Kim Rendfeld's Blog, page 14
June 3, 2016
A Mathematical Approach to Atoms That Works but Is Complex
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses how Werner Heisenberg—he of the Uncertainty Principle—decided to take a mathematical approach to something he couldn’t observe. For those who need a refresher, Heisenberg’s Uncertainty Principle is that we cannot know both the position and momentum of a particle precisely, and that the more we know about one, the less we know about the other.—Kim
By Dean Zollman
 In the last post, I discussed an experimental confirmation of Louis de Broglie’s hypothesis that matter could behave as waves do. In that case, Clinton Davisson and Lester Germer observed interference effects that could occur only if matter had wave-like behavior. In this post, we will back up in time just a little and consider the first of two theoretical developments that also occurred in the mid-1920s. The second approach will be discussed next time.
In the last post, I discussed an experimental confirmation of Louis de Broglie’s hypothesis that matter could behave as waves do. In that case, Clinton Davisson and Lester Germer observed interference effects that could occur only if matter had wave-like behavior. In this post, we will back up in time just a little and consider the first of two theoretical developments that also occurred in the mid-1920s. The second approach will be discussed next time.
In 1925, Werner Heisenberg (1901-1976) was conducting research at the University of Goettingen in Germany. He was working under the direction of Max Born (1882-1970) and trying to resolve some of the mysteries that recent experimental discoveries had revealed. He had just spent about eight months working with Niels Bohr in Copenhagen. Thus, he was building on Bohr’s work and trying to find a fundamental basis to explain the Bohr Model of the Atom and the discrete spectrum of the hydrogen atom. Heisenberg had concluded that he needed to “reinterpret” classical mechanics in order to bring quantum ideas in to the picture.

Werner Heisenberg in about 1927
Heisenberg’s basic approach was to avoid ideas such as orbiting electron because they were not observable. Instead, he pursued a mathematical theory. At one point in a letter to Wolfgang Pauli (1900-1958), he stated, “My entire meager efforts go toward killing off and suitably replacing the concept of orbital paths that one cannot observe.”
To do so, he looked first at a situation that was simpler than an atom—a certain kind of oscillating object whose behavior was not quite periodic. Heisenberg and his colleagues at Goettingen made some progress. They were able to base their theory on a new set of mathematical operations. While this approach showed some promise, it did not involve Planck’s constant and thus did bring in the latest quantum interpretations.
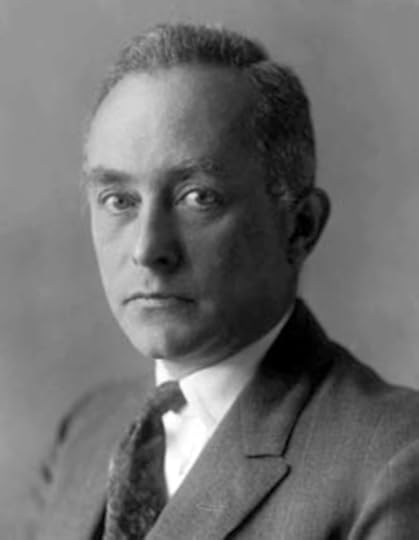
Max Born; in addition to being a Nobel laureate, he was Olivia Newton-John’s maternal grandfather
While working intensely on this issue, Heisenberg suffered an extreme attack of hay fever. He needed to get away from pollen, so on June 7, 1925, he left for the island of Helgoland. This German island in the North Sea has very little vegetation and therefore very little pollen. As he recovered from the hay fever, Heisenberg also had very little distractions. So, he was able to develop a mathematical approach to quantum physics.
When Heisenberg returned to Goettingen, he presented the ideas to Max Born and his young assistant Pascual Jordon (1902-1980). They recognized that Heisenberg’s formulation was quite similar to a relatively new mathematical concept called the matrix. So, they expanded the ideas to create a matrix approach to quantum physics.

Pascual Jordon in the 1920s (unknown photographer, Mondadori Publishers)
While Heisenberg’s matrix mechanics worked to obtain observable results, it was very difficult to use to complete calculations. For example, Wolfgang Pauli obtained the energy levels in the hydrogen atom using matrices and thus connected it back to Bohr’s ideas and the spectrum of hydrogen. However, it took him 40 pages of calculations to get to a result that could be observed in an experiment.
One of Heisenberg’s biographers, David Cassidy, states, “The new mechanics was (and is) nearly incomprehensible to the technically uninitiated.”
Unfortunately, that statement does seem to be true. Part of the reason is that matrix mechanics, as it became to be known, is not easily visualized. During the years that I have been teaching quantum physics to non-science students, I have not found an acceptable way to present this approach to students who have not studied the detail of matrix algebra.
Fortunately, a different method that was developed about the same time is more easily visualized. We will look at that approach and learn about Erwin Schroedinger in the next post and the rivalry that developed between Heisenberg and Schroedinger.
Public domain images via Wikimedia Commons.
Dean Zollman is university distinguished professor of physics at Kansas State University, where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
How Bohr’s Famous Model of the Atom Was Created
Bohr’s Model of the Atom Answers Fundamental Questions – but Raises More
Bohr’s Model of the Atom Draws Critics
‘A First Feeble Ray of Light’ to Explain Electrons’ Orbits
Two Labs across the Atlantic Prove That Electrons Behave Like Waves


May 31, 2016
What If the Abolitionist Had Telekinesis?
I’m happy to welcome author Jessica Knauss to Outtakes as she introduces Awash in Talent, three interconnected novellas of people coping with special abilities. Here, she explains the need for an alternate history.—Kim
By Jessica Knauss
 Awash in Talent follows three narrators through beautiful Providence, Rhode Island, as they deal with the ups and downs of the rare Talents: telekinesis, firestarting, and the ability to see people’s thoughts. As I wrote the novel, the paranormal elements demanded an alternate history.
Awash in Talent follows three narrators through beautiful Providence, Rhode Island, as they deal with the ups and downs of the rare Talents: telekinesis, firestarting, and the ability to see people’s thoughts. As I wrote the novel, the paranormal elements demanded an alternate history.
Providence is so awash in history that I consider the four years I lived there research for Awash in Talent. As Patricia the psychic so joyfully remarks in the final novella, you can’t walk five paces on the East Side without stumbling over a historical marker.
The alternate history of my paranormal Providence follows real history until the late 19th century, when the three Talents came to light. No un-Talented people think of them as strange now. Early on, major adjustments were made in order to segregate Talented children during their school years, and complex legislation watches over Talented adults. Otherwise, there’s a 10 percent chance anyone you might meet on a paranormal Providence street could have one of the three Talents.
I plan to explore that history in the sequel to Awash in Talent. This first book is most concerned with the present situation of the Talents, and, to say it one way, their prehistory. Just because the Talents weren’t discovered before 1870 doesn’t mean Talented people didn’t exist. I thought they must’ve been using their special abilities although they didn’t tell anyone about them: a secret history.
Most of the prehistorical burden in Awash in Talent falls on one remarkable man. In real history, Moses Brown (1738–1836) was one of the wealthy Brown brothers, who founded what would become Brown University. Moses broke away from the family to become Rhode Island’s most influential abolitionist.

1857 portrait of Moses Brown by Martin Johnson Heade (Image courtesy of the Brown University Portrait Collection, Brown University, Providence, R.I., public domain via Wikimedia Commons)
Moses donated the land the Moses Brown School occupies and served as its treasurer until his death at age 98. It maintains its reputation as a fine institution of learning. In my Providence, the school has morphed into the Moses Brown Academy for Telekinesis, the most desirable place for a child to learn about his or her Talent. Kelly the firestarter looks on with envy during an unforgettable visit that changes the course of the second novella.
We learn why Moses Brown’s school specializes in telekinesis in the final novella, when Patricia muses on her favorite misfit Brown brother: He may have been telekinetic! I enjoyed imagining what someone with that power could do for the abolitionist cause. “The stories go that at slave auctions where Moses was present, the shackles mysteriously lifted off the men and women as they were brought to the display area,” Patricia tells us.
When I choose which parts of history to change for my paranormal Providence, I honor real historical research. I also claim issues to highlight and explore in the story. If Moses Brown, defender against slavery, had a Talent, who’s around now to defend Talented people?
About Awash in Talent
So much Talent can kill you.
Welcome to Providence, Rhode Island, home of telekinetics, firestarters, and psychics!
Emily can’t escape her annoyingly Talented telekinetic healer sister without committing a crime.
Kelly must escape her pyrokinesis school and bring Emily’s sister to Boston—her mother’s life depends on it.
Appointments with Emily might drive her psychic therapist insane.
With so much Talent, sometimes it’s all you can do to function in an un-Talented society.
Awash in Talent will be released June 7. It is now available for preorder on Amazon.
Jessica Knauss was born and raised in Northern California but has wandered all over the United States, England, and Spain, including four wonderful years in Providence. She’s been a librarian, a Spanish teacher, and an editor. Get updates on her writing at:
JessicaKnauss.com
Jessica’s blog
Facebook
Twitter
Goodreads
Pinterest
Jessica’s Amazon Author Page


May 25, 2016
What a Pronoun Reveals about a Medieval Duke and Duchess
Waldelen and Flavia’s story of seeking divine intervention from Saint Columbanus struck me in many ways, but one could easily escape notice.
Columbanus’s hagiographer, a monk named Jonas, uses the word they as he describes the sixth century childless couple’s predicament: “Both of them begged of him that he would pray to the Lord on their behalf, for they had great wealth, but no son to whom they could leave it after their death.”
That great wealth was a duchy from the Jura to the Alps. Although the couple specifies a son, Jonas’s use of they rather than he implies Waldelen and Flavia were partners. This doesn’t fit the stereotype of medieval women as chattel. Early medieval times were far from ideal, but women often were strong and influential.
According to Jonas, Columbanus agreed to pray for them under the condition they give their first child to the Church. “Joyfully they promised what he wished, asking only that he would not cease to implore God to have mercy upon them.”
It must have been a tough choice, but the couple made the decision together. For more about a duke and duchess who gave up their miracle baby, see my post at English Historical Fiction Authors.
Source
Medieval Sourcebook: The Life of St. Columban by the Monk Jonas

What Christians in the fourth through sixth centuries wore, according to The History of Costume by Braun & Schneider, c.1861-1880


May 19, 2016
Don’t Go Breaking Her Heart: Breach of Promise Suits
 I am happy to welcome Maria Grace back to Outtakes as she introduces her latest release, The Trouble to Check Her, where Lydia Bennet must face the consequences of running off with Mr. Wickham. Here, Grace tells us how a jilted bride could make her former fiancé pay, literally.—Kim
I am happy to welcome Maria Grace back to Outtakes as she introduces her latest release, The Trouble to Check Her, where Lydia Bennet must face the consequences of running off with Mr. Wickham. Here, Grace tells us how a jilted bride could make her former fiancé pay, literally.—Kim
By Maria Grace
 Even after the decline of arranged marriage after 1780, marriage still remained largely a business transaction. Even the promise to marry was considered an enforceable contract, with a breach of promise suit a possible consequence of a broken engagement.
Even after the decline of arranged marriage after 1780, marriage still remained largely a business transaction. Even the promise to marry was considered an enforceable contract, with a breach of promise suit a possible consequence of a broken engagement.
History
As early as the 15th century, English ecclesiastical courts equated a promise to marry with a legal marriage. By the 1600s, this became part of common law; a contract claim one party could make upon another in civil court suits. (And you thought this was just the stuff of modern daytime television!)
To succeed in such a suit, the plaintiff, usually a woman, had to prove a promise to marry (or in some cases, the clear intention to offer such a promise), that the defendant breached the promise (or the implied promise to promise), and that the plaintiff suffered injury due to the broken promise (or failure to make the implied promise). Don’t think about it too hard, it’ll make your head hurt.
Breach of Promise Claims
A breach of promise suit required a valid betrothal. Promises to marry when both parties were below the age of consent were not valid. Similarly, promises to marry made when one was already married (as in I’ll marry you if/when my current spouse dies—how romantic) or between those who could not legally marry were not enforceable.
If significant and material facts were discovered that could have influenced the agreement, then betrothal could be dissolved without penalty. So issues like misrepresentation of one’s financial state, character, or mental or physical capacity presented valid reasons to end an engagement.
If a betrothal was valid, a breach of promise claim could be presented in court.

Eduard Swoboda’s 19th century painting of a bride jilted at the altar
Reasoning
Why were such claims filed when it seems like it would be far easier, less painful, and less embarrassing for a couple to simply go their separate ways? When a promise to marry was broken, the rejected party, usually female, suffered both social and economic losses.
Socially, an engaged couple was expected to act like an engaged couple. Though it seems unfair in modern eyes, the acceptable behaviors she may have shared with her betrothed, would leave her reputation damaged if he left her. Moreover, though premarital sex was officially frowned upon, it was known that a woman was much more likely to give up her virginity under a promise to marry. But if that promise was not kept, her future search for a husband would be significantly hampered for having broken the code of maidenly modesty.
The loss of reputation translated to serious economic losses, since middle and upper class women did not work outside the home and required a household supported by a husband’s wealth. A woman with a tarnished reputation was unlikely to marry well.

An 1823 illustration of a jilted bride seeking redress from her former fiancé
Damage Awards
Perhaps as a result, a woman was far more likely to win a breach of promise claim than lose one. Middle-class ladies were generally able to obtained larger damage awards than working women, though cases varied greatly. About half of women winning damages obtained £50-£200. (For reference, middle class family of four could live comfortably on £250 a year.)
While these awards could indeed offer assistance to wronged plaintiffs, the system was also ripe for abuse. Jurors were often unduly sympathetic toward jilted women, especially when they were attractive or portrayed as particularly virtuous. Damage awards could easily be swayed by such sympathies, making false claims very tempting. All this sounds so much like modern reality television, doesn’t it?
The more things change, the more they stay the same!
Images in the public domain, via Wikimedia Commons.
About The Trouble to Check Her
Lydia Bennet faces the music…
Running off with Mr. Wickham was a great joke—until everything turned arsey-varsey. That spoilsport Mr. Darcy caught them and packed Lydia off to a hideous boarding school for girls who had lost their virtue.
It would improve her character, he said.
Ridiculous, she said.
Mrs. Drummond, the school’s headmistress, has shocking expectations for the girls. They must share rooms, do chores, attend lessons, and engage in charitable work, no matter how well born they might be. She even forces them to wear mobcaps! Refusal could lead to finding themselves at the receiving end of Mrs. Drummond’s cane—if they were lucky. The unlucky ones could be dismissed and found a position … as a menial servant.
Everything and everyone at the school is uniformly horrid. Lydia hates them all, except possibly the music master, Mr. Amberson, who seems to have the oddest ideas about her. He might just understand her better than she understands herself.
Can she find a way to live up to his strange expectations, or will she spend the rest of her life as a scullery maid?
The Trouble to Check Her is available on Amazon, Barnes and Noble, and Kobo.
About Maria Grace
Though Maria Grace has been writing fiction since she was 10 years old, those early efforts happily reside in a file drawer and are unlikely to see the light of day again, for which many are grateful. After penning five file-drawer novels in high school, she took a break from writing to pursue college and earn her doctorate in educational psychology. After 16 years of university teaching, she returned to her first love, fiction writing.
She has one husband, two graduate degrees and two black belts, three sons, four undergraduate majors, five nieces, six new novels in the works, attended seven period balls, sewn eight Regency era costumes, shared her life with nine cats through the years, and published her 10th book last year.
She can be contacted at author.MariaGrace@gmail.com. You can also connect with her at:
G+
Amazon
Random Bits of Fascination
Austen Variations
English Historical Fiction Authors


May 10, 2016
I Use Real Research to Create Believable Lies
When I first started writing the post for Unusual Historicals’ theme “My Characters Lived in…” I got nervous. Who am I to write a short blog post about Carolingian Francia, a time period that has been the subject of entire books?
Heck, I have some of those books in my home office. I can write my novels because someone else translated the medieval Latin, or studied the archaeology, or read between the lines of royal annals for clues of the truth the anonymous authors didn’t want revealed.
I just picked and chose the information most useful in crafting my fiction, which by its nature is not entirely accurate. Scholars must admit when facts are unknown. We novelists don’t have that luxury if our characters would know that information, and we must resort to making something up.
But I try to get the culture right, and although I would never want to live in eighth and ninth century Europe, its melding of the personal and political, religion and magic, provides too much fodder for an author to resist.
So as I sat down to write the post, I realized I couldn’t include everything about Carolingian Francia. I am still learning about it as I write in this period and must give deeper thought to historical events I read in passing. But I could include a few elements about this society that fascinate me from afar.
For a flavor of the era my characters lived in, see Unusual Historicals.

From a ninth century Psalter (public domain, via Wikimedia Commons)


April 27, 2016
Why I Use the Term ‘Dark Ages’
What should we call that period between roughly 500 and 1000, which includes the setting of my novels?
In my scholarly-like moments, I say early medieval times, but often, I’ll type Dark Ages.
That term riles some readers, and I can understand why. Dark Ages conjures a stereotype of a dim, smoky hall with a bunch of drunken, grunting guys who’d start knife fights at the slightest offense. While early medieval times were hardly ideal by my 21st century standards, I don’t like this oversimplified version of history, and I get irritated with questions like, “But how could those barbarians have art?”
True, most of the population couldn’t read, many children died of disease before age 5, teenage girls were married to older men, and wars were common. But we could say that about a lot of societies and eras. This time period did have its share of intellectuals, trade, art, poetry, and complicated politics, and its women were far from weak willed—they were protectors of their sons’ rights, supporters of political alliances, diplomats, and missionaries.
So why do I use Dark Ages while railing against the connotations? Readers recognize the term easily, especially in the limited space of a title or a social media post, and Dark Ages is more likely to get attention.

From the ninth century Folchard Psalter (public domain, via Wikimedia Commons)


April 20, 2016
Two Labs across the Atlantic Prove That Electrons Behave Like Waves
In this installment on the history of atom theory, physics professor (and my dad) Dean Zollman discusses how two separate teams an ocean apart proved Louis de Broglie right about matter sometimes behaving as waves rather than particles.—Kim
By Dean Zollman
 In the last post, I discussed Louis de Broglie’s radical hypothesis that matter sometimes behaved as waves. De Broglie did not have any direct evidence for his theory. However, he was able to show how his ideas could be connected to Niels Bohr’s model of the atom. Once de Broglie introduced these ideas, both theoretical and experimental progress occurred rather quickly. In this post, I will discuss the experiments that were able to conclude that de Broglie was right. Next time, we will look at the major theoretical advance in the 1920s. (This approach is not quite chronological, but many things happened and influenced each other over a short time.)
In the last post, I discussed Louis de Broglie’s radical hypothesis that matter sometimes behaved as waves. De Broglie did not have any direct evidence for his theory. However, he was able to show how his ideas could be connected to Niels Bohr’s model of the atom. Once de Broglie introduced these ideas, both theoretical and experimental progress occurred rather quickly. In this post, I will discuss the experiments that were able to conclude that de Broglie was right. Next time, we will look at the major theoretical advance in the 1920s. (This approach is not quite chronological, but many things happened and influenced each other over a short time.)
As I have discussed previously, the fundamental property that distinguishes waves from particles is interference. When two particles meet, they bounce off each other. Two waves can reinforce each other (constructive interference), cancel each other (destructive interference), or have some result between these two extremes.
To observe interference experimentally, we need to arrange for waves to move along two different paths and then meet. The distance that each wave travels determines the type of interference. In one method, the wave goes through two openings simultaneously and then combines. In another, the waves reflect off two surfaces that are very close to each other.
This second method is more common in everyday life. When you look at a soap bubble or at a puddle with a thin layer of oil on it, you frequently can see colors, with each color at a different location. The colors are created by some light reflecting from the top of the oil and interfering with light that travels through the oil and reflects from the water. The colors appear because each color has a different wavelength and thus constructive interference occurs at different places. Light passing through opening is less common in everyday life. Although, you may have seen an interference effect by looking at light through a fine lace curtain.

Interference on a soap bubble (by Cosmic73, Creative Commons BY-SA 4.0, via Wikimedia Commons)
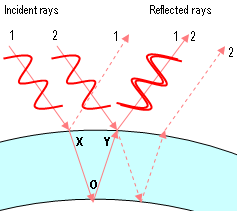
Paths of light for a thickness of a bubble where red light interferes constructively (Theresa Knot, GNU Free Documentation License via Wikimedia Commons)
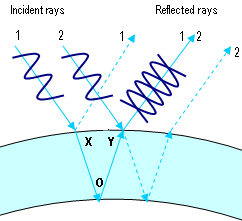
Paths of light for a thickness of a bubble where blue light interferes destructively (Theresa Knot, GNU Free Documentation License via Wikimedia Commons)
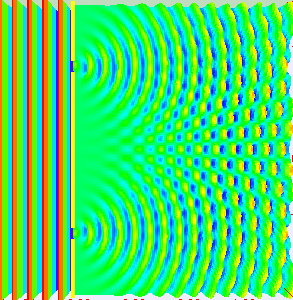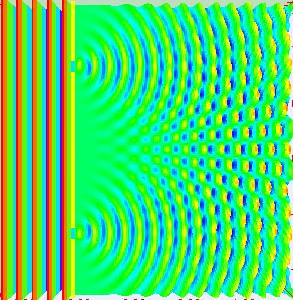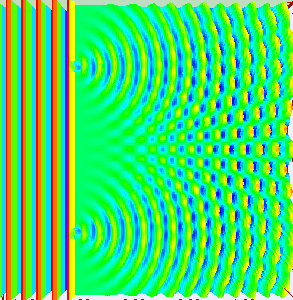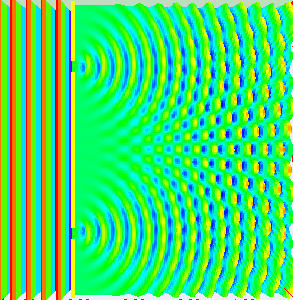
Animation showing light passing through two openings and interfering as the light from the two openings meet. (By Lookang, with many thanks to Fu-Kwun Hwang and authors of Easy Java Simulation: Francisco Esquembre, CC BY-SA 3.0, via Wikimedia Commons)
To show that particles have wave properties, physicists needed to observe some type of interference with particles. Then, they would need to compare the results of the interference with de Broglie’s predictions. The most logical particle to start with was the smallest one known at that time—the electron.
The biggest difference when we consider electrons instead of light is the size of the objects and wavelengths. To cause interference, the sizes need to be approximately the same as the wavelength of the object doing the interfering. The wavelength of light is quite small but within the general range of the thickness of a soap bubble or an oil slick. One can also make openings for light to pass through by using a razor blade carefully. But using de Broglie’s result, we find that the wavelength of an electron is much smaller than that of visible light. It is similar to the wavelength of X-rays.
The solution to this problem is to use crystals. In a crystal, such as table salt, the atoms line up in nice, neat patterns. The layers of atoms create surface from which waves can reflect while the spaces between atoms can be used for opening through which waves can pass. The distances between atoms in the crystal are the sizes we need to observe interference.
Two research efforts—one in the U.S., the other in the U.K.—independently worked on this experiment. In his Nobel Prize lecture one of the experimenters, Clinton Davisson, described the two locations as “… in a large industrial laboratory in the midst of a great city, and in a small university laboratory overlooking a cold and desolate sea.” The experiment in the large industrial laboratory used the reflection method while the one near the cold and desolate sea used transmission through small openings. Both involved crystals.
The effort in the United States took place at Bell Labs. This laboratory was established in 1925 by the old version of AT&T. Its general mission was to conduct research to improve communications. However, a large amount fundamental research also took place. Over many years, many important scientific discoveries were made at Bell Labs, and some significant devices which are now part of our everyday life (and not just telephones) were invented there. The Wikipedia article on Bell Labs lists these achievements and the Nobel Laureates who worked there.
Clinton Davisson (1881–1958) began working for Western Electric, a division of AT&T, during World War I. After the war, he remained with the company and joined Bell Labs when it was founded. Lester Germer (1896–1971) worked for Western Electric for just a couple of months before entering military service in World War I. During the war, he was one of the first airplane pilots to see action on the front. After the war and a short rest, he was rehired by Western Electric and assigned to work with Davisson. They were assigned projects to look at some aspects of metals that were used in parts of the telephone company’s amplifiers. One aspect of this work involved shooting electrons at the metals. They were looking at how the electrons bounced off metals to better understand the metals’ properties. (Physicists use the phrase elastic scattering of electrons rather than bouncing off.) Wave behavior of electrons were not part of the research agenda. And for a while, they did not see any such behavior.

Clinton Davisson (left) and Lester Germer in 1927 hold the apparatus that they used to detect electron interference (public domain via Wikipedia Commons).
[image error]
Here’s a schematic drawing of the Davisson-Germer experiment. The electrons’ speeds are set by the apparatus in the lower left. The movable detector looks at the number of electrons coming off at different angles (by Roshan, CC BY-SA 3.0, via Wikimedia Commons).
Davisson described how they became interested in looking at the details of the electron scattering. “Out of this grew an investigation of the distribution-in-angle of these elastically scattered electrons. And then chance again intervened; it was discovered, purely by accident, that the intensity of elastic scattering varies with the [angle].” This change in intensity with angle was much like the different colors of light appearing at different angles when it reflects from the surfaces on a bubble. The phenomenon implies an interference pattern.
As Davisson and Germer investigated the scattering of electrons from crystals, they completed 21 different experiments. For each experiment, they could accelerate the electrons to a speed that they knew. So they also knew the electron’s momentum. In turn, they could calculate the wavelength of the electron and determine at which angle the constructive interference should occur. They compared their results for angle of the maximum number of electrons with the angle predicted by using de Broglie’s wavelength. For 19 of the experiments, their results matched extremely well with the wavelength predicted by de Broglie.

George Paget Thomson (by Nobel Foundation, public domain, via Wikimedia Commons)
At about the same time George P. Thomson (1892–1975), son of the J.J. Thomson, who discovered the electron, was conducting a similar investigation in Aberdeen, Scotland. His experiment involved transmitting electrons through a crystal. In his Nobel lecture, Thomson said, “A narrow beam of [electrons] was transmitted through a thin film of matter. In the earliest experiment of the late Mr. Reid, this film was of celluloid, in my own experiment of metal. In both, the thickness was of the order of 0.000001 cm. The scattered beam was received on a photographic plate … and when developed showed a pattern of rings … An interference phenomenon is at once suggested.”
The picture below shows the result of a modern version of Thomson’s experiment, one that is done by many college physics majors. The light and dark circle are locations where constructive and destructive interference of electrons (respectively) occur.

A modern version of Thomson’s experiment shows rings similar to the ones that he saw in his photographs (by And1mu, CC BY-SA 4.0, via Wikimedia Commons).
The results indicated that electrons had wave-like behaviors. As with the Davisson-Germer experiment, Thomson accelerated the electrons to a speed that he knew very well. So he could use de Broglie’s hypothesis to determine the wavelength. Using that wavelength, he predicted where the bright rings should occur in the photographs. The agreement between predictions and measurements were “within 1 percent.” G.P. Thomson had shown that indeed electrons behaved as waves.
These two experiments definitively established the wave behavior of matter. This behavior is at the foundation of most of contemporary physics and is critical to the development of many devices that today we take for granted. However, measuring the wave behavior was just one important step. Next time, we will look at the major theoretical advance that was underway at about the same time as these experiments.
Dean Zollman is university distinguished professor of physics at Kansas State University where he has been a faculty member for more than 40 years. During his career he has received four major awards — the American Association of Physics Teachers’ Oersted Medal (2014), the National Science Foundation Director’s Award for Distinguished Teacher Scholars (2004), the Carnegie Foundation for the Advancement of Teaching Doctoral University Professor of the Year (1996), and AAPT’s Robert A. Millikan Medal (1995). His present research concentrates on the teaching and learning of physics and on science teacher preparation.
Previously
What Are Things Made of? Depends on When You Ask.
Ancient Greeks Were the First to Hypothesize Atoms
Religion, Science Clashed over Atoms
Medieval Arabic Scholarship Might Have Preserved Scientific Knowledge
Rediscovering a Roman Poet – and Atom Theory – Centuries Later
Reconciling Atom Theory with Religion
Did Atom Theory Play a Role in Galileo’s Trouble with the Inquisition?
Did Gifted Scientist’s Belief in Atoms Led to His Obscurity?
Does Atom Theory Apply to the Earthly and the Divine?
Isaac Newton: 300 Years Ahead of His Time
Issac Newton and the Philosopher’s Stone
When Chemistry and Physics Split
Mme Lavoisier: Partner in Science, Partner in Life
With Atoms, Proportionality and Simplicity Rule
Despite Evidence of Atoms, 19th Century Skeptics Didn’t Budge
Mission of the First International Scientific Conference: Clear up Confusion
Rivalry over the First Periodic Table
The Puzzle of Dark Lines amid Rainbow Colors
The Colorful Signature of Each Element
Even Scientific Dead Ends Can Contribute to Knowledge
Discovery of the Electron Took Decades and Multiple Scientists
The Accidental Discovery of Radioactivity
Marie Curie: A Determined Scientist
Pierre and Marie Curie Extract Radium – and Pay a High Price
Scientists Delve into Radioactivity and Make Their Own Discoveries
The First Attempts to Visualize Atoms
Did Busy Work Lead to Models for Atoms?
Why Does Ice Melt? The Answer Lies in Physics.
Einstein Explains How a Dim Light Can Release More Energy Than a Bright One
How Bohr’s Famous Model of the Atom Was Created
Bohr’s Model of the Atom Answers Fundamental Questions – but Raises More
Bohr’s Model of the Atom Draws Critics
‘A First Feeble Ray of Light’ to Explain Electrons’ Orbits


April 13, 2016
Did the Queen Help Create Charlemagne’s Intellectual Circle?
When Charlemagne decide to travel to Rome for important business in 780, he took his wife, Hildegard, with him, and she likely did more than provide companionship.
She was an Agilolfing, a member of the powerful, established family that ruled Bavaria. And if she was like most medieval women, she wanted the inheritance to go her sons, not the offspring from Charles’s first marriage.
At that time, Charles had reigned 12 years and was 32, no longer a young man by medieval standards. It might have been time to think about their children’s future. When Charles and Hildegard reached Rome in 781, their two youngest boys, ages 3 and 4, were anointed subkings, and their 6-year-old daughter was betrothed to the child emperor of Byzantium.
Another indication that the royal couple was thinking long term: when they returned to Francia, they brought scholars with them. If they wanted their subjects, and their enemies, to associate their realm with ancient Rome, they needed intellectuals. For more about learned men in Charles’s court, see my post in Unusual Historicals.

A 1499 illustration of Hildegard and Charlemagne (public domain via Wikimedia Commons)


April 5, 2016
Sympathy for the Monster’s Mom
Whoever wrote Beowulf probably didn’t intend for the audience to feel sorry for a killer.
Yet I must admit I sympathize with Gendel’s mother, called a “monstrous hell-bride” and “troll-dam” among other names. Her murderous son is the worst possible enemy for the residents of Heorot Hall—a joyless being who cannot be appeased. So, the only way to stop him is to slay him. When Beowulf fatally wounds Grendel, we understand why Heorot Hall celebrates.
Yet we can also understand why a mother who is “grief-racked and ravenous, desperate for revenge” would make Heorot Hall pay a bloody price.
Grendel and his mother are ingenious literary creations, blending pagan and Christian beliefs. For more, see my post at English Historical Fiction Authors.
Source
Beowulf, translated by Seamus Heaney

From Jean Lang’s A Book of Myths, 1915 (public domain, via Wikimedia Commons)


March 22, 2016
An Ex-First Lady Who Founded Something Bigger
When I was thinking of a real-life character to feature for Unusual Historicals’ “First Ladies” theme, Saint Cuthburga came to mind.
First lady is defined as the wife of a political leader or the first woman recognized for a particular accomplishment. In this case, Cuthburga is the ex-wife of a seventh-century Northumbrian king.
Usually, a divorce in the Dark Ages was an insult to the woman and her family, so it was not done lightly. When Charlemagne parted from his second wife in the eighth century, he ended up going to war with his former father-in-law. As for Himiltrude, the first ex-wife, the consequences of that separation are the subject of my work in progress, Queen of the Darkest Hour. (Whether Himiltrude was a concubine or a wife is a topic that deserves its own post.)
No one questions whether Cuthburga was a queen, although for how long is up for debate. Also the sister of a king, she founded Wimborne Minster at Dorset after she left her husband, and that decision would have an impact beyond her lifetime. Read my post about Cuthburga on Unusual Historicals.

A ninth century Anglo-Saxon woman of rank, from Kretschmer and Rohrbach’s Costumes of All Nations (public domain)






