Jay L. Wile's Blog, page 15
January 6, 2020
More Incredible Dinosaur Soft Tissue Results

An axon from a nerve fiber, found in a triceratops fossil. (image taken from the video discussed below)
The Dinosaur Soft Tissue Research Institute is producing some incredible results. About two months ago, I discussed a video in which the institute’s founder, Mark Armitage, showed some of them. Recently, Armitage posted another video that shows even more results, and once again, they are amazing.
If you don’t have time to watch the entire video, let me summarize what I consider to be the two most amazing things shown. In my previous post, I told you that Armitage shows delicate vein valves that he extracted from soft tissue found in a triceratops fossil. It is amazing that he could get them, since they are so delicate that I end up destroying them when I try to get them from a dissection. More importantly, there is no possible way that such a vein valve could be from any source other than the dinosaur, since no organism that could possibly contaminate the fossil produces such structures. Thus, these vein valves are clearly original tissue from the dinosaur itself.
At 2:49 in this video, he shows not only the vein valve, but he shows that the wispy tissue which covers the valve when it is close is still 100% intact! How does he do that? He traps bacteria underneath the closed valve. The tissue is so thin that you can actually see the bacteria swimming around underneath it, trying to get out! The bacteria are obviously the result of contamination, but there is simply no way that the vein valve can be explained that way. So the video shows incredibly delicate dinosaur tissue (so delicate that you can see through it) that is still soft! That’s strong evidence that the fossil is not millions of years old!
Surprisingly, that’s not the most incredible result shown in the video. To understand that result, you need to know that neurons (the workhorses of the nervous system) are specialized cells that have distinct characteristics. A schematic of a neuron is shown below (image copyright shutterstock.com/logika600)
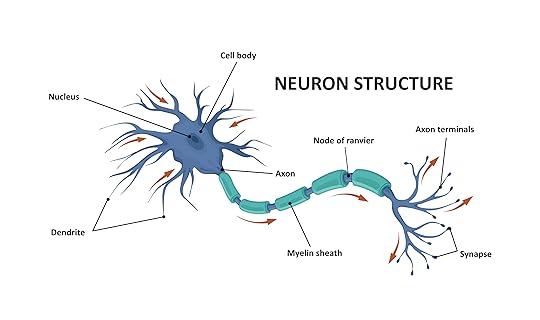
A neuron receives nerve signals in its dendrites, processes them in its cell body, and if needed, sends them down its axon. The axon is microscopically thin, but some can be very long. There are axons in the human body, for example, that run from the base of the spinal cord all the way to big toe of each foot! To increase the speed at which the signal travels, the axon is covered in sections of myelin, and the gaps in between those sections are called nodes of Ranvier. Because of this very specific structure, axons behave in specific ways when viewed using different microscope techniques.
At 5:27, Armitage starts showing several structures that he interprets as axons. Scientists who are committed to these fossils being millions of years old dismiss such structures as remnants of fungi that have contaminated the fossil. However, fungal remnants have specific characteristics, and Armitage does tests to show that the structures he interprets as axons don’t have those characteristics. Instead, they have the characteristics expected of axons! Indeed, he shows images from another scientist that demonstrate how axons from a mouse behave under specific conditions, and then he shows that one of the structures he interprets as an axon from a dinosaur behaves in a similar way.
The most stunning image in the video is the one I show at the top of this article. The image shows what appears to be the sections of myelin that wrap an axon as well as the nodes of Ranvier that exist in between those sections. The arrows point out where the myelin wrappings end and the nodes of Ranvier begin. If these results are confirmed, they represent the first time anyone has isolated soft axons from a dinosaur fossil! Based on what I have seen, I think Armitage makes a very strong case, so I expect the results to be confirmed.
Now, of course, Armitage doesn’t have any research grants like those who are committed to the belief that these fossils are millions of years old, so if you have the means, you might consider donating to the Dinosaur Soft Tissue Research Institute (DSTRI). I suppose that most people who support the organization believe in a young earth, but honestly, even if you think these fossils are millions of years old, you have to agree that the results being produced by the DSTRI are stunning!
December 2, 2019
A Bacterium That “Eats” Carbon Dioxide…and a Creationist Prediction
A false-color scanning electron microscope image of Escherichia coli. The different colors represent bacteria with different traits.
Escherichia coli (E. coli) are the workhorses of the bacterial world. They are found in every human being and most warm-blooded animals, but they are also found in laboratories all over the world. Because they are easy to care for, reproduce quickly, and have a genome that is reasonably well-understood, they are a popular subject of study among biologists. In addition, they end up producing a lot of chemicals that we need but are unable to produce ourselves. For example, insulin is a protein that all people need, but some people don’t make enough of it (or don’t respond well enough to it) to remain healthy. That leads to diabetes, and one treatment for diabetes is regular insulin injections.
While the insulin in pigs and cattle is close to what we find in people (and was used to treat diabetes for a long time), the best insulin for most diabetics is human insulin. Unfortunately, even with the best technology available, we aren’t good enough chemists to make insulin, but simple organisms like bacteria are. As a result, scientists have learned how to insert the human gene for insulin into a bacterium, which allows the bacterium to do the chemistry for us. As a result, much of the insulin used to treat diabetics today is human insulin produced by E. coli bacteria.
Unlike some species of bacteria, however, E. coli have to eat in order to get the energy and raw materials they need to do that chemistry. This ends up producing carbon dioxide as waste. To reduce the buildup of carbon dioxide in the atmosphere, then, it would be nice to produce chemicals like insulin from an organism that does not produce carbon dioxide in order to live. There are technical problems with that, however, so right now, diabetic insulin (and many other medically-related chemicals) adds to humanity’s “carbon footprint.” There are two ways to fix this: Either figure out how to use organisms that don’t have to eat (like organisms that make their own food through photosynthesis) or change E. coli so that it doesn’t have to eat.
In a recent study, scientists have been working on the second alternative and have experienced some success. As a byproduct, they have produced something that can be used to test creationism.
In the paper, the researchers report genetically modifying E. coli to “eat” carbon dioxide instead of the simple sugars that they prefer. This involved inserting genes for enzymes that can convert carbon dioxide to carbon-containing compounds that the E. coli can use. However, even with these additional genes, the E. coli would not use carbon dioxide the way the researchers wanted them to. As a result, they decided to use laboratory evolution to get the job done. They started giving the genetically-modified E. coli a sugar that the bacteria want to eat, but they then started increasing the amount of carbon dioxide in the surroundings while decreasing the amount of sugar given to the bacteria. After about 200 days, they observed that some of the bacteria were not eating the sugar. After about 350 days, they eliminated all sugars from the experiment, and they found that a population could sustain itself. They had successfully produced a population of E. coli that no longer needed sugar as a raw material for their chemical reactions.
Now before you start thinking about the possibilities of chemicals being produced without carbon dioxide emissions, there are two major issues. The chemistry that a bacterium must do to stay alive (and to make the chemicals we want it to make) takes energy, and carbon dioxide cannot be used to produce it. So while the bacteria can use carbon dioxide as the raw material they need to make their chemicals, they still have to get energy. They do that by taking in a one-carbon chemical called formate and burning it. That, of course, produces carbon dioxide – more than what is consumed by the chemistry that is being done. Thus, there is still a net output of carbon dioxide, but that output is less than a normal population of E. coli. The authors suggest making the formate used by the bacteria from carbon dioxide using renewable energy sources, so there is no net carbon dioxide production from the formate. However, there are technical problems associated with scaling that up to what it needs to be for producing medically-related chemicals in the amounts needed to make this economically viable.
The other problem is that right now, these bacteria are not very efficient. They are roughly 54 times less active than normal E. coli, so once again, they cannot be used in a viable way to make medically-related chemicals. Also, they can only exist if 10% of the atmosphere is carbon dioxide. Since the earth’s atmosphere is only 0.04% carbon dioxide, additional energy must be expended to enrich the bacteria’s surroundings with carbon dioxide. As a result, the most interesting aspect of this research isn’t its ability to reduce the medical industry’s carbon footprint. It’s the fact that scientists could radically alter an organism so that it no longer uses what it was designed to use to do its chemistry.
To me, however, there is another very interesting aspect to all of this. Some people (like Bill Nye) claim that creationism does not make testable predictions, which is the principal characteristic of a scientific theory. When they make such statements, they are only displaying their ignorance. In fact, one reason I am a creationist is because of creationism’s success at making testable predictions that are confirmed by the data. This study allows another opportunity to do just that.
We know that part of what produced the bacteria in the study was laboratory evolution. However, exactly how the evolution occurred has not been determined. They can tell you what mutations were produced in the evolutionary part of their experiment, but they can’t say when or how they happened. As a result, it is not known whether this is the kind of evolution that evolutionists require to turn flagellates into philosophers (evolution that is built on random mutations) or the evolution that creationists think is common in nature (evolution built on the genome’s designed ability to mutate in order to adapt to new challenges). Fortunately, there is a way to test this: See how easy it is to recreate the experiment. There are enough mutations in this new version of E. coli to say that if they are random, the odds of reproducing the result is very, very low. It could be done, but it would take many, many attempts. However, if this is the result of mutations that were produced by the genome’s design, then it should be very easy to get the same result. It might not happen every time, but it should happen with only a few attempts.
Now I have to admit that this isn’t much of a prediction. After all, we have seen it before. In Lenski’s long-term evolution experiment, a population of E. coli were produced that could eat a chemical called “citrate” under conditions in which they normally couldn’t eat it. While this was hailed as a result of random mutations filtered by natural selection, intelligent-design scientists have shown that it is very easy to replicate, which rules out random mutations. As a result, it is an example of mutations that the genome was designed to make. It is rather easy to predict that this current experiment is another result of the same process. Nevertheless, I look forward to seeing it confirmed.
November 25, 2019
When Is a Person Actually Dead?
A student recently sent me an article from Live Science that reports on a man who was declared dead by three doctors. Four hours later, as he was being prepped for an autopsy (the marks to guide the autopsy had already been put on him), he started snoring! As of the time the article was written, he was alive and in the intensive care unit of a hospital. The student asked how such a thing could happen. Was it incompetence on the part of the doctors, or is it difficult to tell whether or not a person is dead? I told the student that while I couldn’t address the details of this particular case since I wasn’t involved, I could tell him that there have been cases over the years where the experts were convinced that a person was dead when, in fact, that person wasn’t.
I first heard this kind of story when I was preparing for a talk about miracles. I ran across the case of Emma Brady. She had been declared dead after exhibiting no vital signs. She was placed in a body bag and taken to the morgue. When her children arrived about an hour later to say their goodbyes, they found her gasping for air. The administrator of the hospital said that after the family told a nurse about what they had seen:
Miraculously, the patient exhibited vital signs that were absent previously.
Over the years, I have kept my eye out for stories like this, and while they are rare, they are most certainly not unheard of.
Consider, for example, the story of Steven Thorpe. At age 17, he was in a tragic accident that killed one of the other occupants of the automobile. He was put in a medically-induced coma, and a team of four physicians told his parents that he was brain dead. They suggested that his organs be donated to help others. However, the parents brought in an additional doctor (a neurologist), who demonstrated faint brain activity. The doctors at the hospital agreed to bring him out of the medically-induced coma, and Thorpe recovered. He left the hospital five days later and at the time the article was published, he was alive and well.
Once again, while these stories seem rare, they are not unheard of. In 2008, Zack Dunlap was in an automobile accident and was declared dead 36 hours later. However, he wasn’t dead. In fact, he says that he actually heard his doctors saying that he was dead. The hospital made plans to harvest his organs, since his driver’s license said that he was an organ donor. However, as his family was saying goodbye, one of his cousins (a nurse) decided to pull out his pocket knife, hold Zack’s foot, and scrape the knife against it. Zack pulled his own foot out of his cousin’s hand. The family took it as a sign of life, and they argued that the hospital should treat Zack as if he were alive. He ended up making a full recovery.
The bottom line is that while we have amazing technology and a lot of knowledge about human anatomy and physiology, there are limits to what we can detect and what we can conclude. While it is sometimes very obvious that someone is dead, there are other times when even the experts can be fooled. That’s something all of us need to keep in mind when we deal with life and death issues.
November 18, 2019
No, It’s Not a Picture of an Atom
When I wrote the first edition of my high school chemistry textbook (back in 1994), I discussed the impossibility of seeing atoms. Atoms aren’t just small; they are simply too small to be seen, even with the most powerful microscope. That’s because in order for us to see something, light must bounce off it and enter our eyes. Cells on our retina detect that light, send nerve signals to our brain, and our brain interprets the signals to form an image. Since atoms are roughly 1,000 times smaller than the smallest wavelength of visible light, there is no way for light to bounce off a single atom. As a result, we will never be able to see individual atoms, no matter how powerful a microscope we use.
I said that every year to every general chemistry class I taught at the university level, and no one ever questioned my assertion. But homeschoolers think more critically than most students, so the very first year my chemistry course was published, I got a FAX (yes, a FAX) from a student questioning my statement. The student showed me an image like the one below, which was taken with a scanning tunneling electron microscope at IBM. She asked if it wasn’t a picture of individual atoms:
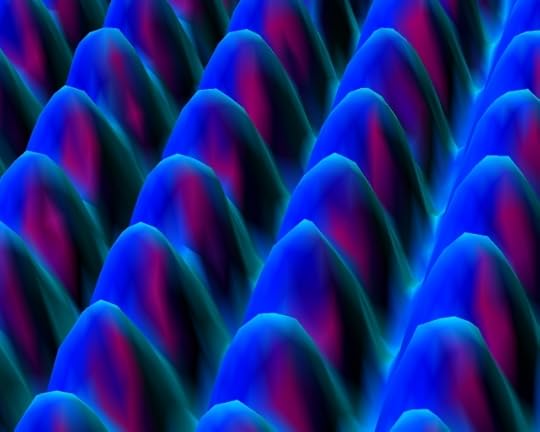
An image of the surface of nickel, as produced by a scanning tunneling electron microscope (click for source)
While the image is generally presented as a “picture” of nickel atoms, it is not. It is a graph that represents data that have been processed through a series of equations which govern the behavior of electrons traveling at high speeds. The equations depend on multiple theories, including special relativity and quantum mechanics. I am pretty confident that those theories are mostly true, so I am pretty confident that this is a realistic representation of atoms on the surface of a nickel foil. However, it is not a picture. Light was not used (high-energy electrons were used), and the image is not a direct representation of the nickel surface. It is a direct representation of data that have been processed through mathematical equations that come from several theories.
Recently, I got a question from a teacher who is using my new chemistry course. A student brought him an article that says scientists have been able to take a picture of a single strontium atom. I told the teacher why it is not a picture of a strontium atom, and then over the weekend, I got the same question on my Facebook page. As a result, I decided to write a quick article (well, as quick an article as I can write) about it.
This is definitely a picture, as it is the result of directly detecting light. And, that light is coming from a single atom. That’s the remarkable aspect of the photograph. Because atoms are so small, it is very hard to isolate a single one, but the experimenter who took the picture was able to do just that. However, it is not a picture of the atom. It is a picture of the visible light that the atom is emitting as a result of being excited. When atoms are excited, they must get rid of that excess energy, and one way they can do that is to emit light. The strontium atom is emitting visible light, which is why a picture of it could be taken.
Once again, however, this is not a picture of the atom; it is a picture of visible light being emitted by the atom. After all, the picture shows that the light is purple. If this were a picture of a strontium atom, it would mean that strontium atoms are purple. However, atoms have no color, because they are smaller than the smallest wavelength of visible light.
If you think I am just being pedantic here, consider the picture below:

Tubes of neon atoms emitting light as a result of being excited by electricity. (click for credit)
Each tube in the picture contains neon gas, and electricity is being passed through the gas, giving the atoms extra energy. To release that energy, they emit light, and the visible wavelengths that are emitted produce the orange color that you see in the picture. Are you seeing all the individual neon atoms in those tubes? Of course not. You are seeing the light that those excited neon atoms are emitting.
The “picture” of the strontium atom is the same thing, except that there is only one atom emitting light, while there are roughly 100,000,000,000,000,000,000,000 neon atoms emitting light in the picture above.
November 13, 2019
A Different Kind of Blog

Upper Waterton Lake in Alberta, Canada. (Photo copyright Kathleen J. Wile)
My wife and I felt led to leave the church we had been attending for 25+ years, and after a lot of searching and praying, we found a wonderful new church. There are lots of ways to serve in this church, and one of them is to contribute to the church’s blog. I will be doing that on a regular basis. My contributions to the blog will come under the heading “Amazed by Him,” because that’s the way I feel when I study Him. As a scientist, I am amazed by His creation, and as a Christian, I am amazed by Him. If you would like, you can read my first article, which was published today:
November 11, 2019
Another Special Effect Based on Chemistry

The very talented Eric Bailey portraying Dr. Jekyll in Jekyll and Hyde: The Musical (edited photo by Michelle Mullins)
I just got finished portraying Sir Danvers Carew in Jekyll and Hyde: The Musical. I had never seen or read the show before, so I didn’t know what to expect. It turns out that it is more of an opera than a musical. Most of the lines are sung, and the music is hauntingly beautiful. The cast was full of incredible singers, so the performances were remarkable. I got the opportunity to sing a duet with the young lady portraying my daughter Emma, who is engaged to be married to Dr. Jekyll. I also got to sing a lovely quartet with Emma, Dr. Jekyll, and John Utterson (Dr. Jekyll’s attorney). The music was incredibly challenging, but with lots of help from my fellow actors, I managed to pull it off.
The superbly-talented director had developed some important imagery for the show. The cast is divided into “the rich” and “the poor.” Dr. Jekyll is part of “the rich,” but when he turns into Mr. Hyde, he is part of “the poor.” The rich obviously wore much better clothes than the poor, but the director wanted something else to symbolize the divide between the two, so he used colors. The set was lit with green when the rich were being highlighted, and all the rich people had a splash of green on their costumes. The set was lit with red when the poor were being highlighted, and all the poor people had red in their costumes.
With this in mind, the director asked me if I could make a “smoking potion” that turns from green (representing Dr. Jekyll) to red (representing Mr. Hyde). I said, “no problem.” Then he added that the actor portraying Jekyll and Hyde must be able to drink the potion. That turned out to be a challenge. However, drawing on my experience writing a chemistry book for homeschooled students, I came up with something that worked pretty well.
Red (sometimes called “purple”) cabbage is loaded with a group of chemicals called anthocyanins. It turns out that these chemicals change color based on the amount of acid in their environment. They are red in acidic solutions, purple in neutral solutions, blue in slightly alkaline solutions, green in more alkaline solutions, and yellow in very alkaline solutions. That’s why red cabbage is reddish purple. The leaves are just a bit acidic, but close to neutral.
So I put lots of red cabbage leaves and water into a blender. After pulverizing the cabbage, I poured the result through a strainer and got a deep blue solution. It was blue because my tap water is slightly alkaline. I then added a very small amount of sodium hydroxide. At high concentrations, the chemical is caustic, but at low concentrations, it is just alkaline enough to make anthocyanins turn green. This mixture isn’t really drinkable, because it is alkaline enough to do damage to your throat and esophagus.
Dropping some dry ice into the green liquid made it look like it was smoking, and then I added clear vinegar. The vinegar turned the mixture acidic enough to make the anthocyanins red. While it is acidic, it is not nearly as acidic as vinegar, so it is pretty sour but safe to drink. The actor said that it tasted like sour asparagus. In the end, here is what it looked like during a dress rehearsal. Please note that using this short clip from the musical to illustrate the effect is considered “fair use” of a copyrighted work.
I did learn one thing in the process of making this effect. It turns out that an alkaline solution slowly deteriorates the anythocyanins so that they do not change back to red. Thus, the effect has a “shelf life.” I had to mix it right before the crew put it on stage, which was about seven minutes before he actually started making the potion. The anthocyanins didn’t change significantly over that time, but if you make the green solution even thirty minutes before it is used, the red color will be very weak.
I wrote about another chemistry-based special effect I developed for Dracula here.
November 6, 2019
Incredibly Fragile Dinosaur Soft Tissue

Two images of the delicate, one-way valves from veins. They were found in in dinosaur soft tissue!
(Image copied from the presentation embedded below)
Mark Armitage and James Solliday at the Dinosaur Soft Tissue Research Institute have been doing some amazing work. On October 5th, Mr. Armitage presented their findings at Lower Columbia College. Apparently, he has not yet received the video of that presentation, so he kindly posted a quick overview of the content. To me, it is astounding:
While everyone should watch all 15 minutes of the presentation, I want to highlight the things that I think are most important.
At 2:29, he shows two images that elicited an audible gasp from me when I first saw them. To understand just how incredible the images are, you need to know that there are one-way valves found in vertebrate veins. This is because the blood pressure in a vein is so low that blood can actually travel backwards. To prevent that, there are delicate, one-way valves throughout the veins. They open when the blood is flowing the correct way, and they close to prevent it flowing backwards. In the left-hand part of the image at the top of the post (copied from the presentation), you see a circle with what looks like a partially-opened tent flap. The circle is the base of the valve, and the “tent flap” is the delicate membrane that opens and closes. In that image, the valve is partly open. On the right-hand side, the valve is fully open.
This is incredible to me, because I have tried to dissect animals and extract these valves. I have never been able to. They are so delicate that I end up destroying them in the dissection process. Now, of course, I am not much of a biologist, and I am even less of an expert at dissection. Nevertheless, my experience with them indicates that they are absurdly delicate. Yet, here they are in a dinosaur fossil! Not only does this give evidence that the fossil is not millions of years old, but it also shows that these are definitely not structures that come from fungi or bacteria which recently invaded the fossil. Bacteria and fungi do not build structures with these delicate, one-way valves! He also presents other evidence that rules out bacterial and fungal contamination.
At 8:22, he shows red blood cells from a fossil that is supposed to be 400 million years old! The cells have the appropriate size and shape for red blood cells. Later on (12:05), he shows a blood vessel from a dinosaur fossil that has not even collapsed! It has an air bubble in it. When he does a stain test to see what is in the blood vessel, the test indicates that there is RNA in the blood vessel!
At 6:47, he shows what appears to be blood clotted in the tissue. He shows how it behaves just like you would expect blood to behave when exposed to polarized light, and he also shows that iron from the blood has not spread into the bone tissue. This is important, because Dr. Mary Schweitzer has proposed that iron might be preserving the soft tissue found in dinosaur bones. There has already been several arguments (see here and here) that seem to invalidate Dr. Schweitzer’s hypothesis, but this observation is the nail in the coffin. Iron can’t be preserving bone tissue if it doesn’t spread into the bone to begin with!
I have said this before and will say it again: It’s a wonderful time to be a young-earth creationist!
NOTE: A commentor made the great suggestion that I post a link if you want to support Mr. Armitage’s research. Here it is:
November 4, 2019
Rat Surgeons?
 I have written previously about Australia’s cane toad problem (here and here). In 1935, cane toads were brought in to control a pest that was feeding on sugar cane in northeastern Queensland. They ended up being ineffective at controlling the pest, and because they have few natural predators there, Australia was ineffective at controlling them. They have been spreading out across Australia since 1935, and there is no end in sight to their population’s expansion.
I have written previously about Australia’s cane toad problem (here and here). In 1935, cane toads were brought in to control a pest that was feeding on sugar cane in northeastern Queensland. They ended up being ineffective at controlling the pest, and because they have few natural predators there, Australia was ineffective at controlling them. They have been spreading out across Australia since 1935, and there is no end in sight to their population’s expansion.
As I have discussed previously, cane toads have already affected wildlife in the areas where they have become established. Because they are large (for toads) and the adults are poisonous to snakes, for example, the average head size of a snake has decreased in those areas with a significant cane toad population. After all, the snakes that have large enough heads to eat the adult toads die. As a result, snakes that can’t eat them (snakes with smaller heads) are significantly more likely to survive. They survive because they cannot eat what would kill them!
But there is another way to survive in the presence of cane toads: figure out a way to eat them without being poisoned. Based on the results of a recent study, it seems that one clever Australian predator has learned to do just that. The authors of the study were intrigued when they started finding cane toad bodies that had what appeared to be surgically-precise incisions on their bodies. They eventually set up some infrared cameras and found that golden-bellied water rats were the ones making the incisions.
It turns out that only the skin and certain organs (like the bile duct) in the frog are poisonous. If a predator can avoid those structures, it can eat the toads without being harmed, and apparently, the water rats have figured that out. The researchers found that the heart and liver had been removed in each dead cane toad, presumably eaten by the rats. In the largest toads, the skin of the legs was also peeled back and the leg muscles were eaten. The authors say that all of this was done with a high level of precision.
The question, of course, is how the rats figured this out. The researchers are not sure. They know that water rats feed on other toad species as well as the younger cane toads that aren’t as poisonous, and it may be that in this area, that’s the way rats eat all the toads they kill. It’s also possible that some rats just stumbled onto this technique and passed it on to their offspring. As the authors note, water rats care for their offspring for weeks after they have been weaned, so it would be easy for the young rats to learn how their parents are eating the toads. The researchers note that for now, this feeding technique is limited to the water rats in certain areas, but they suggest that it might spread as time goes on.
Add the Australian water rat to the ever-growing list of surprisingly clever animals (see here, here, and here.)
October 21, 2019
Is Religion on the Decline in America?

Ruins of the Oxford Terrace Baptist Church in Christchurch, New Zealand. It was destroyed by earthquakes (click for credit)
The secularization thesis holds that the advance of science and modernization leads to a decline in religion. As a result, the more scientifically and technologically advanced a society becomes, the less religious it becomes. One of the strong proponents of this view was Dr. Charles Wright Mills. In his book, The Sociological Imagination (Oxford University Press, 1959), he wrote:
Once the world was filled with the sacred – in thought, practice, and institutional form. After the Reformation and the Renaissance, the forces of modernization swept across the globe and secularization, a corollary historical process, loosened the dominance of the sacred. In due course, the sacred shall disappear altogether except, possibly, in the private realm. (pp. 32-33)
There are many people who believe this, and they think that Europe provides a great example of how it happens. There are huge, magnificent cathedrals in Europe, which are a testimony to how influential Christianity once was. Today, however, many of those cathedrals do not serve as houses of worship. Instead, they are museums of history. Even the ones that are still functioning as houses of worship have tiny congregations that are dwindling year by year.
Despite what happened across Europe, I have always been skeptical of the secularization thesis. Mostly, that’s because science convinced me that there must be a Creator God, which then led me to Christianity. The more I teach science and do scientific research, the more I see the Hand of God in nature. In my mind, advances in science and technology strongly support Christianity. As a result, the secularization thesis makes no sense to me.
I was recently made aware of an article from two years ago that argues against the secularization thesis in the United States. As I read it, I couldn’t help but think that it applies to the world as a whole.
The essential argument made in the paper is that casual religion is on the decline in the United States, but what they call “intense religion” (what I would call “real religion”) has not declined in any significant way. How do they make this argument? They draw on the results of the General Social Survey, which has been polling citizens of the United States on a variety of issues, including religion, since 1972. Using data going back to 1989 (a very common data set used in many other studies) they separate the people who they judge as “intensely” religious from those who they judge “moderately” religious. The results strongly support their argument.
Consider, for example, the simple question of whether or not someone has a religious affiliation. If the person answered “yes,” the follow-up question asked if the affiliation was “strong,” “somewhat strong,” or “not strong.” The authors grouped the “not strong” and “somewhat strong” respondents together and came up with this graph:
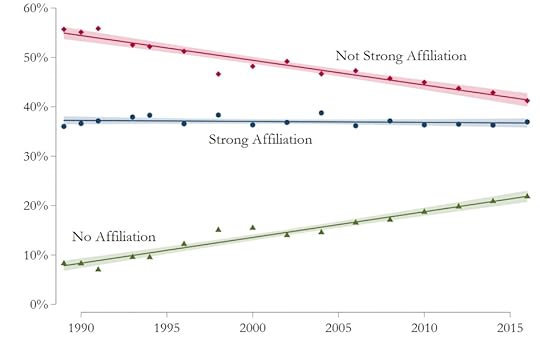
It’s rather easy to see what has happened when it comes to religious affiliation: Many of those who did not strongly affiliate with a religion simply “dropped out.” However, the percentage of Americans that are strongly affiliated with a religion has not changed significantly over this 28-year period.
The paper has several similar graphs, but I would like to share one more. The Survey asks how often the respondent prays. The options have changed a bit over this time period (for example, “never” was added in 2004), but grouping the respondents into one of three categories leads to these results:
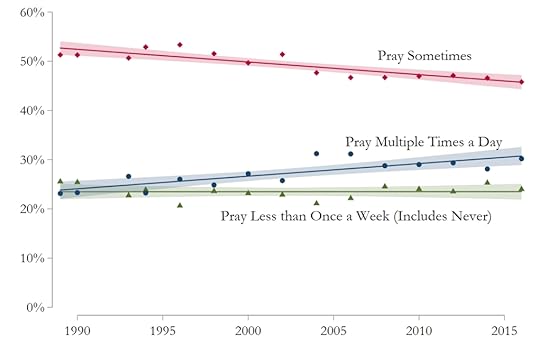
It seems that the percentage of those who casually pray has declined over the years, but the percentage of those who frequently pray has increased.
I encourage you to read the complete report if you have the time and interest, but here is the authors’ conclusion:
We have demonstrated, however, that only moderate religion has declined and that the intensity of American religion is persistent and exceptional. The decline of moderate religion is not, therefore, a pervasive secularization consistent with the secularization thesis. Instead, it appears to be a reaction of moderates against religion that has become too intense, too strict, and too politicized in the face of social change.
I suspect that this conclusion can be applied to the world as a whole. In Europe’s past, for example, you couldn’t be a “respectable” member of society unless you were “religious.” As a result, there were a lot of casually religious people, and therefore religion had a heavy influence on society as a whole. However, as society changed, the social need for religion fell by the wayside, and most of the casually-religious people stopped being religious. This led to what looks like a general loss of religion in Europe, when in fact, it was not. Indeed, in some European countries, serious religion is on the rise.
Yes, science and modernization does reduce casual religion, but I think these authors have made the strong case that at least in the United States, it has not affected the percentage of those who take their religion seriously. I would say that this is probably the case in many other countries as well.
October 14, 2019
Why Do I Include God in My Science Texts?

13th-century illumination from the French Bible Moralisée, depicting Christ (who is God) creating the World.
I was asked that question a couple of days ago, but it wasn’t the first time. Over the years, several people have asked me why I write about God in my science textbooks. After all, science is about facts, while belief in God is about faith. As a result, science doesn’t belong in religious texts, and God doesn’t belong in science texts. That may sound reasonable in today’s world, but it is simply dead wrong. In addition, it demonstrates a shocking level of ignorance about the history of science. Nevertheless, it is a good question, and it deserves a detailed answer.First, there is an obvious philosophical reason: God is the source of all that science studies, so it only makes sense to discuss Him in the context of studying His creation. Consider, for example, teaching a course on U.S. Law. Would you try to teach it without referring to the U.S. Constitution? Perhaps. Maybe there are law professors who do just that, but for me, I can’t imagine discussing U.S. law without discussing the source from which it comes. In the same way, I find it pointless to discuss science without discussing its source.
Second, there are practical reasons to discuss God while teaching students about science. If we emphasize the fact that the things we study as scientists are designed, we give students a superior way in which to view the natural world. Those who want to reject the idea of a Creator God will try to convince students that this world was “thrown together” by random chance. As a result, students get the idea that creation is full of shabby constructions. Of course, nothing could be further from the truth. The designs found in the natural world make our best technology look like garbage. This has led one desperate atheist to write:1
Biology is the study of complicated things that give the appearance of having been designed for a purpose.
Of course, a more reasonable evaluation of the data leads us to the conclusion that biology is the study of things that have been designed for a purpose.
This is very important, because when we understand that biology is the study of designed things, we don’t fall prey to misconceptions that hold back the progress of science. How many lives have been wasted because scientists looked at the primary cilium as a evolutionary vestige rather than an antenna designed to receive signals? How long did scientists delay a more detailed understanding of genetics because of the nonsensical notion of “junk DNA”? How many people needlessly suffer relapses of intestinal bacterial infections because of the silly idea that the appendix is a vestigial organ? A scientist who understands that the natural world is designed is simply better able to interpret what he or she is studying, since it is the more realistic view.
Finally, there is a spiritual reason to include God in science. As Nobel laureate Dr. Arthur Leonard Schawlow once put it:2
But the context of religion is a great background for doing science. In the words of Psalm 19, “The heavens declare the glory of God and the firmament showeth his handiwork.” Thus scientific research is a worshipful act, in that it reveals more of the wonders of God’s creation.
Ever since I became a Christian, science has been a worshipful act for me. There is no way I could write about this worshipful act without including the One who is being worshipped!
REFERENCES
Jay L. Wile's Blog
- Jay L. Wile's profile
- 31 followers



