucleus while others would be farther away. An example is shown in the drawing below. This view of the atom is a representation of the Bohr Model. While it is no longer considered the most correct one, it has appealed to many people for over 100 years. So, it is frequently used when we want to represent something that we cannot see (and even have difficulty comprehending).
When asked to visualize or draw an atom, most of us would probably draw a small central nucleus with electrons orbiting in circles or ellipses. Some of the electrons would be close to the nucleus while others would be farther away. An example is shown in the drawing below. This view of the atom is a representation of the Bohr Model. While it is no longer considered the most correct one, it has appealed to many people for over 100 years. So, it is frequently used when we want to represent something that we cannot see (and even have difficulty comprehending).
[image error]
A schematic illustration of the Bohr Model of the atom (By Sharon Bewick, via Wikimedia Commons under a Creative Commons Attribution-Share Alike 3.0 Unported license )
The Bohr Model builds on the concepts that we have discussed in the previous two posts. It incorporates Max Planck’s ideas that the energy of light is related to its frequency and to Albert Einstein’s concept that light comes in small packets of energy called photons. The model also brings in Ernest Rutherford’s discovery that the atom has a very small but very massive center which has a positive electrical charge. Thus, the model includes many of the important discoveries of the early 20th century.
Niels Bohr (1885-1962) was born in Copenhagen and raised in a family with many intellectual advantages. His father, Christian, was a very accomplished physiologist; his mother, Ellen, was a member of a prominent family in Denmark.

An illustration from a report about Niels Bohr’s doctoral defense. The report was published in the Copenhagen newspaper Dagbladet in May 1911. (Scanned from Niels Bohr: A Centenary Volume edited by A.P. French and P.J. Kennedy)
Bohr attended the University of Copenhagen and received a doctorate in 1911. At the time, Bohr was studying physics at the university, a large fraction of the work in physics was focused on using the recently discovered electron to explain a variety of phenomena. Thus, Bohr’s master’s and PhD studies were about an electron theory of metals. Because he had created a theory involving electrons, Bohr decided to begin his postdoctoral work with the discoverer of the electron, J.J. Thomson. So, he moved to the Cavendish Laboratory in Cambridge.
The collaboration with Thomson did not work out well, and Bohr was rather frustrated with his time in Cambridge. However, he soon met Ernest Rutherford who had recently discovered the structure of the atom and the presence of a nucleus. Rutherford was impressed with Bohr and soon invited him to join the research group at the University of Manchester.
So Why Is This Element So Stable?
Bohr immediately began making significant contributions to work in Rutherford’s lab. One of his early research activities was to understand how alpha particles, which were emitted in some radioactivity, slowed down as they interacted with matter. His collaborator for this work was Charles G. Darwin (1887 –1962), grandson of the Charles Darwin. Issues of stability of the atom were critical to their work as they were to then current models of the atom. As we discussed several months ago, Rutherford had devised a planetary model of the atom. However, this model had a fundamental problem. Based on the laws of electricity and magnetism, the atom was not stable. The electron in orbit should very rapidly radiate energy and end up in the nucleus.

Niels Bohr and Margrethe Nørlund at the time of their engagement in 1910 (public domain, via Wikimedia Commons)
Bohr was also concerned about stability from a different perspective. At a conference in 1922, he described his thoughts as he began thinking about the atom 10 or so years earlier. “My starting point was not at all the idea that an atom is a small scale planetary system and as such governed by the laws of astronomy. I never took things as literally as that. My starting point was rather the stability of matter, a pure miracle when considered from the standpoint of classical physics. By stability, I mean that the same substances always have the same properties.” (Quoted in Pullman, The Atom in the History of Human Thought)
In short, Bohr wondered why all iron (or hydrogen or whatever) atoms were the same as all others of the same element. The laws of physics as they were known in 1912 did not require such consistency in nature. (But nature would have certainly been chaotic without the consistency.)
By June 1912, Bohr was spending all of his research time on a model of the atom. At this time Bohr’s concerns were radioactivity, the periodic table and how atoms bound together to make molecules. He was not yet interested in the spectra of light emitted by the atoms.
However, some other researchers were interested in the light emission process. They were approaching it by applying the results of Planck and Einstein to atomic models that were available. In 1910, Arthur Hass (1884-1941) used Planck’s idea that the energy of an electron vibrating in an atom similar to the Thomson Model was proportional to its frequency. (Recall that the Thomson Model has electrons moving in a uniform positive charge rather than in orbits.)
This concept led John W. Nicholson (1881-1955) to consider a similar idea but using a model of the atom in which all electrons were in orbit outside the positive charge but all at the same distance from the nucleus. Nicholson was interested in explaining the light in stars’ spectra that seemed to have no counterpart on earth. After some work, Nicholson concluded that his model worked best if he required that the angular momentum of the electrons was equal to an integer times Planck’s constant divided by 2 pi.
(A short physics lesson: Angular momentum is a quantity that is useful for any object moving in an orbit. It is equal to mass x speed x radius. Nicholson could equally well have stated a requirement on the energy. It was simpler in terms of the equations to use angular momentum.)
Nicholson concluded that the light in the spectra is created by the atoms when they “run down” (lose energy) by “discrete amounts.” This is the beginning of using Balmer’s discovery of the nature of the spectrum from gases to understand the quantum nature of the atom.)
Bohr built on Nicholson’s idea by adopting the requirement that the angular momentum can have only certain discrete values related to Planck’s constant. However Bohr’s atom has many orbits for the electrons. In the fall 1912, he returned to the University of Copenhagen. He was however still not interested in light emitted by atoms. At the beginning of 1913 Bohr wrote to his brother, “[My] calculations would be valid for the final, chemical state of atoms, whereas Nicholson’s would deal with the atoms sending out radiation, …” And, to Rutherford he wrote “I do not at all deal with the question of calculation of the frequencies corresponding to the lines of the visible spectrum.”

Niels Bohr and Albert Einstein at the 1930 Solvay Conference in Brussels (Photograph by Paul Ehrenfest public domain via Wikimedia Commons)
Let’s Add Some Light
At this time, Bohr had apparently not heard of or had forgotten about Balmer’s famous formula which described the values for the frequencies of the light emitted by the hydrogen atom. Fortunately, H.M. Hansen (1886-1956), a spectroscopy specialist at the University of Copenhagen, reminded Bohr of Balmer’s work. Bohr thought of the emission of light in terms of the electron changing orbits outside the positive nucleus. As the electron moved from one orbit to another closer to the nucleus, it lost energy. That energy appears as light emitted by the atom. Further, that energy was related to the frequency of light by the equation that Planck had invented. He invoked Einstein’s results by assuming that each change in orbit resulted in the emission of one photon (quantum) of light.
[image error]
A schematic showing the process of light emitted by an atom in Bohr’s Model (by Brighterorange, GFDL, CC-BY-SA-3.0, or CC BY-SA 2.5-2.0-1.0, via Wikimedia Commons)
Later, Bohr recalled, “As soon as I saw Balmer’s formula, the whole thing was immediately clear to me.” Balmer’s formula can be written as
Frequency of light = (speed of light) R (1/m2 – 1/n2)
To Balmer, R was a number that he determined experimentally, and m and n were integers. Not only was Bohr able to derive the form of Balmer’s formula, but he determined the value of the until then mysterious R in terms of fundamental measurements such as the mass and charge of the electron, Planck’s constant, and pi.

An animation of a photon of light being emitted and absorbed by a Bohr atom (by Kurzon, CC BY-SA 3.0, via Wikimedia Commons)
Today, Bohr’s work is presented in most texts in a couple of straight forward steps. First Bohr established the rule for the value of the angular momentum. Thus, he established a model of the atom that required the electrons to be in various orbits around, but not all orbits were possible – only those that met his condition on the angular momentum. Then, he derived Balmer’s formula. As is almost always the case, the story was more complex than that.
Bohr’s model of the atom established that the orbits of electrons are restricted to certain values. We can talk about these values in terms of angular momentum, energy or distance from the nucleus – all of them are equivalent. Most importantly, only certain values are allowed. Today we say that the orbits of the electrons are quantized. This fundamentally changed the way that we look at matter. Next time we will look further into Bohr’s atom and some of its refinements. Somewhat later we will consider the models that eventually replaced it.
(Unless otherwise stated quotations from Bohr were taken from Niels Bohr: A Centenary Volume edited by A.P. French and P.J. Kennedy.)
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One


November 24, 2015
When a Tale about Fashion Becomes Subversive
When I read Notker the Stammerer’s biography of Charlemagne, composed about 70 years after the emperor’s death, I get the feeling that he didn’t let facts get in the way of his story.

A 10th century illustration of Notker (public domain, via Wikimedia Commons)
I suspect he fabricated the tale about Charlemagne and his noblemen going on hunt in the clothes they were all wearing right at the moment. Charlemagne had a sheepskin cloak, while the others were adorned in silks and other fragile finery to trek through thorns and rain.
The tale is moralistic about vanity—and subversive. Lay noblemen were expected to flaunt their wealth and chose clothes with pricey materials. The more reliable Einhard has Charlemagne favoring a tunic fringed with silk and a vest of expensive otter and marten furs. The king’s swords had silver and gold hilts. For special occasions, he had embroidered garments, jeweled shoes, a golden buckle for his cloak, and a diadem.
To have a king to wear a sheepskin cloak, something a commoner would have, is a tad rebellious. But Notker does have a point: there are times when the humble item is of great value. See my post on English Historical Fiction Authors about the durability and practicality of this piece of medieval fashion.
Sources
The Monk of Saint Gall: The Life of Charlemagne
Einhard: The Life of Charlemagne


November 18, 2015
The Kindness of a Parisian Stranger
Before my first trip out of the States in 1987, I was warned that the Parisians would be rude. I heard things like “Once you get out of Paris, the French are friendly.” And my first glimpses of the city made me think, “Oh, this is like New York, but they speak French.” For a Jersey girl, that’s no big whoop.
My arrival into France was less than ideal. The airline had lost my luggage and my ears felt like they were stuffed with cotton. On top of that, I had jet lag. Then I noticed a tear in my skirt.
So I must have been a pathetic sight in that little store where I was looking for needle and thread.
I found what I needed, approached the woman at the counter, and said in French what I thought was “I would like to buy these.” The shopkeeper, old enough to be my mother, gave me a puzzled look. I repeated it.
After a moment, a look of understanding dawned on her, and she said the word I had meant to say. At that moment, this teenage American realized that I had said I wanted to sell her something already in her shop.
I felt like an idiot, but this Parisian was very kind. When I removed the wad of money from my pouchette, she maternally explained francs, and that this purchase would cost only a few of them.
This was not at all the rudeness I was expecting. Perhaps, it was that I was 19, female, and harmless looking. Perhaps, it was at least an attempt – clumsy but sincere – to speak the language. Or perhaps it is that there are kind people everywhere.
So as I hear the news from Paris, my heart breaks for this magnificent city and the people who live in it.

By Tristan Nitot (standblog.org) (GFDL, CC-BY-SA-3.0 or CC BY-SA 2.5, via Wikimedia Commons)


November 6, 2015
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses Albert Einstein’s explanation of why a light’s color matters more than its brightness in increasing kinetic energy from electrons ejected from atoms. Not everyone welcomed Einstein’s idea about the nature of light. – Kim
By Dean Zollman
 In this post, I will look at one of Albert Einstein’s (1879-1955) contributions to our understanding of atoms and matter. Of his many contributions to physics and our understanding of nature, this is the only one for which Einstein used the word revolutionary. It was, but so were many of his other ideas.
In this post, I will look at one of Albert Einstein’s (1879-1955) contributions to our understanding of atoms and matter. Of his many contributions to physics and our understanding of nature, this is the only one for which Einstein used the word revolutionary. It was, but so were many of his other ideas.
Before our discussion, it is a good time to mention that November 2015 is the 100th anniversary of Einstein’s publication of his General Theory of Relativity. At the time of its publication, the General Theory was a sophisticated mathematical description of much of the universe and provided a theoretical description of gravity – an academic exercise that improved humankind’s understanding of the universe but seemed to have little or no practical application. However, among other things the General Theory gave us an understanding about how light and radio waves interact with gravity.
Today, the understanding that comes from Einstein’s mathematical description of gravity is a critical component in making our GPSs as accurate as they are. So, the next time you hear complaints about university professors doing research that seems to have no value, think about your GPS “recalculating.” Without research that 100 years ago seemed of interest to only some physicists, the recalculation would not be accurate.
Now, back to earlier in the 20th century. As we discussed last time, Max Planck had addressed the issue of the spectrum of light coming from a so-called blackbody radiator. He had come to the conclusion that light energy came in packets in which the energy was proportional to the frequency of light. He did not have a strong theoretical reason for this conclusion, but it worked.
The blackbody radiator was not a practical device but many common emitters of light approximated it. However, there was another phenomenon – the photoelectric effect – that did not have a good explanation. Einstein would tackle that problem.
UV Rays and Electrons
The photoelectric effect was discovered by Heinrich Hertz (1857-1894), a pioneer in investigating radio waves. Much of the waves he generated were created by causing a spark to jump between two electrodes.
In 1887, Hertz discovered that he could get a stronger spark if he shined ultraviolet light on the electrodes. Later, scientist discovered that this spark was a result of electrons being emitted by the electrodes. Thus, the ultraviolet light was causing the emission of a greater number of electrons than would occur if the UV light were not there. The phenomenon was called the photoelectric effect.

An artist’s conception of Hertz’s discovery of radio waves. The device under the window has two small spherical electrodes in its middle. The wavy line between the spheres represent the spark while wavy lines above and below the spark represent the emission of radio waves. Shining UV light on these spheres increased the intensity of the spark. Hertz is holding a receiver which has a spark between two electrodes. This spark occurs when the radio waves reach the receiver (public domain via Wikimedia Commons).
The basic process of the photoelectric effect is shown in the figure below. Shine UV light on certain metals and electrons are emitted. The number and energies of the electrons could be measured and compared with the properties of the light that was shining on the metal.
[image error]
A modern view of the photoelectric process. UV light strikes a metal and electrons are emitted from the metal (public domain via Wikimedia Commons).
To investigate the photoelectric effect with a simulation visit PhET. There you can vary the parameters and “see” how the electrons are emitted.
Measurements Don’t Match Hypothesis
James Clerk Maxwell (1831-1879) had established that light was a form of electromagnetic wave. That is, light waves consist of vibrating electric and magnetic fields. As the light wave enters the metal, electrons begin to vibrate in response to these changing electric and magnetic fields. The electrical bonds holding the electrons to their atoms are a little like rubber bands. Small vibrations do not enable the electron to escape – the rubber band always pulls it back. Larger vibrations, however, could eventually stretch the rubber band far enough that it breaks. The electron escapes from the atom with whatever energy is left over after the bond has been broken. This extra energy becomes the kinetic energy of the ejected electron.

James Clerk Maxwell with his wife, Katherine, and the family pet (public domain via Wikimedia Commons)
This idea was used to predict what might happen in the photoelectric effect when the intensity (brightness) or frequency (color) of the light source was varied. The intensity of the light source describes the amplitude of the individual waves. Dim light consists of waves with small amplitudes; bright light consists of waves with large amplitudes.
We could imagine metal surfaces for which the amplitude of the waves in dim light is too small to break the electrical bonds holding the electrons. Increasing the intensity of this light should increase the vibration of the electrons, eventually to the point that the electrons break free. Increasing the intensity even further should increase the kinetic energy of the ejected electrons.
On the other hand, the frequency of the light should have little effect on either the ejection of electrons or their kinetic energy. Changing the frequency of the light source simply changes the rate at which wave disturbances reach the metal surface. If the amplitudes of the waves are not sufficient to help the electrons break the bonds, changing the rate at which these waves reach the metal surface should not make much difference. Similarly, changing the frequency of the incident light should not affect the kinetic energy the ejected electrons have.
The problem was that the measurements showed something quite different. The frequency of the light determined the energy of the electrons – the higher the frequency the greater the energy. The amplitude determined the number of electrons that were emitted but had no effect on the energy. The experimenters could shine as bright a red light (low frequency) as they possibly could on a metal and no electrons would be emitted. But shine a very dim violet light on the same metal and electrons would immediately pop out. Maxwell’s wave theory of light could not explain the observations of the photoelectric effect.
Following Planck’s ideas, Albert Einstein proposed that in its interaction with electrons in the photoelectric effect, light could be best described as a stream of small particles, or “energy packets.” Each packet, called a quantum of light or a photon, acts as a unit when it interacts with an electron. Again using Planck’s idea, Einstein suggested that the energy carried by each photon is defined by:
Energy = (Planck’s constant) x (frequency)
Einstein’s concept of the photon provides a solution to the problems posed by the photoelectric effect. Each photon acts as a unit when it interacts with matter. When a photon encounters an electron, it does one of two things: (1) transfers all its energy to the electron and ceases to exist or (2) does nothing and goes on its way. By associating energy with frequency, the photon explains why electrons are ejected by light of one frequency, such as high frequency UV light, but not of another, such as low frequency red light. It also explains why the kinetic energy of the released electrons depends only on the frequency of the incident light.

Albert Einstein in 1905, at the time he proposed a solution to the photoelectric effect (By Lucien Chavan / ETH Zürich, CC BY-SA 3.0, via Wikimedia Commons)
In a letter to Conrad Habicht, Einstein called the photoelectric explanation “Very Revolutionary.”
Acceptance Took Years
Many textbooks, including ours (The Fascination of Physics), imply that once Einstein had published his ideas about connecting Planck’s approach with the photoelectric effect, everything fell into place, and it was quickly accepted by the physics community.
Unfortunately, that did not happen.
Philipp Lenard (1862-1947) proposed some alternative ideas. He saw the effect as a triggering phenomenon. In Lenard’s model, light did not contribute energy to electrons. Instead, the light just selected those electrons which were ready to be emitted by an atom anyway. To continue with the rubber band analogy, the rubber band connecting the nucleus and electron was already stretched. The light just provided the mechanism to release the band and shoot the electrons out. Ideas such as Lenard’s allowed people to hold on to the wave theory of light. So, they ignored or rejected Einstein’s ideas.
The best example to show that it took a long time for Einstein’s idea to be accepted is Planck’s letter supporting Einstein’s nomination to the Prussian Academy of Science. In 1913 (eight years after publication of the photoelectric explanation), Planck wrote, “That [Einstein] may occasionally have missed the mark in his speculations, as for example with his hypothesis of light quanta, ought not be held too much against him …”
Eventually, experiments showed that Einstein was correct. Today, his explanation of the photoelectric effect is well accepted. In 1921, Albert Einstein received the Nobel Prize “for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect.”
Further, the work of Rutherford, Einstein, and Planck set the stage for a model of the atom that persists even today. More about that next time.
A political footnote: The major players – Einstein, Hertz and Lenard – became caught up in the insanity of Nazi Germany. Hertz’s family background was Jewish, but his grandparents had converted to Christianity when Hertz’s father was 7 years old. Heinrich Hertz was a Lutheran. However, that did not stop the Nazis from declaring Hertz as undesirable. His portrait was removed from the Hamburg City Hall because of his Jewish background. (It has long since been restored.) Einstein was Jewish and fled Germany to avoid the persecution. Lenard was on the other side. He was a German Nationalist and joined the Nazi party. He championed “German Science” and worked with Hitler to rid the country of “Jewish fallacies” such as Einstein’s theories. Today, it is difficult to understand how an intelligent person could have held such views.
(The paragraphs about the wave model and the photoelectric effect are adapted from The Fascination of Physics, copyright by J.D. Spears and D. Zollman. They are used by permission of the authors.)
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.


October 28, 2015
Watch Out for the Water. There Might Be Nixies.
Perhaps the most fearful characteristic of water nixies is their allure.
These magical creatures appear as beautiful women, but they trick fathers into giving up their sons or outright kidnap and enslave children who fall into wells. My early medieval characters grew up hearing tales like “The Water Nixie” and “The Nixie of the Mill-Pond”. These beings have such a hold on the imagination, that they, not the protagonists, are in the titles, and what might have frightened listeners most is that the villains, although defeated, are still alive and able to wreak more havoc on mortals.
See my post on Unusual Historicals for more about water sprites that terrified my characters.

Helen Stratton’s illustration of the Lorelei in A Book of Myths (public domain, via Wikimedia Commons)


October 21, 2015
Paying for Goods in the Dark Ages
It’s easy to forget commerce in early medieval times. Yet that sign of civilization existed despite wars, disease, and famine.
In an age when only a few could write, most sales went unrecorded, but those that were used monetary units to say what the trade was worth. A parcel of land was described as being worth 6 deniers or that value in goods such as food and animals.
Despite a lack of formal schooling, early medieval people knew how to bargain, although they often bartered for what they wanted. See my post at English Historical Fiction Authors for a sampling what goods were prized in the Dark Ages. (Hint: Fur was a status symbols in those days, too.)
Source
Daily Life in the World of Charlemagne by Pierre Riche

An early 15th century illustration of a marketplace (public domain via Wikimedia Commons)


October 7, 2015
Christians in the Dark Ages Didn’t Choose Ignorance
It’s easy for modern-day Christians to sneer at believers in early medieval times.
After all, the folk did not question Saint Augustine’s teachings that babies who died unbaptized would be damned but receive the lightest of punishments. Although these children did nothing wrong, he reasoned they were born with original sin, which only baptism could remove.
One common response to my posts about this subject is something like: how could the early medieval faithful believe a teaching that goes against the Bible?
Most early medieval Christians could not look up Scripture for themselves. The vast majority couldn’t read. Only wealthy parents could afford tutors for their sons and daughter rather than have their children do farm work. Schools run by the Church were mainly to train the clergy.
Besides, only an elite few could afford books. Commoners needed their land for crops, not a herd of sheep whose skins would become pages. To peasants, sheepskin was something to wear, not read.
And then there was the labor to hand write the Latin, add artwork, and bind the books. Bibles were as inaccessible to peasants as yachts are to most of us.
So most of the laity, especially commoners, had no choice but to know the Bible only through murals and statues and sermons. They believed what the priests told them and little beyond that.
Today, we have mass-produced Bibles in multiple languages and multiple translations of a language. Heck, we can even Google a verse. Darn near everyone in the First World can look up a concept quickly and easily, study it, and argue about it, and that process cultivates a more mature understanding of the faith.
But many 21st century Christians have Bibles that remain unread on their shelves. Many who profess to follow the faith choose to be as ignorant as their counterparts in the Dark Ages. And that should distress us more.

From a manuscript crafted in 820 (Public domain, via Wikimedia Commons)


September 30, 2015
How Did They Measure Horses in the Dark Ages?
When Fastrada, my heroine in Queen of the Darkest Hour, is comparing the Avars’ horses to her husband Charles’s Roman military horses, I have her think the Avarian steeds are about a foot shorter. Although I rarely employ this unit of measure in fiction, the Carolingians did use feet.

From a ninth century Carolingian manuscript (public domain, via Wikimedia Commons)
Now all you horse lovers are cringing. As S.K. Keogh, author of the Jack Mallory series and one of my astute critique partners pointed out, horses are measured in hands. Today, thanks to Henry VIII, a hand is standardized at 4 inches.
But what about people who lived centuries earlier? Did the folk in early medieval times use hands to measure the height of their horses?
Well, this unit of measure goes way, way back. The Egyptians used it, and Ezekiel 40:43 (New International Version) uses “handbreadth” to describe the length of double pronged hooks.
But what about my people? Here, I consult the Romans, whom Charlemagne tried to emulate. Turns out, the Romans used palms. They had two forms: the great palm, which is length of the hand, or 12 fingerbreadths, and the small palm, the breath of the hand, about four digits. A palm as a unit of measure varied with the size of the hand.
So would a Germanic woman who grew up east of the Rhine think in terms of palms or something else? And what about modern readers who don’t measure livestock by hands or palms?
Fastrada might have used palms as a unit of measure, especially when bargaining with merchants. For the non-equestrian modern readers, though, I might use “four palm-breaths.” Perhaps, it’s not exact, but it will be simpler to visualize.
Just to complicate matters, the average early medieval horse was shorter, about 4 ½ feet at the withers, I mean 13 ½ hands. In this current draft of my work-in-progress, here is how I’m describing these animals:
Whispering about the Avars, she and Meginfrid strode toward the courtyard. At the entryway, Meginfrid marched ahead to announce her to their foreign guests, who bowed when she stepped outside. Surrounded by their Frankish and Bavarian escort, the Avars had dismounted from their armored steeds. The sturdy horses were the same height as most Frankish animals, about four palm-breadths shorter than Charles’s Roman military horses. Even though the withers of the Avarian beasts only reached Fastrada’s breastbone, they were majestic with gold discs on their bridles and feathers from gold objects just above their broad, convex foreheads. And there were those objects hanging from the saddles, what Charles had called stirrups.
Sources
A Mathematical and Philosophical Dictionary: Containing an Explanation of the Terms, and an Account of the Several Subjects, Comprized Under the Heads Mathematics, Astronomy, and Philosophy Both Natural and Experimental, by Charles Hutton
Dictionary of Greek and Roman Antiquities, edited by William Smith
Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures, edited by Helaine Selin
A General Dictionary of Commerce, Trade, and Manufactures: Exhibiting Their Present State in Every Part of the World; and Carefully Comp. from the Latest and Best Authorities, by Thomas Mortimer


September 22, 2015
Why Does Ice Melt? The Answer Lies in Physics.
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses the contributions of Max Planck seeking an explanation for why energy goes only one way (for example, ice starts to melt in a warm room instead of becoming even colder). – Kim
By Dean Zollman
 Ernest Rutherford’s discovery of the nucleus provided a basic structure for the atom. However, to explain some of the observations of the 19th century required additional discoveries. In particular, the light emitted from atoms could not be explained by a simple model of an electron orbiting a nucleus. (Recall that each element emits a unique set of colors when the element is heated or, for gases, has electricity passed through it.) To get the additional tools to explain this phenomenon, we need to back up to the end of the 19th century and follow a thread that was developing simultaneously with the study of radioactivity and the nucleus.
Ernest Rutherford’s discovery of the nucleus provided a basic structure for the atom. However, to explain some of the observations of the 19th century required additional discoveries. In particular, the light emitted from atoms could not be explained by a simple model of an electron orbiting a nucleus. (Recall that each element emits a unique set of colors when the element is heated or, for gases, has electricity passed through it.) To get the additional tools to explain this phenomenon, we need to back up to the end of the 19th century and follow a thread that was developing simultaneously with the study of radioactivity and the nucleus.
During the last half of the 19th century, some physicists were attempting to explain the behavior of matter, primarily gases, by applying Newton’s Laws to particles in the gases. Some of them were assuming that matter was made of small solid objects (atoms) that bounced off each other. This bouncing and motion could be described by using the principles that Newton had laid out. Others thought that while the basic known laws of mechanics would still work, it was better to approach the issue from the perspective on energy. All of this research is called statistical mechanics and is still an active area of research.
One observation that statistical mechanics needs to explain is the one way energy naturally flows. If I put a cup of ice on a table in a warm room, the ice always gets warmer. It never becomes even colder by transferring its internal energy to the room, which would then become even warmer. To make the ice colder, we must intervene (e.g. put it in the freezer).
In the 19th century, physicists were divided over whether this observation was a fundamental law of physics based on energy considerations or something based on the average (statistical) behavior of the particles making up the ice and surrounding air. (Statistical wins in the long run.)
Black Bodies That Aren’t Black

Max Planck in 1878 (public domain via Wikimedia Commons)
Max Planck (1858-1947) began considering these arguments in the 1880s. At that time, he was an advocate of the energy perspective and thought that the concept of atoms was not consistent with one-way flow of energy. He had written that “in spite of the great successes of the atomistic theory in the past, we will finally have to give it up and to decide in favor of the assumption of continuous matter.” (Quoted in Kragh, Physics World, December 2000)
To develop an explanation for the behavior of energy flow, Planck began looking for ways to combine mechanics and electrodynamics, the theory that describes electromagnetic phenomenon. A particular problem that Planck tackled was that of the so-called black body radiation. The physicist’s black bodies are rather poorly named because they are not necessarily black. These objects do absorb all electromagnetic radiation (including light) that strikes them. But a good absorber of radiation is also a good emitter. So the body may appear to be any color depending on the energy being emitted by it. Stars, including the sun, and a potter’s kiln are examples of black bodies that are definitely not black.
[image error]
An artist’s rendition of what we might see when looking at the spectrum of a radiating black body that would be somewhat orange. The horizontal scale is wavelength in nanometers. Human eyes see only about 400 to 700 nanometers, so this drawing shows much more than we can see (via Wikimedia, licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license).
Each black body has a characteristic color that depends on its temperature. But not all of the light is emitted at that one color. Instead, light of many colors (and therefore energies) is emitted. The distribution of colors determines how we perceive the object. When the burner on your electric stove is glowing red, it is emitting more red light than other colors but is still emitting some of the other colors.
By the late 19th century, physicists had investigated the colors emitted by many different objects. They could draw graphs of the intensity of light versus wavelength or frequency, which is related to color. The problem was that there was no good theoretical background to explain these curves. By applying statistical methods and electrodynamics to the material in a black body, Planck was attempting to create such a foundation.
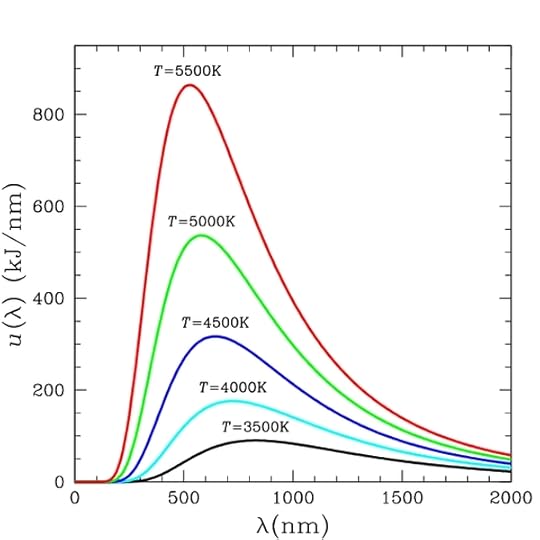
The black body radiation curves that Planck was seeking to explain. T in the graphs is the temperature associated with the object, called the black body temperature (public domain via Wikimedia Commons).
Two nice interactive graphs are available on the web. To see an interactive graph with as indication of the color that we see for stars as the temperature changes, go to McGraw Hill Education. To “create” similar graphs for a variety of object including an oven, light bulb, and the sun go to PhET Interactive Simulations. You will need to zoom in to see the graphs for the earth and the oven.
A Revolutionary Formula
Planck’s attempts were at first not very successful. He would get part of the curve right but not all of it. In the autumn of 1900, he tried a new idea. He thought of the light-emitting object as containing a large number of electrically charged oscillators. Each oscillating charge would emit radiation. There is nothing new so far. James Clerk Maxwell had shown that oscillating charges emit electromagnetic radiation. (In a couple of months, we will see how this fact came to haunt physicists.) Now, Planck added a twist. Instead of emitting any frequency, Planck’s oscillators could emit only multiples of some fundamental frequency. Mathematically, energy of the emitted light is expressed as
Energy =n x h x (frequency of light)
The symbol n is an integer number. Today, we refer to h as Planck’s constant.
With this additional hypothesis, Planck was able to match the intensity versus frequency spectrum for black bodies at all temperatures.
Max Planck’s Nobel portrait (public domain via Wikimedia Commons)
Creating a formula to match an observation is not such a big deal. However, the idea underlying Planck’s assumption started a revolution is physics. His assumption says the light energy is emitted in discrete amounts. If we have an electrically charged object that is oscillating with a frequency f, the object can emit light with energies of only n times h times f. All other frequencies are somehow forbidden. Light comes out of the object is discrete small packets. Those packets are now called quanta or photons. From this beginning, quantum mechanics was built and changed our fundamental understanding of the nature of matter. Historians of science discuss whether Planck understood the importance of his discovery. Some argue that his formula worked, but Planck had little understanding as to why. Others think that he did see the deep meaning that allows only certain energies to be emitted. Of course, there are historians who are in between these two views.
By the time Planck received the Nobel Prize, much progress had been made in using Planck’s hypothesis to describe a variety of phenomena. In his Nobel lecture, Planck stated that science “owes [current knowledge] to the unceasing progress of the researchers who have put the quantum of action to the service of their investigations. A. Einstein made the first breakthrough in this domain.” We will look at Einstein’s contribution to this progress next time.
A few side notes:
Many textbooks state the Planck discovered quanta because he was trying explain something called the “ultraviolet catastrophe.” In fact, I introduced Planck’s result to my classes in this way for many years. The “catastrophe” was that some equations predicted that black bodies should emitted an infinite amount of radiation at low wavelengths (high frequencies). This does not seem to be the case. Instead Planck was bringing to together various known laws of physics and needed one new hypothesis.
In 1878, Philipp von Jolly (1809-1884), Planck’s physics teacher at the University of Munich, told the young Max Planck to pursue some other discipline. Jolly believed that there was nothing new to be discovered in physics and “all that remains is to fill a few holes.” Planck was interested in understanding nature as it was, so he studied physics anyway and created some monstrous holes that still need to be filled.
Planck’s fourth son, Erwin, was involved in the July 20, 1944, attempt to assassinate Hitler. A few years ago, this event was depicted in the film Valkyrie. Erwin Planck was executed in January 1945 for his role in the attempt.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?





