Jay L. Wile's Blog, page 32
May 9, 2017
Read, PZ, Read!
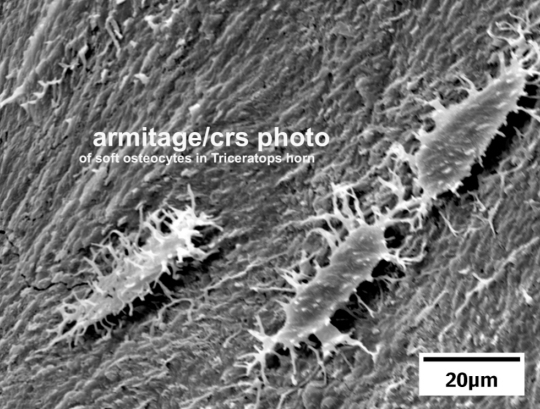
Electron microscope image of three soft bone cells from a dinosaur fossil
(image by Mark Armitage)
As I indicate in my “Links to Investigate” list on the right, one of the few blogs I read regularly is written by Dr. PZ Myers. While I disagree with nearly everything he writes, I do think he provides an entertaining, “new atheist” perspective on science. Of course, if you dislike foul language, it is best to avoid his blog. Some people are adept at defending their point of view with reason and intelligence, while others specialize in crude insults. It is rather obvious which camp Dr. Myers is in.
Imagine my delight when I was doing my “light reading” this past weekend and found that a blog post of mine was the focus of one of his diatribes! The post is entitled, “Think, creationists, think,” and it is an attempt to discredit Mark Armitage’s excellent work of isolating soft cells from a dinosaur fossil. The problem, of course, is that Dr. Myers didn’t actually bother to read up on the issue, and as a result, he makes some rather silly statements.
For example, when discussing the carbon-14 found in Armitage’s Triceratops fossil, Dr. Myers claims that someone with a PhD in nuclear chemistry (like me) should know that the presence of carbon-14 in the fossil means nothing. After all,
C14 dating uses the ratio of carbon isotopes; it can’t be used on material above about 50,000 years because the quantity of carbon-14 is too low to be reliable, not because it’s nonexistent. If the bone was really young, you wouldn’t just be reporting that there was some C14 in it, you’d be reporting an age derived from a ratio.
Well, had Dr. Myers bothered to click on the link given in my post, he would have seen that an age was reported: 41,010 ± 220 years. As I state in that link, this is well within the accepted range of carbon-14 dating, and it is younger than many other carbon-14 dates published in the literature. In addition, the process used to make the sample ready for dating has been spelled out in the peer-reviewed literature, and it is designed to free the sample of all contamination except for carbon that comes from the original fossil. Now as I said in my original post, it’s possible that the reading comes from contamination. However, I find that unlikely, given the process used on the sample, the cellular evidence that Armitage found, and the fact that such carbon-14 dates are common in all manner of fossils that are supposedly millions of years old or older.
Not only did Dr. Myers not take the time to read about the carbon-14 evidence, he didn’t take the time to carefully read my blog post, because he seems confused about what it says. He says that I claim there was no chemical process needed to isolate the bone cells, but then I turn around and say that there was a chemical process needed. However, in my original post, I clearly am discussing completely different images in each case. I begin by discussing the first image in Armitage’s Microscopy Today article, which is from his Triceratops horn. I tell the reader that no chemical process was needed to obtain the soft, stretchy fibrillar bone tissue that was shown in the image, which comes from a light microscope. I then inform the reader that bone cells (osteocytes) can be seen embedded in the tissue.
After that, I discuss several other images, including images from a different fossil (a Triceratops rib). Then I discuss the final two images. In that discussion, I indicate that a chemical process was used to isolate individual bone cells so that they are free of any surrounding tissue. Obviously, then, no chemical process was required to get the light microscope image of osteocytes embedded in the bone tissue. However, a chemical process was required to isolate them so that their individual images, free of the surrounding tissue, could be shown.
Dr. Myers’s main complaint, however, is that all of this is the result of contamination. To bolster this claim, he quotes me quoting Armitage. As Armitage admits, plant roots, fungal hyphae, and insect remains are found in the fossil. Dr. Myers replies:
Unbelievable. Utterly unbelievable. He just compromised his own results, and Wile obliviously calls this the “most important part of the article.” I agree, but not for the reasons Wile thinks.
Armitage did not compromise his own results. He simply wrote truthfully about his fossil. In addition, anyone with a basic understanding of histology would know why plant roots, fungal hyphae, and insect remains do not compromise his results in any way. Based on all the visual evidence, the cells he found are osteocytes. They are not only the shape and size one expects from osteocytes, they have the filipodial extensions that are characteristic of osteocytes. They also have the cell-to-cell junctions one expects in groups of osteocytes. Thus, they cannot be the result of contamination, since plants, fungi, and insects do not have osteocytes.
Of course, had Dr. Myers bothered to read my post carefully, or had he read Armitage’s peer-reviewed article, or had he read Armitage’s Microscopy Today article, he should have realized all that. But then again, why bother to read, when you aren’t willing to consider the evidence in the first place?
May 5, 2017
Scientist Isolates Individual Dinosaur Cells!

A single bone cell isolated from a Triceratops fossil. (Photo by Mark Armitage)
If you follow young-earth creationism at all, you probably know about Mark Armitage. In 2013, he and Kevin Anderson wrote a paper in which they imaged soft bone cells from a dinosaur fossil that is supposedly 65 million years old. This challenges the old-earth dogma pronounced by the High Priests of Science, and Armitage was happy to discuss that fact with students at the university where he worked. However, to teach such heresy is an excommunicable offense, so the Inquisition fired him from his university position. He eventually won a lawsuit against them.
Despite the hard work of the Inquisition, Armitage is still investigating his amazing discovery. A couple of years ago, for example, a sample of the fossil was analyzed for carbon-14 content. If it really is 65 million years old, there should be no carbon-14 in the fossil. Nevertheless, carbon-14 was found. Of course, there is always the chance that the carbon-14 is the result of contamination, but combined with the presence of soft bone cells, it seems obvious to me that the fossil is significantly younger than 65 million years!
Somehow, I missed the latest update on Armitage’s incredible work. In the January, 2016 issue of Microscopy Today, he published more results from his painstaking analysis, and they are truly amazing.
He starts off his paper with a microscope image of the soft tissue that was found in his fossil Triceratops horn. This soft tissue didn’t require any chemical procedure to isolate. It was simply there, inside the horn. He describes the sample as “soft, stretchy fibrillar bone,” and the light microscope image clearly shows the bone cells embedded in the tissue. Thus, this isn’t some biofilm left behind by bacteria or some other form of contamination. This is soft bone tissue from the horn itself, as evidenced by the bone cells embedded therein.
He then shows two stunning images from a Triceratops rib. The first clearly shows a soft blood vessel extending outward, and the other shows spherical microstructures found inside a blood vessel. The microstructures are consistent with red blood cells! He also shows several images from the horn. They show many intact, soft bone cells with tiny extensions that are also soft.
While all of these images are incredible, he saves the best for last. Using a six-week process involving a weak acid, dialysis tubing, and distilled water, he was able to isolate individual bone cells. Look at the photo at the top of this post. It is of an individual Triceratops bone cell, as seen with a standard light microscope. The final two images of Armitage’s paper show two bone cells like the one above. They are free of any surrounding tissue, and one of them shows what appears to be the cell’s nucleus! If I hadn’t been told that these cells came from a Triceratops fossil, I would think they had come from a living animal’s bone tissue.
While the images themselves are stunning, and his accomplishment in isolating individual dinosaur bone cells is quite noteworthy, I found one of his statements to be the most important part of the article:
The remarkable preservation of delicate ultrastructures such as filopodia and cell-to-cell junctions (white arrows, Figures 6 and 7) has resisted a simple explanation despite hypothesized temporal limits on molecular preservation over millions of years. In the case of soft vessels recovered from dinosaur femur specimens, it seems reasonable that these tissues were sequestered from the elements and from biological scavenging activity because of deep encapsulation within compact bone. Within the Triceratops horn, however, which was highly vascular, no sequestration was likely because all of the vessels were openly exposed to air, soil, water, scavengers, dissolved salts and minerals, and the freeze-thaw cycle and heat of Montana seasonal weather; yet a high degree of preservation persists. While plant roots, fungal hyphae, and insect remains were all found traversing the horn, soft fibrillar sheets of bone and well-preserved osteocytes remain.
In other words, for some soft tissue discoveries, such as the work done by Schweitzer and others, you can imagine that the tissues were at least protected from decomposition because they were found inside an intact bone. That wasn’t the case for his specimen, however. Plant roots, along with the remains of insects and fungi, show that the interior of this fossil was exposed to the elements. Nevertheless, soft bone tissue and soft cells from the Triceratops are still there!
Mark Armitage’s work is some of the most important research being done from a creationist perspective right now, and his facility is a unique creationist resource. I hope he can find enough financial support to keep it going!
May 1, 2017
No, I Did Not Write Apologia’s Elementary Science Books
The cover from one of the many excellent elementary science books authored by Jeannie Fulbright. Her name is magnified so you can see it clearly.
It has been a while since I have been able to blog, because I have been busy speaking at homeschooling conventions. This past weekend, for example, I spoke at the MassHope convention in Worcester, Massachusetts. I haven’t been at that convention for several years, and it was great to be back! It is an excellent event, with lots of great speakers and a fine facility.I had an experience at the convention that I would like to share, because some version of it has occurred over and over again for many, many years. While its frequency has decreased, it is still something that happens regularly at the homeschool events which I attend. In between my talks, I was sitting at my publisher’s booth so I could speak with people one-on-one. A mother came up to me with a confused look on her face. She looked down at my elementary books (which were on the table), looked up at me, and the following conversation took place:
Mother: What are these?
Me: They are my elementary science courses.
Mother: But they aren’t from Apologia.
Me: No. They are published by Berean Builders
Mother: But you wrote the Apologia books.
Me: Apologia publishes most of my junior high and high school courses, but Berean Builders publishes my elementary courses and my high school chemistry course. I did not write the Apologia elementary courses.
Mother: Of course you did.
Me: No, I did not. They were written by Jeannie Fulbright.
Mother: (still a bit confused) We use the Apologia elementary books, and I thought you wrote them.
Me: No, Jeannie Fulbright wrote them. She and I have similar writing styles, but she wrote the Apologia elementary courses.
To some extent, I understand the confusion. My wife and I started Apologia with my high school science courses. As a result, my name became strongly associated with the company. Eventually, however, it started publishing the works of other authors, including Jeannie Fulbright’s excellent elementary courses. Her name has always been on the cover (shown above), spine, and title page of each of her books. When I was still with Apologia, it at least made sense that people who had never used her books would think that I had written them, since I was still the “face” of the company.
However, my wife and I sold the business in 2008. I had planned to stay with the company for ten years, but I quit after only two. So it has been more than 8 years since I have worked for Apologia. Nevertheless, despite the fact that I do not work for Apologia, and despite the fact that Jeannie Fulbright’s name is on all its elementary books, people still seem to think that I have written them. That is simply not true. I did edit most of her books, but that is all. The books are a result of Jeannie Fulbright’s hard work and obvious talent.
Now please understand why I am writing this. It’s not because I dislike her books. In fact, I think that her elementary science books are excellent. She has a way of explaining scientific truths that is easy to understand and enthusiastic. Many children throughout the years have told me how much they enjoy her books, and I can see why. Because her books are so good, it is wrong for me to get credit for them. She should get the credit that she so richly deserves.
Well, if her books are so great, why would I write my own series of elementary courses? Because like most curricula, her books suit one kind of student very well, but there are other kinds of students. I wrote my books to meet the needs of at least some of those students. So her books are not better than my books, and my books are not better than her books. Our books are different, and they are both excellent – for different kinds of students.
How do these two sets of books meet the needs of different students? First, her books are topical. The book pictured above, for example, covers the animals that live on land. The student spends an entire year studying just those kinds of animals. For some students, that is ideal. They love “diving into” one subject and studying it for a long time. My books follow history. Instead of concentrating on a subject, the books discuss science as it was discovered. This means you are always studying a different scientific topic, but you are actually seeing how it was figured out. For students who do not like concentrating on one thing for a long time, and for students who love history, my courses are more ideal.
Second, Jeannie’s books are written like “living books.” They are designed for the family to sit together and read aloud. There are some hands-on activities, but they are not the focus of each book. In addition, some of those hands-on activities are long projects that might take several days to complete. My books, on the other hand, are focused on the hands-on activities. Every day you do science, you do something with your hands, and usually, the activity is short and to the point. As a result, there is less reading and more “doing” in my books. For some students, Jeannie’s approach is better. For others, my approach is better.
Third, Jeannie’s books are free-flowing. Her lessons are each a chapter long, so families must decide where to start and stop each time they want to do science. There are reading schedules to help them make that decision, but in the end, it is something that has to be determined. In my books, the lessons are already determined. Each day you do science, you start at the beginning of the lesson, and you end when the lesson ends. There are 90 lessons in each book, 12 of which are “challenge lessons” that can be skipped.
So when I talk with homeschooling parents about elementary science, I don’t just say, “You should use my courses.” I try to get to know what kind of children they have, and then I recommend what I think is best for them. Sometimes, it’s my books. Sometimes, it’s Jeannie’s books. Sometimes, it’s a completely different curriculum altogether. While I recommend a wide variety of curricula, the only ones I have written are the ones that have my name on the cover.
April 17, 2017
A Series of Unorthodox Easter Skits

Me as the devil and Emma as my right-hand demon. (photo by Kim Williams)
Easter is the most important holiday in Christendom. As Scripture tells us, “and if Christ has not been raised, then our preaching is vain, your faith also is vain.” (1 Corinthians 15:14). In the church I attend, we always try to do something very special on Easter Sunday, and this Easter’s service was particularly meaningful to me. More than a month ago, the pastor asked if I could work up a series of skits that would augment his sermon. We had done something very similar for the service on Christmas day, and the congregation really seemed to appreciate it. So the pastor and I exchanged some ideas, and I ended up writing a series of very unorthodox skits that we presented throughout his message.His sermon was based on three gardens (Eden, Gethsemane, and the Garden Tomb). His overall message was that the tragedy of what happened at the Garden of Eden has been erased by the sacrifice that started at the Garden of Gethsemane and the victory of the resurrection that took place at the Garden Tomb. It’s difficult to write skits about such well-known events, so I often try to write from a unique perspective. With the pastor’s permission, I decided to write these skits from the Devil’s perspective. The four skits will appear below.
This was all laden with emotion for me, because it was the first time I had done a skit since my right-hand man in the church’s drama ministry passed away. I wanted to do something that made it clear how important this step was, so I hesitantly asked his teenage daughter, Emma, if she would do the skits with me. She agreed and did a great job. I really couldn’t have asked for it to go much better, and the congregation appreciated both the content of the skits and the significance of the event.
Feel free to use these skits in any way that the Lord leads. If possible, I would like a credit, but the most important thing is to use them to minister to the Body of Christ.
(Since these skits occurred during the sermon, the set had to be simple and something that could be left on stage. There also had to be a transition between the pastor speaking and the skits themselves. Since our church lighting system has black light, I purchased a cheap black light poster that had some deformed skulls on it, as shown in the picture above. I took an old end table and painted it with black light paint. I did the same with an old phone that was put the table. I also worked with the lighting so that when the pastor preached, the congregation’s lights were on and he was in a pool of light. The table, phone, and poster were behind him, along with a fancy chair. They weren’t in the pastor’s light, so they were de-emphasized while he was speaking. When each skit started, the pastor’s lights and the congregation’s lights went down, and the black lights came up. This made the poster, table, and phone glow, indicating that the congregation was being “taken” into hell. When we came out, the acting lights came up, and the scene began.)
Skit #1: Shortly After the Garden of Eden
(A young demon comes in and sits in the chair. She puts her feet up and sighs, indicating that this is where she thinks she should be. She hears footsteps and immediately jumps out of the chair and stands at attention. The Devil enters.)
Demon: (trying to be nonchalant) How did it go?
Devil: Better than I could have imagined! These things the Enemy created…what do you call them?
Demon: People.
Devil: Right…people. People are stupid!
Demon: How so?
Devil: They had this amazing garden. It was lush and beautiful, and the climate was perfect. They had more food than they could ever eat, and they even had animals they could play with. It was incredible. The Enemy gave them just one rule. Just one, simple rule.
Demon: What was it?
Devil: There was one particular tree they couldn’t eat from. There were hundreds of trees in the garden, all with luscious fruits, and they just had to stay away from that one tree. So you know what my job was?
Demon: To get them to eat from that tree.
Devil: Of course. And I did it. It was a piece of cake.
Demon: How?
Devil: Well these….what do you call them again?
Demon: People.
Devil: Right. These people are easily confused. First, I just sowed a little doubt. I asked, “Did he really tell you not to eat from that tree?” While they were thinking about that, I pounced on their desire for power. I said, “You know, the only reason he doesn’t want you to eat from the tree is because you will become like gods yourselves.” That’s all it took. They ate!
Demon: Congratulations, Boss!
Devil: You should have seen the Enemy! He was so upset. He kicked them out of the garden, and that made them miserable. (truly enjoying the memory) I had no idea how much I would enjoy making them miserable! Yes, I think I am going to enjoy making….(looking to the demon to remember the word)…people…miserable for the next few thousand years!
Demon: So…what do we do now?
Devil: Well, the Enemy said something about someone coming along to crush my head, but honestly, I think he was just trying to save face. (proudly) It’s clear I really blindsided him. Nevertheless, it’s better to be safe than sorry. You’re speciality is seeing into the future, right?
Demon: Right.
Devil: So what have you seen lately?
Demon: Well…it’s all a bit hazy, and I’m not sure exactly what it means, but in the future, there are going to be two words – TWO WORDS – that will be associated with a lot of pain and misery.
Devil: (Brightening) Really? What are they?
Demon: United Airlines
Devil: Ah…Well…you need to keep an eye on that. Also, start looking into the future to see if the Enemy really is going to try to send someone who will crush my head.
Demon: Yes, boss.
Devil: Now realize that I am putting you in charge of this, and when I put a demon in charge of something, I expect it to be done right. Do you understand?
Demon: I understand.
Skit #2: Shortly After the Garden of Gethsemane
(When the acting lights come up, the phone rings. I used a few seconds of an instrumental version of the chorus from “Highway to Hell.” If you don’t use something like that, the reference to the ringtone needs to be cut.)
Devil: (picking up the phone) I really love that ringtone. (like a help desk operator) This is the Devil, how may I harm you today? (pause) What do you mean you’re down 10 degrees? That’s not acceptable. I’ll call engineering. (hangs up, dials a number) Engineering? Torment just called. They say they’re down 10 degrees. Now you know as well as I do that there is a sweet spot when it comes to burning souls. It can’t be too cold; it can’t be too hot. It needs to be just right, and torment says they’re 10 degrees below that sweet spot. I want it fixed. (pause) I don’t care what’s going on down there. You have an entire lake of fire. I think you can figure out how to give them 10 more degrees. I better not hear from torment again. (hangs up) Why do I have to do everything around here?
(demon enters)
Devil: What are you doing here? You’re supposed to be keeping watch on the Enemy’s son.
Demon: I don’t think I need to anymore.
Devil: Why?
Demon: He’s been taken into custody.
Devil: What?
Demon: Guards came and took him into custody. They locked him up. He’s not free any more.
Devil: Did the disciples put up a fight?
Demon: Not really. (amused by the memory) This one guy, Peter, he pulled out this sword. (chuckling) He clearly didn’t know how to use it. He was swinging it around and actually managed to cut a guard’s ear off!
Devil: That’s cool!
Demon: (really into it) Yeah. There was blood everywhere. It was awesome. But then the Enemy’s son told him to stop fighting and actually healed the guy’s ear. Stuck it right back on his head.
Devil: Leave it to the Enemy’s son to ruin a perfectly good maiming.
Demon: I know, right?
Devil: So did the disciples follow him when he got taken away?
Demon: No. They just scattered. I decided to follow that Peter guy, cause he’s so funny. He did follow the Enemy’s son, but when another person asked him if he knew the Enemy’s son, he said no.
Devil: Wow. It sounds like they’re completely demoralized. I doubt there will be a rescue attempt. Does that mean it’s all over? Have we won? You know, I have to admit that I owe this all to you. You were the one who looked into the future and saw this coming. That allowed us to make our plan. Boy, do I love it when a plan comes together! (pause, not sure if he has really won) But you know, the Enemy is tricky. He may have something up his sleeve. You go back and keep an eye on the Enemy’s son. Don’t come back here until you are CERTAIN that he won’t be a problem anymore.
Demon: Yes, sir!
(Acting lights out, black light still up. Devil and demon exit. Then the preaching lights come back up.)
Skit #3: Shortly After the Cross
(The Devil is sitting in his chair, and once the acting lights are up, the demon enters.)
Devil: (Looks up and is surprised) Does this mean it’s over?
Demon: Yes.
Devil: You’re sure?
Demon: Yes. He’s dead.
Devil: (really surprised) Dead?
Demon: Yep. They put him in a tomb and closed it up.
Devil: (starting to think he has won) Wow. There’s no coming back from that!
Demon: Exactly.
Devil: (really getting happy) So that’s it. We won. I can’t believe it. We won again. I have to let everyone know about this. (picks up phone and hits a key). Intercom. For those of you keeping score, it’s the Devil 2, the Enemy 0! (He hangs up phone and starts to come down from the high. He looks into the distance and sighs.)
Demon: What’s the matter?
Devil: Well…I’ve just been doing this for so long…fighting the Enemy’s plan…I don’t know what to do now. I mean, what is there left to do?
Demon: (brightens up) You should take a vacation.
Devil: (This makes him happy, too.) What a great idea! That’s exactly what I need. A vacation. I have been working so hard at this for thousands of years. I need a break. (picks up the phone) Kitchen, I am taking a little trip, and I’ll need some supplies. I want some deviled eggs, some devil’s food cake, extra hot salsa, and all the Mexican food you’ve got. I’ll be down in a few minutes. (He hangs up the phone and looks at the demon.) Well…now it’s time for your reward. I told you before, I couldn’t have done this without you. So…(indicates the chair) take a seat!
Demon: (realizes this is a promotion) All right!
Devil: You are now the Acting Devil!
Demon: I am honored! (The devil starts to leave, but she calls him back) Wait a minute. In case I need you, where will you be?
Devil: Where else? I’m going down to Georgia!
(I had the sound people play the intro to “Devil went down to Georgia” by Charlie Daniels after the devil said that line and exited. It got a good laugh. Acting lights out, black light still up. Demon exits. Then the preaching lights come back up.)
Skit #4: Shortly After the Resurrection
(The demon is sitting on the chair enjoying her position. The phone rings again, and if you are using a fun ringtone, the demon enjoys it.)
Demon: What a ringtone! (answers the phone) Acting Devil, how may I harm you today? (pause) What do you mean he’s alive? (pause) Shouldn’t he be in the tomb? (pause) Then where is he? (pause) Yes, the boss needs to know about this. Why don’t you tell him? (pause) What do you mean I’m Acting devil? (pause then sigh) I suppose you’re right. Someone has to tell him. (hangs up the phone and stands) He is not going to like this. (looks around and then out into the audience) How did I not see THIS coming?
(Acting lights out, black light still up. Demon exits. Then the preaching lights come back up.)
April 10, 2017
Can a Homeschooled Child Take an Extra Year of High School?

A group of high school graduates (click for credit)
This past weekend, I spoke at the Alberta Home Education Convention in Canada. As far as I know, it is the largest home education convention in Canada, and I think I have spoken there only once before, way back in the year 2000. It was really wonderful to go back. I met several parents who told me they remembered me from 17 years ago, and that I encouraged them to continue on in their homeschool journey. Their children are now in high school, at university, or in the real world, and they are very happy with their decision to continue homeschooling.
One of the kind souls who drove me around actually told me his son’s story, which is worth retelling here. He graduated homeschool many years ago and wanted to attend a major Canadian university. At that time, the university did not accept homeschool applicants. However, the student’s family knew someone on the inside, and that person was able to convince the university to accept him. At first, the university did not allow him to take any courses related to his desired major, because the administrators thought that homeschooled students “just played with Play-Doh all day.” As is generally the case, this homeschool graduate excelled, and the university quickly changed its tune. After he graduated with a 4.0 GPA and a pile of honors, the university asked him to help them write their admissions policy for homeschooled students.
I spoke several times at the convention, and the audiences were very appreciative. I always try to leave time at the end of my talks for questions from the audience, and I succeeded for every talk except one. Many of the questions related to very specific cases, but I got one question that I think could apply to everyone, so I decided to discuss it here. At the end of one of my talks, I was asked whether or not a homeschooled student could take a fifth year of high school. The mother thought that for one of her children, an extra year of high school would do a lot of good, but she was concerned that it might look odd to a university.
I told this mother that I think one of the great benefits of homeschooling is that you can tailor your child’s education to match his or her needs. One of the many weaknesses of today’s standard educational system is its “one size fits all” mentality. It is nonsensical to think that a single textbook and a single teacher’s methodology will meet the educational needs of all the students in a given class. It is even more nonsensical to think that all students mature at the same rate and therefore should spend exactly the same amount of time in school. Some students are quick to learn and quick to mature, and they should probably leave school early. Others are more deliberate in their approach to studies and life, and they should probably stay in school longer.
As a homeschooling parent, you know your child better than any teacher or bureaucrat. As a result, you are the best one to determine how long your student should be educated before going off to trade school, university, or the real world. If you think your student would really benefit from a fifth (or sixth or seventh) year of high school, then you should trust your parental judgement. If you think your student is ready for his or her next step in life at the age of 16, then once again, you should trust your parental judgement. A high school graduate needs a specific set of skills (educational, social, and practical) in order to pursue his or her life goals, and as a parent, you should keep your child in school long enough to acquire those skills, but not a second more!
Now, of course, the mother who asked the question does have a legitimate concern. If her child’s next step is university, will a fifth (or sixth or seventh) year of high school look odd? In my experience, the answer is, “no.” Universities have plenty of students who graduate after four years of high school. They tend to look on “nontraditional” students more favorably, as long as the “nontraditional” students meet their entrance requirements. Especially if you can use the extra year (or two or three) of high school to give the student experiences that will make him or her stand out, that will probably make the student more attractive to the unviersity. For example, a student who takes a fifth year of high school to volunteer full time at a veterinary clinic would be an attractive premed student to many universities.
If you have concerns about a university’s view of more than four years in high school, however, there is a way you can make the extra year (or years) less obvious. While many student transcripts are chronological (like this one, for example), some are arranged by subject (like this one). If you arrange your transcript by subject, it will be harder to notice that the student took more than four years to complete high school. Once again, I think a high school experience of more than four years will make a student slightly more attractive to a university. However, it’s possible that for certain universities, it won’t. If you think your child’s university of choice is one of those, a transcript arranged by subject is the way to go.
But here is the bottom line. Your job as a home educator is not to get your student into the university of his or her choice. Your job is to give your student the skills he or she needs to become the adult that God wants him or her to be. If your prayerful decision is that your child needs a fifth (or sixth or seventh) year of high school, then it is your obligation to do that.
April 3, 2017
Scientist Realizes Important Flaw in Radioactive Dating
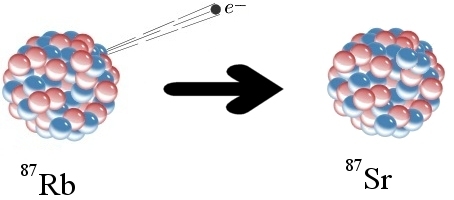
In beta decay, a neutron turns into a proton by emitting a beta particle, which is an electron (click for credit)
As someone who has studied radioactivity in detail, I have always been a bit amused by the assertion that radioactive dating is a precise way to determine the age of an object. This false notion is often promoted when radioactive dates are listed with utterly unrealistic error bars. In this report, for example, we are told that using one radioactive dating technique, a lunar rock sample is 4,283 million years old, plus or minus 23 million years old. In other words, there is a 95% certainty that the age is somewhere between 4,283 + 23 million years and 4,283 – 23 million years. That’s just over half a percent error in something that is multiple billions of years old.
Of course, that error estimate is complete nonsense. It refers to one specific source of error – the uncertainty in the measurement of the amounts of various atoms used in the analysis. Most likely, that is the least important source of error. If those rocks really have been sitting around on the moon for billions of years, I suspect that the the wide range of physical and chemical processes which occurred over that time period had a much more profound effect on the uncertainty of the age determination. This is best illustrated by the radioactive age of a sample of diamonds from Zaire. Their age was measured to be 6.0 +/- 0.3 billion years old. Do you see the problem? Those who are committed to an ancient age for the earth currently believe that it is 4.6 billion years old. Obviously, then, the minimum error in that measurement is 1.4 billion years, not 0.3 billion years!
Such uncertainties are usually glossed over, especially when radioactive dates are communicated to the public and, more importantly, to students. Generally, we are told that scientists have ways to analyze the object they are dating so as to eliminate the uncertainties due to unknown processes that occurred in the past. One way this is done in many radioactive dating techniques is to use an isochron. However, a recent paper by Dr. Robert B. Hayes has pointed out a problem with isochrons that has, until now, not been considered.
To understand the problem, let’s start with an example of how radioactive dating works. The elements rubidium and strontium are found in many rocks. One form of rubidium (Rb-87) is radioactive. As illustrated above, a neutron in a Rb-87 atom can eject an electron (often called a beta particle), which has a negative charge. Since a neutron has no charge, it must become positively charged after emitting an electron. In fact, it becomes a proton. This changes the chemical identity of the atom. It is no longer Rb-87; it is strontium-87 (Sr-87). Sr-87 is not radioactive, so the change is permanent.
We know how long it takes Rb-87 to turn into Sr-87, so in principle, if we analyze the amount of Rb-87 and Sr-87 in a rock, we should be able to tell how long the decay has been occurring. Of course, there are all sorts of uncertainties involved. How much Sr-87 was in the rock when it first formed? Was Rb-87 or Sr-87 added to the rock by some unknown process? Was one of them removed from the rock by some unknown process?
The isochron is supposed to take care of such issues. Essentially, rather than looking at the amounts of Rb-87 and Sr-87, we look at their ratios compared to Sr-86. The ratio of Sr-87 to Sr-86 is graphed versus the ratio of Rb-87 to Sr-86 for several different parts of the rock. How does that help? Sr-86 is another stable form of strontium, but it isn’t produced by the radioactive decay of Rb-86. Thus, it provides an independent analysis of the rock that does not depend on the radioactive decay that is being studied.
The amount of Sr-87 that was already in the rock when it formed, for example, should be proportional to the amount of Sr-86 that is currently there. Since the data are divided by the amount of Sr-86, the initial amount of Sr-87 is cancelled out in the analysis. If some process brought Sr-87 into the rock, it probably brought different amounts of the atom into different parts of the rock, so the ratio of Sr-87 to Sr-86 won’t stay consistent from one part of the rock to another. If a consistent isochron is generated, however, we can be “certain” that no process interfered with the relative amounts of Rb-87 and Sr-87, so the radioactive date is a good one.
Here’s where Dr. Hayes’s paper comes in. He says that there is one process that has been overlooked in all these isochron analyses: diffusion. Atoms and molecules naturally move around, and they do so in such as way as to even out their concentrations. A helium balloon, for example, will deflate over time, because the helium atoms diffuse through the balloon and into the surrounding air. Well, diffusion depends on the mass of the thing that is diffusing. Sr-86 diffuses more quickly than Sr-87, and that has never been taken into account when isochrons are analyzed.
No problem. Now that Dr. Hayes has brought it up, we can take it into account, right? Perhaps, but it’s rather tricky, because the rate of diffusion depends on the specific chemical and physical environment of each individual rock. If the effects of diffusion can be taken into account, it will require an elaborate model that will most certainly require elaborate assumptions. Dr. Hayes suggests a couple of other approaches that might work, but its not clear how well.
So what does this mean? If you believe the earth is very old, then most likely, all of the radioactive dates based on isochrons are probably overestimates. How bad are the overestimates? I have no idea, and I don’t think anyone else does, either. However, it will probably be dependent on the age. I would think that the older the sample, the larger the overestimate. However, it’s important to note that some radioactive dates (like those that come from carbon-14) don’t use the isochron method, so they aren’t affected by this particular flaw.
As a young-earth creationist, I look at this issue in a different way. As I have stated previously, we just don’t know a lot about radioactive decay. Certainly not enough to justify the incredibly unscientific extrapolation necessary in an old-earth framework. This newly-pointed-out flaw in the isochron method is a stark reminder of that. A good isochron was supposed to be rock-solid evidence (pun intended) that the radioactive date is reliable. We now know that it is not.
I suspect that this flaw is not the last one that will be uncovered.
March 31, 2017
I Will Be Teaching Online Classes!

Your high school student can have me as a teacher!
After teaching university classes for a couple of years, I have remembered that I really enjoy teaching. However, due to scheduling issues, I won’t be able to teach at the university this year. Nevertheless, I have officially “caught the bug,” so I decided to get my teaching “fix” with online courses. If you would like your student to have me as a teacher for the upcoming academic year, this is your chance!I will be teaching biology, chemistry and physics. Not surprisingly, we will use the textbooks I have authored: Exploring Creation with Biology, Discovering Design with Chemistry, and Exploring Creation with Physics. Each course will consist of a weekly 90-minute videoconference where I get together with 20-25 students and discuss the material that is covered in the text. Classes start the week of September 11 and meet every week except for the week of November 20th (Thanksgiving break), the weeks of December 25th and January 1st (Christmas break), and the week of March 19th (spring break). Classes end on May 16th.
Students will be expected to have read the material that will be discussed in class so that they can ask questions about the things they don’t understand. In addition to answering any questions the students have, I will show cool videos (like this one) that illustrate the scientific concepts which are being covered, discuss the more difficult material, give students tips on how to remember things, and share my views on the relevant scientific breakthroughs that are currently happening. I am really looking forward to it!
One thing to note is that these are “honors” classes, which means that they are more academically challenging than a normal high school class but are not at the AP or CLEP level. Students will be expected to do experiments at home, but I will grade their laboratory notebook entries. Students in chemistry and physics will be expected to do all of the experiments in the course. For biology, students who do not care about having an “honors” course will be expected to do the experiments that use household items as well as the dissection experiments. Students who want an “honors” level of biology will be expected to do all the experiments, even the ones requiring a microscope and its associated kit.
March 27, 2017
More on the Flat Earth
The logo of the 2013 Flat Earth Society
(click for credit)
I love attending that convention. Not only is it close to home, but the organization that runs it is incredible, and the speakers they invite are usually quite wonderful. I don’t always get to attend, because I am often asked to speak at a different convention that same weekend. However, this year, I had no previous commitments, so I went to the convention to sit at my publisher’s booth and give a brief talk about my new award-winning elementary science series. At the end of my talk, a homeschooling mother asked to speak with me about the fact that some people in her family were beginning to believe that the earth is flat. She asked what she could do help debunk that notion.
I talked with her for a while and gave her a couple of resources, and I also gave her my e-mail address in the hopes that her family members would send me any questions they had on the issue. However, as I started thinking about our talk, I decided it would be best to produce a page where I could gather some of the resources that clearly show the earth is not flat. It’s rather ironic that an idea which could be easily refuted more than 2,000 years ago still requires refuting today. Nevertheless, I am happy to do my part.
Because of the many conspiracy theories that are necessary to prop up the idea of a flat earth, I will not utilize such organizations as NASA and the European Space Agency. Since national governments are so good at keeping secrets, it’s clear that such agencies can “hide the truth of the flat earth” from us unsuspecting rubes. Instead, I will rely on some Christian resources that suggest specific observations you can make yourself to confirm that the earth is really a sphere. After all, it doesn’t take fancy equipment to confirm observations made before Christ was born!
Dr. Danny Faulkner is the best resource on this issue. He has a Ph.D. in astronomy, but what is probably more important for conspiracy-minded people is that he is a young-earth creationist. Thus, he clearly doesn’t “toe the party line” when it comes to science. If he is independent enough to evaluate the evidence for the age of the earth and come up with a conclusion that is far different from the scientific consensus, he could clearly do the same when it comes to the shape of the earth. However, he has spent a lot of effort showing people that the evidence demonstrates quite clearly that the earth is a sphere.
In this long article, for example, he discusses lunar eclipses, the stars we see from different parts of the earth, the appearance of distant objects across large bodies of water, and the testimony of Christians who have seen the earth from outer space. In this article, he discusses the appearance of distant objects across large bodies of water in much more detail. He has a series of pictures (figures 4-15) of two ships traveling away from him. He demonstrates that the hulls of the ships disappear from the camera’s view long before the tops of the ships, which is inconsistent with a flat earth. You don’t have to trust the pictures, because they could be Photoshopped. Instead, you can go to a port city and make the observation yourself. Its one of the many observations made by ancient, uneducated sailors which convinced them of a spherical earth.
In this article, Dr. Faulkner made a simple observation that anyone can make. He chose a night when the moon was going to pass in front of a star. This is called a lunar occultation, and there are websites that help you find out when one will occur in your area. By watching the occultation, you can see that moon doesn’t have the properties that flat-earthers need it to have in order to be consistent with other observations.
In this article, Dr. Faulkner takes the model that is currently fashionable among flat-earthers and makes an easy-to-evaluate prediction. He then makes observations with a telescope to evaluate the prediction and shows that it is falsified. While he used a fairly nice telescope, you can do the same observation with an inexpensive one, as long as you get a filter for viewing the sun.
Dr. Robert Carter and Dr. Jonathan Sarfati have also written a long article about the flat earth. Like Dr. Faulkner, they are both young-earth creationists, so they are willing to make conclusions that are opposed to the scientific consensus if they are convinced by the evidence. They bring up many of the issues that Dr. Faulkner does, but they add some others. They even have a suggestion for an experiment that you can do on social media of you have at least one friend who lives in the opposite hemisphere of the earth.
To round out the list of resources, here are a few that deal with the mistaken notion that the Bible says the earth is flat. In this article, James Patrick Holding discusses the Hebrew in the Old Testament and how it relates to the shape of the earth. He has a more detailed article here. Tony Breedon also has an article that shows the Bible doesn’t teach a flat earth.
I hope these resources help those who have heard flat-earth arguments and have found them convincing to one degree or another.
March 23, 2017
Dr. Patrick Briney, Atheist-Turned-Christian

Dr. Briney presenting design evidence for creation (click for source)
As many readers probably know, I was once an atheist but was “argued into the Kingdom.” Because of this, I tend to collect stories of other atheists who have become Christians. What intrigues me about these stories is that few of them are alike. God seems to use many different means to call people to Him, which is both wonderful and fascinating. Every now and again, however, I find a story that is similar to mine. Recently, I learned about Dr. Patrick Briney, and while there are some differences between his journey and mine, there are some similarities as well.
In his personal story, he talks about wanting to be a medical doctor from an early age. When he went to university to start pursuing his dream, however, something happened. A young lady who eventually became his wife called him to tell him that she had become a Christian, and she put him in contact with a person on his campus, the University of California, Irvine. According to Dr. Briney, this
…led to Bible studies, discovering answers, and eventually my salvation about two years later.
In this version of his story, he is short on the details, but according to another article he wrote, creation science played a role in this process. As I read that article, I couldn’t help but notice the similarities (and differences) between his story and mine.
He was an atheist when he went to university. That’s slightly different from me, as I was an atheist until my late high school years but was a young Christian when I went to university. However, it seems that the design found in nature was an important part of his realization that atheism isn’t a scientifically reasonable position. That’s also what first drove me away from atheism. To this day, I find it hard to understand anyone who has studied the natural sciences to any great extent and is still an atheist. As I scientist, I think the data point unmistakably to a Creator.
There is at least one important difference in our stories. Even as a high school student, I recognized that evolution was a terrible theory for biological origins. Thus, while I was an atheist, I was not an evolutionist. I simply thought that science had not yet come up with a robust theory of origins. It seems Briney was a committed evolutionist, and the evidence against evolution was another important factor in his journey away from atheism.
He became a Christian at university, and then decided to go on and get a Ph.D. in microbiology. This leads to another difference between our stories. I went to graduate school and studied nuclear chemistry with the plan of becoming a university professor, while he went to graduate school with the plan of being a campus minister. He thought that by pursuing and earning a Ph.D., he would not only be more integrated into the academic community, but he would also be better able to minister to those who were experiencing the university environment.
This brings us to another similarity between Dr. Briney and myself. Even though I planned to be a university professor and did so on a full-time basis for a while (and still do so on a part-time basis), I also ended up in an academic ministry. While Dr. Briney focuses on university students, I focus on homeschooled students, many of whom will attend university. Nevertheless, we are both trying to show students how studying science is really studying the Creator’s works.
Many great scientists of the past understood this, and some great scientists of the present understand it. Dr. Briney and I share the goal of helping future scientists to understand it. That’s probably the most important similarity in our stories!
March 20, 2017
An Excellent Observation about Postmodernism

Cartoon by Judy Horacek (click for her website)
I was first exposed to postmodernism when I went to university. If you don’t recognize the term, it is rather hard to define, mostly because there are so many variants of it. However, it generally refers to the idea that there are very few (if any) objective truths. Most of the things we hold to be “true” are only true for our experiences. Someone with a completely different set of experiences might come up with a completely different sent of “truths,” and those “truths” are just as valid as the “truths” that we come up with.
Consider, for example, the insightful cartoon above. The first panel shows an artist who has apparently come up with something he thinks is amazing. Because he sees that it is good, he considers himself to be a genius. The second panel shows a postmodern artist, who says that there is no such thing as a genius, because that category is dependent on culture. Of course, he thinks he is a genius for recognizing this fact!
Now, when it comes to art there is a measure of truth here. What is beautiful to one person might be quite unpleasant to someone else. As the old maxim states, beauty is, indeed, in the eye of the beholder. However, I think it is possible to recognize the genius of an artist, even if you don’t find his or her art appealing. A postmodernist would not agree. Moreover, a strict postmodernist would apply this idea of “truth” everywhere, even in science. According to the postmodernist, a “scientific fact” isn’t a fact at all. It is a social construct, and it might be quite different in another culture or society.
Obviously, I think postmodernism is mostly nonsense. Apparently, so does Andrew Klavan. In a previous post, I discussed his book, The Great Good Thing: A Secular Jew Comes to Faith in Christ. I noted that he makes some very profound statements, and one of them required its own blog post. Well, I got distracted (probably by something shiny) and only now remembered that I wanted to blog about it. As you might have guessed, it has to do with postmodernism.
In chapter 8, he discusses the “mad scene” in Shakespeare’s Hamlet. In that scene, the title character (Hamlet) is pretending to be insane. Klavan writes:
When he’s asked what he’s reading, he answers weirdly, “Words, words, word.” He talks about how his internal moods seem to transform outer reality so that he can never be sure what the world is really like. Morality especially has come to seem to him completely dependent on his own opinions. “There is nothing either good or bad, but thinking makes it so,” he says.
How wild was this? Shakespeare had predicted postmodernism and moral relativism hundreds of years before they came into being! Like Hamlet, the postmodernists were declaring that language did not describe the world around us…Like Hamlet, the postmodernists announced that what we thought was reality was just a construct of our minds…And like Hamlet, the postmodernists had dismissed the notion of absolute morality…
But there was one big difference. Hamlet said these things when he was pretending to be mad. My professors said them and pretended to be sane.
Indeed.
Jay L. Wile's Blog
- Jay L. Wile's profile
- 31 followers





