Kim Rendfeld's Blog, page 15
March 8, 2016
Dreams of a Garden as Spring Draws Near
As I wrote a post about early spring gardens in the Dark Ages for English Historical Fiction Authors, one thought came to mind: I want one. Well the garden, not the Dark Ages.
The backyard in our prior house was too shady for many vegetables, so we grew summer tomatoes, peppers, and a few herbs in pots. I slipped some Swiss chard along a walk one year and have planted herbs and garlic in the front, but it’s not the same. I miss the lettuces and greens, kale, and peas. I long for asparagus and strawberry patches.
When we moved, we chose a property with a good sized backyard and a lot of sunshine. But the yard is grass rather than garden beds, so we will need to dig first. That means waiting for warmer weather and drier soil. Maybe next year, we’ll sow crops that can tolerate a few frosts, and I’ll see seedlings of those vegetables I described earlier.
Of course the stakes for me and my husband are a heck of lot lower than the imaginary friends I write about. For us, this is waiting a little longer to pursue a hobby. For them, it was life or death.

A 16th century illustration of what happens in March (public domain via Wikimedia Commons).


March 4, 2016
‘A First Feeble Ray of Light’ to Explain Electrons’ Orbits
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses the work of Louis de Broglie, who sought to explain why electrons adhere to certain orbits. As a grad student, de Broglie looked to the properties of waves. And even Albert Einstein weighed in. – Kim
By Dean Zollman
 To create a model that worked, Niels Bohr needed to assume that the electron could orbit the nucleus at only certain radii and that all other radii were forbidden. In the two previous posts, I mentioned that this assumption was a major concern. Bohr could not justify the assumption but used it in order to make the model match the data had been collected for more than half a century.
To create a model that worked, Niels Bohr needed to assume that the electron could orbit the nucleus at only certain radii and that all other radii were forbidden. In the two previous posts, I mentioned that this assumption was a major concern. Bohr could not justify the assumption but used it in order to make the model match the data had been collected for more than half a century.
A justification was forthcoming in the 1920s. In this post, I will discuss this research. However, the underlying reason for the discrete orbits very quickly became much more than just a justification for Bohr’s model. It was the beginning of a revolution in the understanding of atoms and other small objects. And it basically put the Bohr model “out of business” as a serious contender for the model of atoms. More about that revolution will come next few posts. First we need to learn about the critical step to get the revolution started.
From his name alone, you know that Louis-Victor-Pierre-Raymond, seventh duke of Broglie (1892–1987) came from an aristocratic French family. In the 1920s, he was not yet the seventh duke and used the somewhat less pretentious name Louis de Broglie. As a student, he began graduate studies in history but decided that theoretical physics was more interesting. He was motivated in part by his elder brother, the sixth duke of Broglie, an experimental physicist.
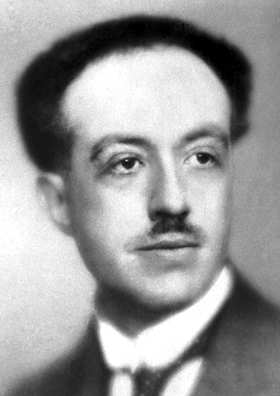
Louis de Broglie in 1929 (by Nobel Foundation, public domain, via Wikimedia Commons)
“I was attracted to theoretical physics by the mystery enshrouding the structure of matter and the structure of radiations, a mystery which deepened as the strange quantum concept introduced by Planck in 1900 in his research on black-body radiation continued to encroach on the whole domain of physics,” he said during his Nobel lecture.
For his PhD dissertation, de Broglie was greatly influenced by the work of Planck and Einstein. A few months ago, I discussed how Einstein had explained the photoelectric effect by assuming that light sometimes acted as if it came in small packets (photons) of energy rather than in waves. One of Einstein’s conclusions was that the energy of the photon was equal to Planck’s constant times its frequency. In symbols:
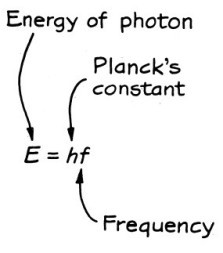
The relation between the energy and frequency of a photon. (From The Fascination of Physics by Jacqueline Spears and Dean Zollman, used with permission.)
By applying Planck’s hypothesis, Einstein concluded that light which is normally considered a wave sometimes acted like a particle with its energy given by the equation above.
De Broglie thought that if light could sometimes act as particles, then maybe particles could sometimes act as waves. If objects like electrons behaved like waves, they needed to have a wavelength. Using Einstein’s equation and his special theory of relativity, de Broglie derived an equation for the wave length of any object that has a mass. It says the wavelength is inversely proportional to the mass times the velocity (the momentum). In symbols:
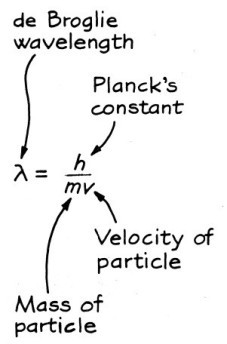
The relation among the wavelength, mass and velocity of an object with mass. (From The Fascination of Physics by Jacqueline Spears and Dean Zollman, used with permission.)
In those days, it was not uncommon for a graduate student to work on his or her own and deliver a completed dissertation to the examining committee. Basically, de Broglie worked in this way. However, his hypothesis was not received well by the committee. Certainly part of the problem that the committee saw was that de Broglie could not refer to a single experiment that supported his work.
A member of the committee was Paul Langevin (1872–1946), a prominent physicist in the 1920s. De Broglie later said that Langevin was “probably a bit stunned by the novelty of my ideas.” Even though he may have been stunned, Langevin sent a copy of the dissertation to Einstein for his review. Einstein rather quickly responded, “I believe it is a first feeble ray of light on this worst of our physics enigmas.” De Broglie wrote in the German translation of his dissertation, “Einstein from the beginning has supported my thesis.” (De Broglie’s dissertation is also available in a modern translation into English at the Fondation Louis de Broglie site. However, it has some heavy duty mathematics.) With Einstein’s endorsement, de Broglie received his PhD.
To explain why the electron in an atom could have only certain orbits, de Broglie relied on the interference property of waves. As shown in the figure below when two waves meet, the result is a bigger wave or a smaller one. On the left side of the figure is the result when the troughs and the crests of the two waves match up. The result shown at the top is a bigger wave. This effect is called constructive interference. On the right, the crests of one wave matches with the troughs of the second. As shown at the top of this one, the two waves cancel each other. This one is destructive interference.
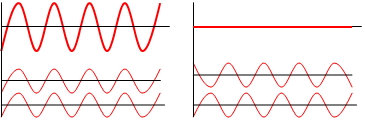
Interference of two waves (by Haade, GFDL or CC-BY-SA-3.0, via Wikimedia Commons
For the electrons in atoms, the wave needs to exist as they move in a circle. The drawing below show different situations for these circular waves. For the waves in (a) each of the waves can fit on a circle and close on themselves. When they come back around they act like the waves on the left in the figure above. Toughs meet troughs and crests meet crests, and we have constructive interference. So they add nicely. Because the wave of the electron is not zero, we can conclude that the electron can exist in these two orbits.
However, when the wave in (b) comes back around the troughs will eventually meet crests. They act like the waves on the right of the figure above. Thus, the wave is canceled (destructive interference), so the electron cannot exist in the orbit because its wave is zero. The net result is that only certain radii can meet the condition that an electron’s wave just fits nicely on the circumference of the circle. Only when the electron’s wave can exist, can the electron be in that orbit. Thus, de Broglie’s electron waves give a reason for Bohr’s orbits.
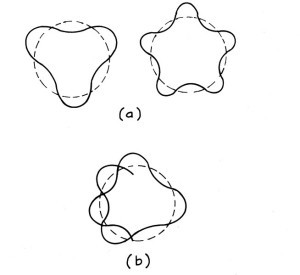
De Broglie waves on a circle. Part (a) shows two waves which can fit on a circle and close on themselves. Part (b) shows one that does not. In (b) when the wave comes back around the troughs and crests meet. The result will be no wave of this wavelength can exist in a circle with that radius. (From The Fascination of Physics by Jacqueline Spears and Dean Zollman, used with permission)
A result of a detailed analysis of the electron orbits and several other phenomena provided evidence that de Broglie was on to something. However, the explanations of the Bohr orbits and other phenomena were indirect evidence of the wave behavior of matter. Constructive and destructive interference can only occur with waves; particles cannot do it. If electrons really have wave properties, physicists should be able to devise an experiment in which they can directly observe the constructive and destructive interference of electrons. Within a few years of publication of de Broglie’s dissertation such experiments were completed on both sides of the Atlantic. There are some interesting stories connected to the experiments, so I will save them for next time.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
How Bohr’s Famous Model of the Atom Was Created
Bohr’s Model of the Atom Answers Fundamental Questions – but Raises More
Bohr’s Model of the Atom Draws Critics


February 24, 2016
Did King Pepin and Queen Bertrada Love Each Other?
We don’t know how Bertrada of Laon felt about marrying Pepin, mayor of the palace. Was she overjoyed to join a Frankish family more powerful than the royal Merovingians? Or did she see herself fulfilling her duty to her kin as a partner in building an alliance? What did she think of Pepin as a man?
Whatever her sentiments during the nuptials in 744, she was not about to give up on her relationship with her husband when he sought to end the childless union two years later. At least, I think that’s what Pepin tried when he asked the pope about illicit marriages – he and Bertrada shared a set of great-grandparents.
Had Bertrada been willing to go quietly, she could have taken the veil, perhaps even held out for her own abbey to rule. But she didn’t. And the pope told Pepin canon law prohibited remarriage after a divorce.
So Pepin and Bertrada stayed together, and the story has a surprisingly happy ending, the reason I’ve chosen to write about them as an unlikely romance at Unusual Historicals.

Bertrada’s and Pepin’s tombs at Saint-Denis, France (photo by Roi Boshi (CC BY-SA 3.0 or GFDL, via Wikimedia Commons)


February 10, 2016
How Did Willehad Feel When He Had to Flee?
Would history classes have been more interesting if we occasionally paused and asked, “What was going through their mind?”
Often there is no way to know for sure. So this question invites speculation rather than the cold memorization of names, dates, and events. But it does make the person we’re studying human and real to us.
This question occurred to me as I read about Saint Willehad, an eighth century priest and missionary to pagan Frisia and Saxony. What was he thinking in 782 when he had to flee from pagan Saxons who burned churches he founded and killed his followers? Did he feel betrayed that the very people he was trying to help turned against him? Or did he feel like he had somehow failed those lost souls?
Thinking about Willehad’s point of view by its nature excludes others – that the Saxons had valid grievances like forced conversion and exorbitant tithes.
In the 21st century, it’s easy to find fault with both sides and such objectivity is important to get a balanced picture of what happened. But it also distances us from the people, complete with virtues and biases, who inhabited our past.
For about the history of Saint Willehad and my speculation, read my post on English Historical Fiction Authors.

An 1880 fountain honoring Saint Willehad, next to the cathedral in Bremen, Germany, was melted down in 1942 (photo shot around 1900, public domain via Wikimedia Commons).


February 5, 2016
Bohr’s Model of the Atom Draws Critics
In this installment of the history of atom theory, physics professor (and my dad) Dean Zollman discusses the problems physicists had with Bohr’s model – and they could get downright catty. – Kim
By Dean Zollman
 If you look at a description of the Bohr model of the atom in most textbooks or many popularized accounts, you could easily get the impression that it was an immediate success. Physicists were troubled by some of its assumptions, but it worked, so they moved on. However, like several other developments that I have discussed in these posts, the history is more complex. Many physicists were not just unhappy with some of the assumptions, they were sure the entire model was absolutely wrong.
If you look at a description of the Bohr model of the atom in most textbooks or many popularized accounts, you could easily get the impression that it was an immediate success. Physicists were troubled by some of its assumptions, but it worked, so they moved on. However, like several other developments that I have discussed in these posts, the history is more complex. Many physicists were not just unhappy with some of the assumptions, they were sure the entire model was absolutely wrong.
Basically the Bohr model of the atom was an attempt to use the classical physics of Newton, Maxwell, and others but incorporate the quantum ideas of Planck and Einstein. Thus, it was the beginning of a revolution but not as great as the revolution that was to come soon. As a result of Bohr’s quantum-classical blended model, five major concerns arose.
An accelerating charged particle will emit continuous radiation. An electron moving in a circle is accelerating. Yet in Bohr’s model the orbiting electrons did not radiate. If it did, the electron would spiral quickly into the nucleus. (I discussed this one in a previous post.) Bohr had no logical reason for the nonradiating electron, but without it, atoms would not exist.
In the early 20th century, electromagnetic radiation such as radio waves was created by shaking electrons back and forth. The frequency of the shaking determined the frequency of the wave. For radio, microwaves, etc., we still do that. A radio station that broadcasts at 99.5 megahertz shakes electrons in its antenna 99,500,000 times per second. Scientists in 1913 expected that light was emitted in the same way, so an electron in an atom would vibrate to emit light. However, in the Bohr model nothing vibrated; the light just appeared as the electron changed orbits.
Even worse, these “quantum jumps” in orbit seemed to occur without a cause. Once an electron was in orbit with an energy higher than the lowest possible energy, it would at some time move to a lower energy, but Bohr could not ascribe a cause to that event.
Then, physicists asked: how does the electron “know” where it is going? When it leaves one orbit, it gives off a photon of light. The energy of that photon is determined by the energy that the electron will have when it lands in the lower orbit. It seems that the electron knows what will happen to it before the event is finished.
And, finally, what happens to the electron in between the time it leaves one orbit and the time it gets to the second orbit?
So, there were some rather good reasons to wonder if Bohr was making up a story that just did not fit with the known laws of physics. However, for many people something was more important than these concerns. The Bohr model worked in a way no other model of the atom had. Bohr had been able to reproduce the equation for the spectral lines that had been discovered by Balmer. He had also explained several recently observed effects. That was enough for some physicists, but not for all.

Arnold Sommerfeld in 1897 (public domain via Wikimedia Commons)
Arnold Sommerfeld (1888-1951) at Ludwig Maximilian University in Munich did see the positive side of Bohr’s model. He and his colleagues made modifications to the model by assuming that the electron would move in elliptical orbits rather than circles. (Then, the electrons are moving in a manner similar to the planets around the sun.)
This change made the connection between the model and recent accurate measurements even better than the model with circular orbits. Thus. These refinements to create the Bohr-Sommerfeld model gave the model more credibility than it had before.
[image error] The Bohr-Sommerfeld model of the atom (by Pieter Kuiper, public domain via Wikimedia Commons)

Paul Ehrenfest (public domain via Wikimedia Commons)
However, not everyone was happy with this turn of events. Paul Ehrenfest (1880-1933) wrote to Sommerfeld to congratulate him on this work. His statement was somewhat a back-handed compliment. He said, “Even I consider it horrible that this success will help the preliminary, but still completely monstrous, Bohr model on to new triumphs, I nevertheless heartily wish physics at Munich further success along this path!” Earlier Ehrenfest had written to Hendrik Lorentz (1853-1928), “Bohr’s work on the quantum theory of the Balmer formula, has driven me to despair. If this is the way to reach the goal, I must give up doing physics.” Clearly, Ehrenfest was not a fan of the Bohr model.
One of the least diplomatic critics of Bohr was Johannes Stark (1874-1957). In 1919, Stark received the Noble Prize for two discoveries. One of these discoveries, which bears his name, is that spectral lines will split into more than one line when the atoms that emit them are in an intense electric field. Other physicists were able to use the Bohr-Sommerfeld model of the atom to explain why this splitting occurs. That did not, however, stop Stark from taking an intellectual shot at Bohr.
During Stark’s Nobel Lecture, he stated Planck’s hypothesis “forms the starting point of Bohr’s theory of the emission of serial lines. Although I myself once stood on the threshold of this theory, and although the final formulae give a series of frequency relationships in the spectral series which agree well with observed facts, I am nevertheless unable to believe it, because in its provisions it postulates suppositions which contradict, not only Maxwell’s theory, but the very spirit of physics. This criticism is directed not at Planck’s quantum of action, but at the hypotheses of Bohr which are bound up with it.”
In other words, Planck’s ideas are OK, but Bohr misused them and must be wrong. I have not found any record of Stark’s thoughts when Bohr was the recipient of the Nobel Prize three years later.
Stark’s choices in politics were also not very wise. With Philipp Lenard (1862-1947) he led the Deutsche Physik (German physics, sometimes called Aryan Physics) movement during the Nazi period in Germany.
There were also many critics of Bohr’s model in England, including J.J. Thompson (1856-1940), the discoverer of the electron, and John William Nicholson (1881-1955), who developed his own atomic model.
All of the criticisms were eventually silenced when Bohr’s model was replaced by modern quantum theory. We will get to that in a couple of months. But, first we will look at an explanation for why the electrons do not radiate while moving in an orbit. This hypothesis partially helped give a foundation to one of Bohr’s assumptions and laid more of the groundwork for quantum theory. It even involves a French prince.
(Several of the quotations in this post are reproduced from a series of papers written or co-written by Helge Kragh.)
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
How Bohr’s Famous Model of the Atom Was Created
Bohr’s Model of the Atom Answers Fundamental Questions – but Raises More


January 27, 2016
When a Saint Chooses God over Family
In the sixth century, Irish Saint Columbanus got an A on the faith test, but I can’t help but feel sorry for his mother.
His calling to serve God meant leaving all women behind – even her. If we are to believe his hagiographer, Columbanus quoted Matthew: “He that loveth father or mother more than me is not worthy of me: and he that loveth son or daughter more than me is not worthy of me” (King James version).
He was proving he loved God more than anything or anyone else, so much that he sacrificed his relationship with his own family. And he confirmed her second worst fear, saying that they would never see each other again in this life. Assuming she was a faithful medieval woman, her worst fear would be for him to wind up in hell and be separated from him forever.
I would like to think Columbanus struggled with his decision, but his fear of sin was strong. See my post on English Historical Fiction Authors for more.

Medieval image of Saint Columbanus (public domain, via Wikimedia Commons)


January 21, 2016
Sin Is Good for the Bottom Line
If medieval Christians obeyed the Church on when they could have intimacy, the religion would have died out.
Even husbands and wives could only “meet” on certain days. Forbidden were Sundays, Wednesdays, and Fridays; that time of the month; during pregnancy plus a month or more after the birth; 40 days before Christmas; 40 days before and eight days after Easter; eight days after Pentecost; the eves of great feasts; and five days before Communion (maybe that’s why it was only once a year for most people).
One layman probably spoke for many when he said God gave men and women those parts below the waist so that married couples could have relations and so what if the motive was pleasure.
I’m not sure how the Church came up with its list of “no sex allowed” days. If I were a cynical person, I would say it would be a good way to drum up revenue.
If you sinned, you had to confess, then do penance, usually prayer, fasting, abstaining from meat and wine, or some combination. Or you could give alms in the form of money or land.
So while the Church could abhor relations outside matrimony and married couples meeting with each other on the wrong days, it could also make a tidy sum from the sinners.
This is why the current draft of Queen of the Darkest Hour has an uncle deciding to introduce his nephew to his favorite harlot and distract him from the queen’s maid: “Yes, it is different. You’re using a whore instead of ruining a virgin, and that’s better than making an enemy of the queen. Besides, the Church would have little funds if men did not swive whores and repent. I’ll give you some coins for penance afterward.”
Source
Daily Life in the World of Charlemagne by Pierre Riché

The Seven Deadly Sins and the Four Last Things – Lust by Hieronymus Bosch, 1485, oil on panel (public domain via Wikimedia Commons)


January 7, 2016
Lessons Learned from Rereading Grimm’s Folk Tales
The folk tales collected by the brothers Grimm deal with the most powerful motives of all of us: love, envy, revenge, greed, hunger. All in a few pages. Perhaps that is why they continue to fascinate me long into adulthood.
For the past few weeks, I’ve been reading a recent translation of the stories. For research. Really. My characters would have told stories like these to entertain each other and teach their kids moral truths.
Forget the Disney versions. The punishments for the villains are brutal, and sometimes the villain is moronic enough to pronounce their own sentence, thinking the description of their actions is hypothetical. And there are a few real downers where everyone dies.
The tales have their share of humor, often at the expense of the not-so-bright. The guy or gal who’s nicknamed “Clever” often isn’t. Or they are devious.
Sometimes the damsel in distress is rescued by the prince, but many times the heroine is saving the hero. And she is a heck of a lot more loyal, even when her beloved has forgotten her (usually because he’s been bewitched). As adult, I often find myself on the side of the bride who wants to end a marriage she’s been forced into, even though there’s only one way to do that.
Despite the magic and supernatural, I feel like I know these people sometimes. The guy who chronically complains, even about the way things are done in heaven. The wife who paces around the mill pond where her husband disappeared, sometimes calling his name, sometimes sobbing softly. The hero or heroine who brave all for love.
And those moral lessons hold true today:
Your wits are a powerful weapon.
Stupidity and greed will make you suffer and might kill you.
Treachery and rudeness will come back to bite you.
Your kind heart, hard work, fidelity, and courage will always triumph, no matter what other people say.

Illustration for “Snow White and Rose Red,” by Hermann Vogel (public domain image via Wikimedia Commons)


January 1, 2016
Bohr’s Model of the Atom Answers Fundamental Questions – but Raises More
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman explains the science behind Bohr’s Model of the Atom. As so often happens in science, Bohr’s work contributed to our knowledge of matter in its tiniest parts, but more questions arose. – Kim
By Dean Zollman
 Last time, I discussed the historical perspective on the development of the Bohr Model of the Atom. This discovery is critical to the development of the modern quantum view of matter. So, this time I deviate some from the historical approach and discuss the physics of Niels Bohr’s model. Understanding the ideas that go into the model is not complex. Bohr’s genius was putting all of the parts together and adding one critical assumption.
Last time, I discussed the historical perspective on the development of the Bohr Model of the Atom. This discovery is critical to the development of the modern quantum view of matter. So, this time I deviate some from the historical approach and discuss the physics of Niels Bohr’s model. Understanding the ideas that go into the model is not complex. Bohr’s genius was putting all of the parts together and adding one critical assumption.
Bohr had a large foundation on which to build. He knew:
Newton’s basic laws of motion.
Light is a form of energy, so when an atom emits light, it must decrease its own energy.
Planck and Einstein had showed that light came in energy packets (photons). The value of the energy is a constant, called Planck’s constant, time the frequency of the light.
Rutherford had discovered the atom is composed of a positive charge (nucleus) at the center and negative charges (electrons) around the positive charge.
Positive and negative charges attract each other and energy can be stored in the electrical attraction.
Each atom emits a spectrum of light, and that spectrum is unique to each element.
Balmer had been able to describe the spectrum of hydrogen with a not-too-complex equation. (As I mentioned last time, Bohr learned or rediscovered this fact somewhat late in his research on an atomic model.)
To these concepts, Bohr added:
The electron can occupy only certain orbits around the nucleus.
While in these orbits, the electrons does not emit or absorb light.
Light is emitted or absorbed when electrons changes orbits.
These ideas built on those of others, but Bohr applied them in a unique way.
Why Don’t Things Fall Apart?
Limiting the electrons to certain orbits was the critical step. Bohr knew that an electrical charge moving in a circle should radiate light continuously. Such a process would result in all colors of light being emitted, and the electron crashing into the nucleus in a very short time. He needed a reason for the atom to be stable.
To create that situation, he assumed that the electron could have only certain values for its angular momentum. The angular momentum for an object traveling in a circle is its mass times speed times radius of the circle. Bohr’s assumed that this quantity had to be an integer number times Planck’s constant divided by 2 pi. While moving in these allowed orbits, electrons do not emit any electromagnetic energy.
When moving from one orbit to another, however, the electrons absorb or release energy. If the electron moves to an orbit farther from the nucleus, it absorbs an amount of energy equal to the energy of a photon in one of the absorption lines. This energy is also the difference in the energy of the electron in the orbits. When the electron jumps to an orbit closer to the nucleus, it releases an amount of energy equal to the energy of a photon in one of the emission lines. Allowed orbits restrict the possible jumps to just a few. Consequently, the light spectrum emitted by atoms would be discrete. Since Bohr’s model uses the same orbits to explain both emission and absorption of energy, it predicts emission and absorption lines that have identical energies.
In proposing that electrons move only in allowed orbits, Bohr was suggesting that the energy of the electron can be measured, or quantized. In any given orbit, an electron has a certain amount of kinetic energy associated with its motion and electrical potential energy associated with the electrical force of attraction exerted by the nucleus. If electrons move only in allowed orbits, their energy takes on selected, or discrete, values. Bohr saw the same quantization of energy in the atom that Planck saw in the radiation emitted by solids and Einstein saw in the photoelectric effect. At the atomic level, energy appears in discrete amounts.
Measuring How Light Is Absorbed or Emitted
To make these ideas quantitative, Bohr combined this idea with the equations for energy of an orbiting electrical charge and Newton’s Laws. After some algebra, he had an equation for the values of the possible energies that an electron orbiting a hydrogen nucleus could have. The light was emitted or absorbed when the electron moved from one orbit to another. So the energy of the photon was equal to the difference between those energies. To obtain the frequencies of light emitted or absorbed by hydrogen atoms, Bohr applied the Planck-Einstein energy of a photon.

An animation of photon of light being emitted and absorbed by a Bohr atom (by Kurzon, CC BY-SA 3.0, via Wikimedia Commons)
The energies associated with each allowed orbit provide a useful way of describing what happens as atoms absorb or emit light. The energy-level diagram below provides a convenient way to describe the energy emitted or absorbed by a hydrogen atom. The lowest red line is called the n = 1 energy and corresponds to the energy of an electron when it is moving in the smallest orbit, the orbit closest to the nucleus. This is often referred to as the ground state of the electron because it represents the smallest energy the electron can have. The n = 2 line corresponds to the energy the electron has in the next orbit, n = 3 in the third orbit, and so on. Collectively, the energy levels above ground state are called excited states. The energy of the electron increases as we move to higher excited states.
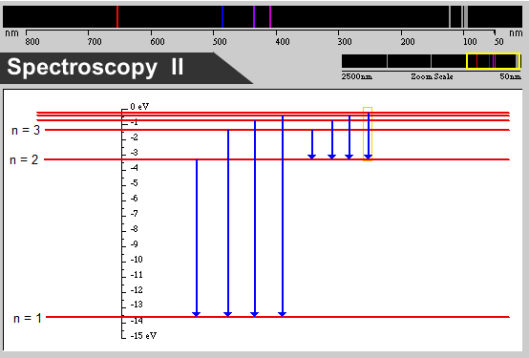
An energy level diagram for the emission of ultraviolent and visible light from hydrogen. (Created with a program from the Visual Quantum Mechanics project, Dean Zollman, principal investigator)
The distance between states is proportional to the energy difference between states. So, for example, the energy difference between n = 3 and n = 2 is smaller than that between n = 2 and n = 1. The blue arrows in the energy level diagram indicate that the electron has changed energy from an excited state to a lower energy state. At the top of the diagram is the spectrum of hydrogen. The lines indicate the colors that would be emitted by these changes. All of the photons emitted by changes to the n = 1 energy are in the ultraviolet and are drawn in gray on the right side of the spectrum. The transitions to the n = 2 energy are visible and shown by their approximate colors. These are the spectral lines that Balmer saw.
[image error]
A schematic using the Bohr Model of transitions for orbiting electrons. (From Nusha, GFDL or CC-BY-SA-3.0, via Wikimedia Commons)
In a sample of hydrogen gas at room temperature, each atom is most likely to have its electron in its ground state. An external source – heat, light, or electricity – can provide energy to these electrons. When we pass white light through a sample of hydrogen gas for example, electrons “select” photons of energy needed to move into excited states.
As shown in the diagram below electrons might move from n = 1 to n = 2, from n = 1 to n = 3, from n = 2 to n = 3, and so forth. Because the energy levels are the same as for emission, the only change in the diagram is the direction of the arrows. The electrons are moving from lower energy to higher energy. The photons with energies equivalent to each of these jumps are absorbed while the others are ignored. Consequently, the light that emerges is the full spectrum minus the photons with frequencies used to move electrons into excited states. We see a discrete absorption spectrum.
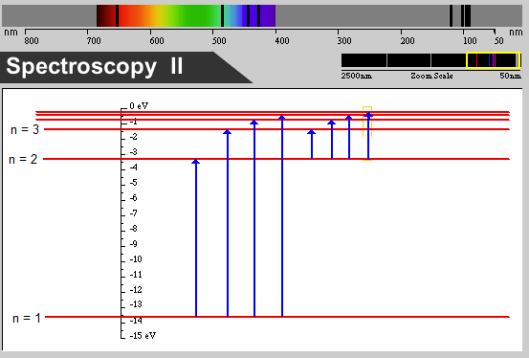
An energy level diagram for the absorption of ultraviolent and visible light by hydrogen. (Created with a program from the Visual Quantum Mechanics Project, Dean Zollman, principal investigator)
Limitations to Bohr’s Model
One of the successes of Bohr’s model is that he could calculate the energies of all of the levels in the hydrogen atom. The n = 1 (ground state) energy is -13.6 electron volts. (The minus sign is a notation to indicate that the electron is being attracted to the nucleus.) The next one, n = 2, is -3.4 electron volts. In general the energy of the nth level is -13.6 eV/n2. (An electron volt (eV) is a very small unit of energy used in atomic physics.) Bohr was able to obtain these numbers by doing some algebra and getting a result that depends only on constants such as the electrical charges on the electron and proton and Planck’s constant. Likewise, he could determine the radius of the hydrogen atom. He did not need Balmer’s data from observations. So, he had a model that seemed quite complete.
In addition to explaining the discrete spectra found for chemical elements, Bohr’s model also resolves the problem of why atoms do not collapse. The electron can lose only specific amounts of energy equivalent to the jumps in the energy-level diagram. When it reaches lowest energy, the electron can neither lose more energy nor move closer to the nucleus. The stability of the atom is assured. Bohr’s model of the atom moved several pieces of the atomic puzzle into place. It did, however, leave some questions unanswered.
While the concept of allowed orbits explains both the stability of the atom and discrete emission and absorption spectra, it is not based on any known principles of physics. There seems to be no reason for electrons to jump instantaneously from one energy state to another, shunning all energies in between.
Furthermore, additional observations revealed limitations to the model. While it was enormously accurate in describing the hydrogen spectrum, Bohr’s model was far less successful in describing the spectra produced by more complex atoms. Moreover, the intensity of individual spectral lines varied. Some lines were brighter than others – an observation for which Bohr’s model offered no explanation. The resolution to these problems lay in yet another model of the atom – the quantum mechanical model. We will move toward learning about it next time.

In 1927, the world’s top physicists met at a Solvay Conference to discuss issues related to quantum physics and the atom. Niels Bohr is on the far right in the second row. Physics was very much a European man’s occupation in those days. The only woman participant was Marie Skłodowska Curie, third from the left on the front row. An American, Arthur Compton, is the third person to the left of Bohr. (Public domain via Wikipedia Commons. For an annotated, interactive version go to Wikimedia Commons.)
Much of the material in this post was adapted from Fascination of Physics by Spears and Zollman. It is used by permission.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
How Bohr’s Famous Model of the Atom Was Created


December 23, 2015
A New Home with an Unburied Saint Joseph Statue
I meant to write this post almost a week ago, and I have Saint Joseph to thank for my tardiness.
Before my husband and I moved, I wrote a post for English Historical Fiction Authors about the various origin stories for burying a Saint Joseph statue to sell property. Then we resumed packing.
The post for EHFA ran on time. The post I was planning for Outtakes didn’t get written. We were at that stage in the packing where we were lucky if we labeled the boxes with the rooms they should go to. No time for the sorting what to keep or trash and write detailed lists as we did at the start of the process.
So we are now in our new home busily unpacking boxes and Saint Joseph’s statue is watching over this chaos from the mantle. Did he sell our house? I don’t know, but both times I’ve buried him, the property sold just in time to avoid our paying two mortgages.






