Jay L. Wile's Blog, page 43
December 10, 2015
Deep Wisdom About Adoption

Katie Davis and her 13 adopted daughters on her wedding day.
About four years ago I read a book that touched me more deeply than I can describe. It is entitled Kisses from Katie and was written by Katie Davis, who is one of the most amazing people about whom I have ever read (and I have read about a lot of people). At the ripe old age of 16, she decided that God was calling her to be a missionary. During her senior year in high school, she did some part-time missionary work in Uganda, and after she graduated high school, she went back there to do full-time missionary work. I blogged about her book, and I encourage all those who follow Christ to read it. It is a remarkable tale of what can happen when a person listens to God’s still small voice (1 Kings 19:12) and follows His lead.
One of the many reasons I was touched by Katie’s story is that we share something in common; we are both adoptive parents. I wrote an article about how my wife and I adopted our daughter, and it barely compares to Katie’s story. My wife and I had a comfortable home, a good dual income, and the young lady we adopted was a healthy teen who attended our church. Katie had no husband, was doing missionary work (for which there is never enough money), and the 14 daughters she ended up adopting were unhealthy strangers with whom Katie didn’t even share a common culture. However, she has the heart of Christ, and that’s all it really takes. If you are wondering why there are only 13 daughters in the picture at the top of this post, one of them was taken back by the birth parent after Katie had lovingly nursed the little girl back to health.
Because her book touched me so deeply, I read her blog from time to time. She doesn’t write very often (I can’t imagine how she finds any time to write), so I don’t visit it very often. However, I recently went there to catch up, and I read an incredibly touching post that I simply had to share. It is written to the adoptive mother who doesn’t really feel like a mother, and the message of the article resonated with me, because it mirrors my own experience as an adoptive parent.
Most of the people who have observed my daughter and I together for any length of would call me a doting father. I am wrapped around her little finger, and there is simply nothing that can be done about that. Why? The answer is simple: I love her. It’s important to note, however, that such intense, emotional love didn’t happen right away. Katie describes this masterfully:
From the moment I met my children I loved them in the way that a heart feels they must love another human being, especially one in need of care. I felt that God made it clear to me that I was to raise them and this intensified my love into a fierce, protective, sacrificial love, but it didn’t change the fact that it takes some time to make strangers into family.
That’s exactly right. At first, we weren’t even considering adopting Dawn. We just knew that she needed a safe place to heal, and we provided that, because the Lord was leading us to do that. Once we decided to adopt her, that kind of “caring love” intensified into something much more sacrificial, but it still didn’t make me into a doting father. As Katie says, it takes time to make a stranger into a member of your family.
As I have written previously, God molded my heart and my wife’s heart around our little girl so that now, she is an inseparable part of our little family. But that didn’t happen overnight. It didn’t even happen over the course of a few months. As Katie writes:
Love is a thing that grows.
If you have recently adopted a child, give your love the time it needs to grow. I assure you, it is well worth the wait!
December 7, 2015
Yet Another Global Warming Alarmist Prediction Has Been Falsified
One species of coccolithophore
(click for credit)
The picture shown above is of an ocean-dwelling microscopic organism known as a coccolithophore. It makes its own food via photosynthesis, and it also makes the “plates” that you see covering it. It makes them by absorbing bicarbonate (HCO3–) and calcium from ocean and making calcium carbonate (CaCO3). When coccolithophores die, their calcium carbonate plates sink to the bottom of the ocean, making deposits of chalk.
Now it turns out that this process of making plates out of calcium carbonate is influenced by the acidity of the water. The more acidic the water, the harder it is for coccolithophores (and all organisms that do the same chemistry) to make calcium carbonate. Well, increasing levels of carbon dioxide leads to increasing acidity (technically, lowering alkalinity) of ocean water, since carbon dioxide can react with water to form carbonic acid. It is therefore assumed that rising carbon dioxide levels in the atmosphere will harm coccolithophores. As one book on biodiversity puts it:1
Higher atmospheric carbon dioxide levels, by making oceans more acidic, could reduce coccolithophore populations (by interfering with their skeletal formation), thereby reducing a major CO2 sink and leading to still higher levels of atmospheric CO2 concentrations.
Fortunately, actual scientific observations demonstrate precisely the opposite.
Sara Rivero-Calle and her colleagues studied coccolithophore populations over a 45-year period (from 1965 to 2010). They showed that coccolithophore populations have increased tenfold over that time period. What caused the increase? Here’s what they say:2
Our study shows a long-term basin-scale increase in coccolithophores and suggests that increasing CO2 and temperature have accelerated the growth of a phytoplankton group that is important for carbon cycling.
In other words, the prediction made by global warming alarmists is exactly opposite of reality. Rather than dwindling as a result of increasing carbon dioxide levels, coccolithophore populations have exploded.
These results illustrate something even more important about the earth’s climate. Many global warming alarmists think that the earth was pieced together haphazardly as a result of random interactions guided by the laws of physics. As a result, they don’t think it has the ability to withstand significant changes. Indeed, they think the earth is so poorly constructed that small changes can cause it to completely spiral out of control.
Consider, for example, the quote I gave from the book on biodiversity. It claims that increasing carbon dioxide levels will result in the decline of coccolithophores, which would cause even more increases in carbon dioxide. This is called a positive feedback loop – a change produces a series of events which increase the severity of that change. Most global warming alarmists think that the earth has lots of positive feedback loops.
Because I think the earth is designed, I expect precisely the opposite. I expect the earth to be full of negative feedback loops, where a change produces a series of events that work to reduce the severity of the change. All well-designed systems contain negative feedback loops, and the earth is no exception (see here, here, here, and here). The change in coccolithophore populations in response to increasing carbon dioxide is just one more example of such a negative feedback loop.
When coccolithophore populations increase, photosynthesis increases. This, of course, reduces atmospheric carbon dioxide. That’s a classic negative feedback loop – more carbon dioxide produces more photosynthetic creatures, which reduces carbon dioxide. In addition, remember what happens when coccolithophores die. Their calcium carbonate plates fall to the bottom of the ocean. The calcium carbonate contains carbon, so the more coccolithophores that die, the more carbon gets sent to the bottom of the ocean, removing it from the cycle that could put it back into the atmosphere in the form of carbon dioxide.3
But this negative feedback loop is even more impressive, because not only do increases in coccolithophore populations decrease carbon dioxide, they decrease the temperature of the planet! One very important factor that affects the temperature of any planet is its albedo, which is the amount of light reflected by the planet’s surface. The higher the albedo, the more light gets reflected, and the cooler the planet. Guess what increasing coccolithophore populations do to the earth’s albedo? They increase it.4 Thus, even if increasing carbon dioxide produces increasing temperature, it will also produce an increasing albedo, which will fight against an increasing temperature!
So the fact that coccolithophore populations have increased in response to carbon dioxide levels in the atmosphere not only falsifies yet another prediction made by global warming alarmists, but it also demonstrates how well-designed the earth is. The more I study the earth, the more I come to appreciate that fact.
REFERENCES
December 3, 2015
Soft Blood Vessels from a Dinosaur Fossil

Blood vessels taken from a dinosaur fossil.
(Image credit: M. Schweitzer, North Carolina State University.)
In 2005, Dr. Mary Schweitzer shocked the paleontology community by reporting that she had found soft tissue in a Tyrannosaurus fossil that was thought to be 65 million years old.1 One type of soft tissue structure found appeared to be branching blood vessels like those shown in the picture above. The idea that blood vessels could have remained soft for millions of years is contrary to everything we currently know about biomolecules and their decay, so many in the paleontology community searched for some other explanation of Dr. Schweitzer’s find.
For example, its possible that despite its appearance, the structure isn’t composed of blood vessels at all. Instead, it could be the result of a recent colonization of bacteria or fungi. After all, when the blood vessels in a bone decay away, the “tunnels” in which they were housed remain. What if rather recently, a colony of bacteria took up residence in those same tunnels? These organisms often leave a slime (called a biofilm) behind, and if they left it in those “tunnels,” the biofilm would take the shape of the “tunnels.” That would make the biofilm look and behave a lot like blood vessels.
Such an explanation seemed to get some support back in 2008, when a major article was published in PLoS ONE.2 In that article, researchers reported on a survey they had done of many dinosaur bones. They found several examples of what appeared to be soft tissue in those bones, and they submitted some of those samples for carbon dating. The dating indicated that the samples were of very recent origin. In addition, they compared their samples to modern biofilms and modern collagen (a protein not made by bacteria). Their samples of apparent tissue resembled modern biofilms much more than modern collagen, so they concluded:
When biofilms coat a substrate, and that substrate is subsequently removed, the biofilm will retain much of the original morphology. This can explain the quantity and similarity of structures found in fossil bone and indicates that these structures are unlikely to be preserved dinosaurian tissues but the product of common bacterial activities.
Since then, however, the analysis of a variety of soft tissue found in dinosaur bones has lent a lot of support to the idea that these tissues are not biofilms, but are genuine dinosaur tissue (see here, here, here, and here). It seems that the definitive paper has finally been published, showing, at minimum, that soft blood vessels found in one dinosaur bone do come from the dinosaur itself.
In the Journal of Proteome Research, Timothy Cleland and his colleagues studied a leg fossil from a Brachylophosaurus canadensis specimen, which is supposed to be 80 million years old.3 They got rid of the hard materials in the leg fossil and examined the soft tissue that remained. They found proteins that were quite consistent with blood vessels. For example, one of the proteins they found was myosin, which is a common component of the muscles associated with blood vessels.
The researchers then did the same procedure on modern chicken and ostrich bones. The proteins they found from those bones were very similar to the proteins found in the dinosaur fossil. They conclude:
When all data are taken into consideration, the most parsimonious explanation is that these vessels, derived from demineralized dinosaur bone, are endogenous.
In other words, these really are the soft remains of dinosaur blood vessels.
Now it’s important to note that Dr. Schweitzer (who is a part of this study) has suggested a mechanism by which soft tissue might be preserved over millions of years.4 I have discussed why I don’t think her proposed mechanism can explain such remarkable preservation (see here and here). Instead, based on my understanding of the thermodynamics of large biomolecules, it is much more reasonable to interpret this soft tissue as evidence that these bones aren’t millions of years old.
Regardless of whether or not I am right on that point, the results presented in this paper are very important. They should put to rest any remaining doubt that some dinosaur fossils do, indeed, hold soft tissue from the dinosaurs themselves.
REFERENCES
November 30, 2015
Another Atheist-Turned-Christian

Dr. Sarah Salviander has a Ph.D. in astrophysics and is currently a research fellow at the University of Texas Department of Astronomy.
As regular readers of this blog know, I collect interesting stories about atheists who have become Christians. This is partly because I was once an atheist myself, and it is partly because I find it fascinating how God reveals Himself to people in so many different ways. Recently, I ran across the testimony of Dr. Sarah Salviander, who holds an earned Ph.D. in astrophysics and is a research fellow at the University of Texas Department of Astronomy. She has a healthy list of publications in the peer-reviewed literature and characterizes herself as a scientist, apologist, and author.In her testimony, she says that her parents were atheists who preferred the term “agnostic” and that religion played no part in her life as she grew up. Indeed, only three of the people she met by the time she was 25 had identified themselves as Christians. She says:
My view of Christianity was negative from an early age, and by the time I was in my twenties, I was actively hostile toward Christianity. Looking back, I realized a lot of this was the unconscious absorption of the general hostility toward Christianity that is common in places like Canada and Europe; my hostility certainly wasn’t based on actually knowing anything about Christianity. I had come to believe that Christianity made people weak and foolish; I thought it was philosophically trivial.
This is something Dr. Salviander and I had in common. When I was an atheist, I viewed religion as a crutch. It was okay for people who didn’t have the intellectual fortitude to face reality, but for someone who was knowledgeable about science and philosophy, it was absurd. Like Dr. Salviander, I eventually learned how wrong such a position is.
It turns out that we have another thing in common: we are both fans of science fiction. She was heavily influenced by Star Wars and Star Trek, as was I. I still love those shows, although I wish the second installment of the Star Wars movies had never been made. Science fiction, along with Carl Sagan’s television show Cosmos, caused her to know at a young age that she wanted to be a space scientist. Today, she has fulfilled that dream, but she is studying space from a perspective that she didn’t have back then.
What caused that change in perspective? The very science she was studying. As she learned more and more about the secrets of the universe, she became more and more amazed. As she says:
Without knowing it, I was awakening to what Psalm 19 tells us so clearly, “The heavens declare the glory of God; the skies proclaim the work of his hands.”
She even surprised herself when she finally came to the realization that science had caused her to believe in God. For a while, that’s as far as it went. She was happy to study science from the perspective that what she was studying had been designed. However, she didn’t care to experience the Designer in any personal way.
Eventually, she met, fell in love with, and married a Christian. This, by itself, did not cause her to become a Christian, but it offered her a measure of comfort. Then, during a time when she and her husband were separated due to career issues, she ran across a book called The Science of God by Dr. Gerald Schroeder. I read that book myself many years ago and was struck by Dr. Schroeder’s original thinking. More recently, I read his second book, Genesis and the Big Bang. While I disagree with some of his points, I found the book interesting enough to write a two-part review (see here and here). I get the impression that Dr. Salviander agrees with Dr. Schroeder more than I do, which (of course) is fine.
She says that Dr. Schroeder’s book helped her realize that the book of Genesis is the inspired Word of God. As a result, she started investigating other parts of the Bible, including the Gospels. She came to the conclusion that the Gospels are true based solely on the facts. Indeed, she says that her emotions fought against her becoming a Christian, but she was so compelled by the facts that she had no choice but to convert. She says:
Maybe that sounds coldly logical. It did to me, and for that reason, I sometimes worried whether my faith was real. And then I had a chance to find out a couple of years ago.
She goes on to recount three devastating things that happened in her life during a single year. Thankfully, she found out that her faith is quite real, as she credits it for helping her to make it through those tragic experiences.
While her testimony is long, I found it to be riveting. If you have the time, I strongly encourage you to read the entire thing.
November 16, 2015
Mathematics and Science
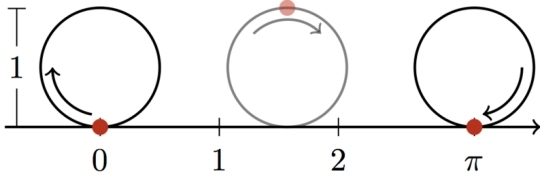
This is one way to visualize the meaning of the irrational number “pi.” If a wheel has a diameter of 1, it will travel a distance of pi when it makes one complete revolution. (click for credit)
Was mathematics discovered or invented? That might seem like an odd question, but it is an important one. I haven’t seen any official poll on the matter, but I suspect that most mathematicians, philosophers, and scientists would say that it must have been invented. After all, math is a tool. We use it for accounting, parceling out land, etc. Surely people invented this tool and then improved on it over time. If that’s really true, however, there is a deep mystery that is awfully hard to explain. Nobel laureate Dr. Eugene Wigner (a theoretical physicist and mathematician) put it this way:
The first point is that the enormous usefulness of mathematics in the natural sciences is something bordering on the mysterious and that there is no rational explanation for it.
Think about it. We didn’t invent the natural world. We simply study it. If we invented mathematics, why does it play such an integral role in our understanding of the natural world?
In my opinion, there is no mystery as to why mathematics is so useful in the natural sciences. That’s because I don’t think we invented it; I think we discovered it. Indeed, I think it is the language of creation. As Galileo wrote:
[The universe] cannot be read until we have learnt the language and become familiar with the characters in which it is written. It is written in mathematical language, and the letters are triangles, circles and other geometrical figures, without which means it is humanly impossible to comprehend a single word.
I was reminded of Galileo’s wise words when I read a short paper by two professors from my alma mater, the University of Rochester.
The paper discusses using a quantum mechanical technique known as variational computation to determine the energy levels in an atom. The quantum mechanical model is (so far) the most accurate model of the atom. However, the mathematics are very complex. As a result, the equations cannot be solved in an exact manner for most atoms. That’s where variational computation comes in. It allows you to approximate the solutions for the equations so that you can apply the quantum mechanical model to any atom. The more approximations you make with variational computation, the more precise your solutions become.
In mathematics, there are also things that must be approximated. One of them is the value for pi, the ratio of the distance around a circle to the diameter of that circle. The drawing at the top of this post gives you a way to visualize what pi means. If a wheel has a diameter of 1 meter, it will travel a distance of pi meters when it makes one complete rotation without slipping. It turns out that the value of pi cannot be calculated in an exact manner. Instead, its value must be approximated. If you do a few approximations, you get 3.14. If you do a few more, you get 3.1416. If you do a few more, you get 3.14159. The more approximations you do, the more accurate decimal places you will get, and therefore, the more precise your value for pi will become. However, you will never get an exact value for pi, because there isn’t one. The decimal places go on forever. Because of that, pi is called an irrational number.
How does this relate to the paper I am discussing? Well, the authors decided to apply variational computation to a system that doesn’t require it: the hydrogen atom. This atom is simple enough that the equations of the quantum mechanical model can be solved exactly, so there is no need to approximate their solutions. Nevertheless, the authors decided to approximate the solutions anyway. When they did, they noticed something. Their solution ended up containing an equation that had been developed by a natural philosopher named John Wallis back in 1655, more than 200 years before quantum mechanics was proposed!
Back then, Wallis developed one of the means by which mathematicians can approximate the value of pi. It turns out that this same approximation simply “falls out” of quantum mechanics when variational computation is applied to the hydrogen atom. In other words, the physics of the hydrogen atom contains one of the means by which we determine pi! As the authors state:
The existence of such a derivation indicates that there are striking connections between well-established physics and pure mathematics that are remarkably beautiful yet still to be discovered.
Indeed. Modern science is still demonstrating the truth of what Galileo wrote nearly 400 years ago.
November 11, 2015
In Honor of Veterans Day

My dad (Howard Edmund Wile) as a naval aviator.
I don’t typically do many holiday posts, and when I do, they are about Christian holidays. However, later on today I am going to attend a Veterans Day ceremony in which my father, Howard Edmund Wile, will be honored for his service. He is 89 years old and was in the Navy during World War II, the Korean War, and the Vietnam War. He was a turret gunner on a PV2 Harpoon and an aviation ordnanceman on four different aircraft carriers. He retired with the rank of Chief Petty Officer. This, of course, has made me think about Veterans Day and how important it is to remember those who have served. In honor of all veterans, I give you “A Nation’s Strength” by Ralph Waldo Emerson.What makes a nation’s pillars high
And its foundations strong?
What makes it mighty to defy
The foes that round it throng?
It is not gold. Its kingdoms grand
Go down in battle shock;
Its shafts are laid on sinking sand,
Not on abiding rock.
Is it the sword? Ask the red dust
Of empires passed away;
The blood has turned their stones to rust,
Their glory to decay.
And is it pride? Ah, that bright crown
Has seemed to nations sweet;
But God has struck its luster down
In ashes at his feet.
Not gold but only men can make
A people great and strong;
Men who for truth and honor’s sake
Stand fast and suffer long.
Brave men who work while others sleep,
Who dare while others fly…
They build a nation’s pillars deep
And lift them to the sky.
November 9, 2015
A Chemistry-Based Special Effect for Stage

Me as Abraham Van Helsing, using the special effect.
(Credit: Lucas Collett, a fellow actor in the production.)
This post is a bit different from the standard fare you find on my blog, but it is about two of my passions: acting and chemistry. As longtime readers are probably aware, acting is a hobby of mine. I write Christian Dramas, most of which are best characterized as “skits,” and I also act in them. In addition, I do some community theater. Most of it is done at Anderson’s Mainstage Theatre as well as a new local group, The Alley Theatre. However, I have done some shows with other groups. In 2006, for example, I played Maurice (Belle’s Father) in Indianapolis Civic Theatre’s production of Beauty and the Beast. You can see a couple of scenes from the show here and here. The young lady who played Belle is one of the best actresses with whom I have ever worked, and I have worked with a lot of actresses!
In any event, I recently played the role of Abraham Van Helsing in Anderson Mainstage Theatre’s production of Dracula. The script is a relatively new one, but I think it is the best one I have seen. In the scene where Van Helsing confronts Dracula with a crucifix, the script says that Dracula is suppose to touch the crucifix, and it is supposed to briefly catch fire. I asked the director if she wanted that effect, and she said, “Yes, as long as it doesn’t look stupid.” I took that as a challenge.
Based on my past experience in the theater, I know of a substance called “flash cotton,” and based on my knowledge of chemistry, I understand that it mostly consists of a chemical called nitrocellulose. I had learned to make it for another show I was in years and years ago, so rather than buying it, I made it myself. The recipe is really simple, as long as you can get the ingredients.
To make the nitrocellulose, I cooled 50 mL of concentrated sulfuric acid and 50 mL of concentrated nitric acid down to 0 degrees Celsius. Once cooled, I mixed them together and kept the mixture in an ice bath so it would stay at 0 degrees Celsius. I pulled the cotton balls into several pieces and put those pieces in the acid mixture. I used a glass stirring rod (no metal!) to mix the cotton with the acid and let the cotton soak for 4 minutes. I then used two glass stirring rods like chopsticks to remove the cotton and place it in a beaker of water. I stirred the cotton in the water, pulled it out, and put it in another beaker of water. I did that twice more, so that the cotton was washed in water a total of four times. I then put the cotton in a saturated solution of baking soda and water in order to neutralize any acid that had not been washed out of the cotton. Once the cotton stopped bubbling, I knew all the acid had been neutralized. I pulled it out of the solution, let it dry, and the result was nitrocellulose.
Now, the great thing about nitrocellulose is that it ignites easily and burns quickly. Since it burns quickly, you don’t need much of it in order to produce a really nice flame. So now that I had the fuel, the only thing I had to do was make an igniter and put the igniter and fuel into a cross. I used a Dremel tool to cut a depression in the back of the cross near the bottom. That served to hold the battery. I then cut a trough in the back of the crosspiece. That trough served to hold the nitrocellulose. Here’s what that looked like:

The back of the cross is blackened because I took this picture after doing the effect in four rehearsals and six performances. The cross never caught fire. The black is mostly residue from the nitrocellulose burning.
I attached a cap to a 9-volt battery. The cap had two wires that were stripped at the end. I ran one wire all the way up to the trough. The other wire I cut a few centimeters from the cap, stripped the end, and wound the bare wire into an oval. I then took another wire, stripped both ends, wound the bare wire on one end into an oval, and placed it next to the first wire oval. I then ran the other end of that wire up into the trough.
Probably the most important thing comes next. You need run a filament between the wires, and that filament needs to get really hot when electricity flows through it. In the theater, I was taught to use nickel chromium (nichrome) wire. However, it is typically thin and breaks easily, which can make the system unreliable. One of my colleagues at Anderson University suggested using a strand from a stainless steel scouring pad. That proved to be perfect! It got red-hot when electricity was passed through it, but it was also strong, so it didn’t break until after the nitrocellulose was ignited.
I secured everything with masking tape, covering the wires and the battery. Since masking tape looks a bit like wood, this also served to “hide” everything from the audience. Here is what it looked like at this point:
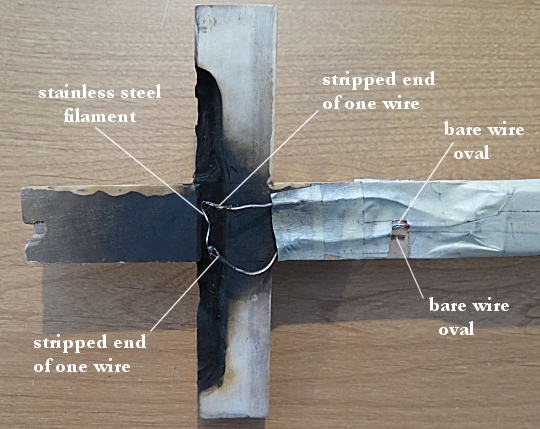
The only thing left was to add the fuel. I covered the filament with nitrocellulose and filled rest of the trough with it. I then put masking tape behind the nitrocellulose to hold it in place. Here is what the back of the cross ended up looking like:

To ignite the fuel, I taped a strip of aluminum foil to my thumb. When Dracula touched the cross, I pushed the foil against the two ovals made of bare wire. That completed the circuit, allowing electricity to heat the filament and ignite the nitrocellulose, producing the effect pictured at the top of this post.
Ignition and the full burn took less than a second, and it never failed.
November 5, 2015
Put Away the Laptop. Take Longhand Notes.

Medical students using laptops in class (click for credit)
In the fall of 2014, I taught a chemistry class at Anderson University. While I had been a guest lecturer for several university-level courses over the years, it was the first time in 19 years that I had taught an entire, semester-long class. As a result, I experienced some things I had never experienced before, and one of them was students using laptops to take notes. It wasn’t incredibly common in my class, but every day, a few students would come in, sit down, and open up their laptops.
I wasn’t crazy about the students using laptops in my class, mostly because I think they can be distracting for the students using them. If a student sees an e-mail message or Facebook notification, it is easy for the student to flip over to those things rather than concentrate on what is happening in class. However, I strongly believe that at some point, you need to start treating students as adults. Thus, I didn’t tell them that they couldn’t use laptops. I did note who the laptop users were and, when I processed test scores, I would compare the average of the students using laptops to the average of the rest of the class. Each test, the laptop users had a lower test score average.
Of course, my sample size was very small. As a result, the statistical error kept me from making any firm conclusion regarding laptop use in class and performance on the tests, but the results did correlate with my “gut feeling” regarding laptop usage, so I became even more convinced that laptop usage in class harms a student’s performance. Little did I know that there is actually a large body of statistically-significant research on the subject, and the studies are in general agreement: taking notes with your laptop is simply not as effective as taking notes longhand.
I learned this by reading a very interesting study that was published in the journal Psychological Science. As is typical with such reports, the paper starts off with a review of the literature. It cites three studies that show laptop-note-takers have decreased academic performance compared to longhand-note-takers, and one study that shows that the students themselves are less satisfied with their education when they use laptops to take notes. Of course, the “obvious” explanation for this is that the students who use laptops in class are being distracted by the other things available on their computers. However, the study linked above shows that even when distractions are removed from the computer, students still get less out of class when they use a laptop to take notes.
The researchers studied three different groups of university students in three different trials. Each trial involved the students viewing a lecture on a monitor. They were instructed to use the note-taking strategy that they used in class, and they were told that they would be tested on the content of the lectures. Students who used a laptop were given one that had no games and no access to the internet. In the first trial, students who took notes on the computer and students who took notes on paper scored roughly the same on the factual questions asked about the lecture, but the students who took notes on paper scored significantly better on the conceptual questions.
One thing the researchers found when analyzing the data from the first trial was that the students who took notes with their laptops used more words in their notes and were much more likely to quote parts of the lecture verbatim. Thus, in their second trial, they instructed some of the students to specifically avoid trying to transcribe the lecture. They thought that this might reduce the problems associated with laptop-note-taking. However, there was only a small decrease in the verbatim content of the laptop users who were told to avoid it, and in the end, the same test results were found: the laptop-note-takers did worse on conceptual questions than the longhand-note-takers.
In the final trial, the researchers thought that perhaps the poorer performance of the laptop-note-takers could be improved by letting them study their notes. After all, if the notes contain more words and more verbatim content from the lecture, that should allow the students who use laptops to study more effectively. In this trial, however, the longhand-note-takers did even better compared to the laptop-note-takers! Those who were able to study their longhand notes outperformed those who studied their laptop notes in both factual and conceptual questions!
Here is how the authors sum up their results:
The studies we report here show that laptop use can negatively affect performance on educational assessments, even — or perhaps especially — when the computer is used for its intended function of easier note taking. Although more notes are beneficial, at least to a point, if the notes are taken indiscriminately or by mindlessly transcribing content, as is more likely the case on a laptop than when notes are taken longhand, the benefit disappears.
I think that statement is instructive to students in two ways: First, stop using your laptop in class. It is likely to affect your performance negatively, even if you can avoid the distractions it presents. Second, even when you are taking notes longhand, don’t mindlessly transcribe content. Think about what you are learning and put it in your own words!
November 2, 2015
Did We Really Save the Ozone Layer?
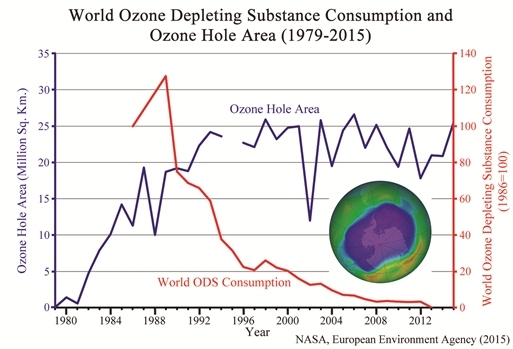
Ozone hole size and worldwide use of ozone depleting substances (click for credit)
A reader sent me this article and asked for my thoughts on it. It discusses the fact that the ozone “hole” over Antarctica grew 22% from 2014 to 2015. It presents the graph shown above, which demonstrates that despite the fact that the worldwide use of chemicals known to destroy ozone has dropped to nearly zero, the size of the ozone hole has not really decreased. It points out two studies that claim the ozone hole will shrink in size by either 2020 or 2040, and it concludes with this sentence:
But the longer the hole persists, the greater the likelihood that the ozone layer is dominated by natural factors, not human CFC emissions.
So what’s the story? By banning the use of CFCs, which we know can destroy ozone in the ozone layer, did we really fix the ozone “hole” problem? Or did we, as this story seems to imply, try to fix something that is probably the result of earth’s natural variability?
The first thing you need to know is that the ozone “hole” isn’t really a hole. It is a reduction in the amount of ozone that exists within the ozone layer, a portion of earth’s atmosphere that is roughly 15-35 kilometers above the surface of the earth. While all portions of the atmosphere have some ozone in them, this portion has the highest levels. Ozone’s molecular structure allows it to absorb some of the ultraviolet light that comes from the sun. That’s good for us, because ultraviolet light is energetic enough to kill living tissue. You can therefore think of the ozone layer as a “shield” that protects us from most of the sun’s ultraviolet light.
The amount of ozone in the ozone layer is measured using Dobson Units (DU). The larger the number of Dobson Units, the more ozone there is in the ozone layer. Globally, the average amount of ozone in the ozone layer is about 300 DU, but in Antarctica, that number fluctuates significantly with the seasons. While there are times the amount of ozone in the ozone layer above Antarctica is 300 DU and higher, there are also times it is significantly lower. The lowest recorded level of ozone in the ozone layer above Antarctica was in September of 1994, when there were only 74 DU of ozone. That reduction of ozone is what scientists refer to as the ozone “hole.”
Why does the amount of ozone in the ozone layer above Antarctica change with the seasons? Because during Antarctica’s winter, the polar vortex develops. This large ring of wind blows above the continent, and it produces a series of events that eventually lead to ozone destruction. When the polar vortex breaks up (usually in October), ozone destruction stops, and the level of ozone in the ozone layer above Antarctica rises again. So when we talk about the ozone “hole,” we are talking about a seasonal drop in ozone levels in the ozone layer above Antarctica. (There is a similar “hole” above the Artic, but it is small compared to the one above Antarctica.)
Now that you have all that information under your belt, you are ready for what I think is the most illustrative graph related to the ozone hole. Every year, NASA records the maximum size of the ozone hole as well as the minimum level of ozone found there. The following graph shows you both. The maximum size of the ozone hole in square kilometers is given in blue, and the minimum level of ozone in the hole is given in red. Please note that to make the graph easier to read, I divided the level of ozone by 2:
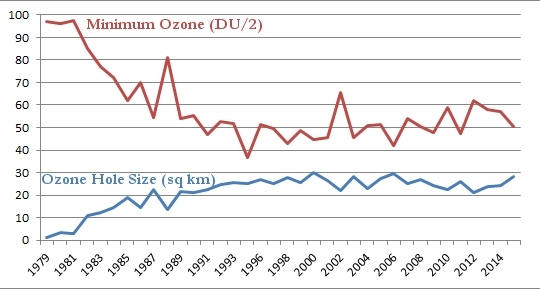
As was shown in the graph at the top of this post, the size of the ozone hole grew in a shaky but steady fashion until about 1998, and it has remained roughly the same since. Thus, while the ozone hole is no longer growing, it isn’t shrinking, either. Look, however, at the minimum level of ozone in the hole. It declined in a shaky but steady fashion until 1994, but it now seems to be growing in a shaky but steady fashion. The growth is very slow, but it seems unmistakable. So while the size of the ozone hole isn’t decreasing, the level of ozone in the ozone hole is rising.
My interpretation of the data, then, is that the ozone hole is recovering, but very slowly. Why is the recovery so slow? Based on the chemistry of CFCs, this is exactly what you would expect. One of the reasons CFCs became so popular is that they are fairly inert. They don’t like to react with much of anything on the surface of the earth. As a result, they are not toxic. However, because of that, they also don’t decay very quickly. This means that even though we have stopped using CFCs, there are still a lot of them in the environment, and it will take a long time for them to decay away. Until they do, they will continue to destroy ozone when the polar vortex forms.
In the end, then, I do think the data tell us that the reduction in CFC use has helped the ozone layer. However, due to the chemical nature of CFCs, it will take a while for this to have a strong effect on the depth and size of the ozone hole.
October 29, 2015
Two Good Points Made by the Director of the Vatican Observatory
Brother Guy J. Consolmagno, the new director of the Vatican Observatory
(click for credit)
I ran across an interview with him in the October 2nd issue of the journal Science. While I am sure there are a lot of things about which Brother Consolmagno and I disagree (theologically and scientifically), I found two statements that he made in that interview with which I wholeheartedly agree. The first was in answer to the question, “Does God get in the way of doing good astronomy?”
Just the opposite. He is the reason we do astronomy. I would say that is true even if you don’t believe in God. We do it first of all because we can, because the universe acts according to laws. That is a religious idea…You also have to believe that the universe is real and not an illusion. You have to believe that the universe is so good that it is worth spending your life studying it, even if you don’t become rich or famous.
The interview ended with this quote from him:
If you think you already know everything about the world, you are not a good scientist, and if you think you know all there is to know about God, then your religious faith is at fault.
Jay L. Wile's Blog
- Jay L. Wile's profile
- 31 followers