Deborah Blum's Blog, page 5
May 1, 2011
A lost girl, remembered
 When I was researching my book, The Poisoner's Handbook, I started by making a list of famous homicidal poisons: cyanide and strychnine, arsenic and antimony and…and…the resulting catalog quickly outgrew my plans for a book of relatively modest length. How would I decide which toxic substances belonged in my particular "handbook"?
When I was researching my book, The Poisoner's Handbook, I started by making a list of famous homicidal poisons: cyanide and strychnine, arsenic and antimony and…and…the resulting catalog quickly outgrew my plans for a book of relatively modest length. How would I decide which toxic substances belonged in my particular "handbook"?
Since my story was of two somewhat renegade scientists trying to establish – or more accurately, invent – the profession of forensic toxicology in Prohibition-era New York, I started researching poison homicides in that time period. I focused on murders from about 1918 to 1935 in that remarkable city. I wasn't looking for famous cases – it was murder as a fact of everyday life that interested me. Those small forgotten stories, the cases that haunted me, the lives altered that I couldn't forget, ended up defining my poisonous history of early 20th century America.
And that's why the chapter on arsenic began with a long-forgotten mass murder:
The weather, that summer of 1922, held steady at what the newspapers like to call "fair", the skies a gas-flame blue, the temperatures hovering near 80 degrees. On the last day of July, as Lillian Goetz's mother would forever recall, the morning was another warm one. She offered to make her daughter a box lunch, but Lillian refused. It was too hot to eat a real lunch; she'd just grab a quick sandwich at a lunch counter.
The 17-year-old daughter worked as a stenographer in a dress goods firm, occupying a small set of offices in the Townsend Building, at the bustling corner of 25th and Broadway. There were plenty of quick eateries nearby, tucked among the offices and shops and small hotels. Lillian, like many of her co-workers, often just stepped over to Shelbourne Restaurant and Bakery, just a half block south on Broadway.
The Shelbourne catered to the office trade, opening in the morning, closing in the early afternoon. Stenographers and secretaries in their bright summer hats and stylish short skirts, businessmen and office managers in their dark tailored suits crowded daily along its wooden counters and small square tables, hurrying through a meal of coffee, hot soup with fresh-baked rolls, sandwiches, and slices of the bakery's renowned peach cake and berry pie.
According to police reports, on July 31, Lillian ordered a tongue sandwich, coffee, and a slice of huckleberry pie. It was the pie that killed her.
Five other people died as well and more than 60 went to the hospital that day, the scream of ambulances down Broadway was so constant that people called the police department thinking the city had caught fire. The lead suspect was a baker at the Shelbourne, who'd caught a false rumor that he was about to be fired.
Arsenic, at the time, was remarkably easy to acquire. It was used in popular rodent  poisons (my favorite had the very direct name Rough on Rats). It was used as a tonic, in brands such as Fowler's Solution. It was beloved by poison murderers because it was odorless and mostly tasteless. In a white powdery form, like arsenic trioxide, it folded almost invisibly into pastry dough.
poisons (my favorite had the very direct name Rough on Rats). It was used as a tonic, in brands such as Fowler's Solution. It was beloved by poison murderers because it was odorless and mostly tasteless. In a white powdery form, like arsenic trioxide, it folded almost invisibly into pastry dough.
Today, thanks to improved regulations, arsenic cannot be so casually acquired. Nor is it in the same homicidal demand. Forensic toxicology has made arsenic far too detectable a means of death. It's been identifiable in a corpse for well over 100 years, these days, in the barest trace amounts. And as a metallic element, it remains in the body (notably in the hair) for centuries. It serves, in fact, as a indelible marker of murder.
The fascinating, twisted story of arsenic then was an obvious choice for my book. The tale of little Lillian Goetz maybe less so. But there was this moment of heartbreak that just stayed with me. I read countless news stories about the Shelbourne killer. There's a moment, in one of them, in which her mother, Anna Goetz, is talking to the police about that rejected box lunch, caught at that point in which she knows, she's sure, that she could saved her daughter's life if she'd only insisted on that homemade meal.
Oh, I could see myself – the working mother of two boys – caught in that same moment, replaying that loop in which I might have rescued my child, could have kept her alive, kept him alive, if I'd only done things differently. One of the tasks that I'd set for myself in the book was – despite my real fascination with the wicked chemistry of poisons – to never glorify the subject. Poisoners represent human evil in my story. A lost child like Lillian reminds us of that, should remind us of that.
Still, when I received an e-mail recently with the subject line "Lillian Goetz", I had a moment where I worried that someone in the family didn't agree with me. In that, I was wonderfully wrong. The message came from Lillian's nephew Steve Goetz, a physiology teacher, and he wrote: When I began the chapter in your book covering arsenic, I was amazed to see Lillian Goetz's story featured. I had never realized that her death was a part of such a large and publicized event. Lillian was my aunt, my father Nelson's older sister. Her death by poison was rarely mentioned in the family, and most of the details were vague.
But though they rarely spoke of her, she was always there, a ghost in the house. Her death rewrote the way they lived. Steve was born in 1943 at Bronx Hospital, the facility that treated the dying girl: When my Grandfather, Lillian's father William Goetz, visited my mother who had given birth to me in 1943 at Bronx Hospital, he told her how very sad he felt revisiting the place. After Lillian's death, her parents (William and Annie) discarded all religious items in their house and were non-observant Jews from that point on. My grandmother Annie rarely left her apartment as long as I knew her, and my 98 year old mother told me the other day that that was also true since at least the early 1930′s, when she first met Annie.
Steve also sent me the photograph that I've put at the top of this post. His 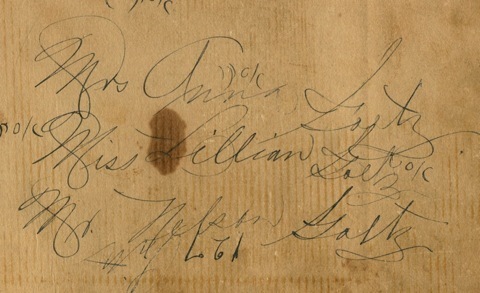 grandmother, Annie Goetz is in the middle, with a very young Lillian holding one hand and her brother Nelson (Steve's father) holding the other. He even sent an image of the back of the photo, all names carefully written in the that lovely cursive handwriting of the past with its lacy capital letters.
grandmother, Annie Goetz is in the middle, with a very young Lillian holding one hand and her brother Nelson (Steve's father) holding the other. He even sent an image of the back of the photo, all names carefully written in the that lovely cursive handwriting of the past with its lacy capital letters.
I've found myself studying their serious faces, taken during an era when people so rarely smiled for photographs. I've pondered Lillian's sober little face under that white hat, imagined her growing up into a dedicated, responsible young woman. But I know that doesn't really do her justice.
I wrote back to Steve Goetz, asking him if I could share the photo and family information and he answered me in the kindest way: "I had rarely thought about Lillian for most of my life – she seemed to be such a distant figure. I want to thank you for bringing her to life for me as a real person, in way she had never existed for me before. My only tenuous link to her is her copy of the Rubaiyat of Omar Khayyam, which I've had for many years. It contains a bookmark, a cut-out yellowed newspaper column called Our Rhyming Optimist. Aline Michaelis published 6 poems a week for her column from 1917 for the next 17 years. The poem Lillian saved is called "You Have Come Back."
Since I learned about, and was given the book, I've been intrigued that my aunt, coming from a family that seemed not to place a high priority on education or reading, should have this book of poetry. I've always felt that she must have been an interesting and sensitive person, that I would have liked to have gotten to know."
So this one's for you, Lillian. In remembrance, and regret. And a wish that you'd never ended up in my book.

April 28, 2011
Chemical-Free Chemistry
The other day my friends at JAYFK posted a story about an innovative new idea in chemistry sets for young science students.
Yes, folks, the idea is that young scholars should learn about the science of chemistry through doing, um, experiments that are, um, chemical free.
I've been puzzling over this idea. How would a budding chemist do these "chemical free" experiments when everything in the box from plastic to paper is made of chemical compounds? One could imagine that these small scientists might put water in the plastic test tubes pictured below. But, no, that would be H2O. Another known chemical compound.

Photo courtesy of JAYFK
As the folks at JAYFK note, I've been writing – some might say ranting – about the use of the phrase chemical-free for some time now. I even proposed that since this is the International Year of Chemistry, we declare a ban on the phrase declare an official ban on phrase "chemical free."
Yes, it's an advertising phrase, yes, it theoretically indicates a product free of industrial chemicals. But it doesn't just sell organic grapefruit. It sells the idea that all chemicals are evil and it sells the idea, apparently, that one can label a chemistry set as full of "chemical-free" options.
And it absolutely deserves its star billing in JAYFK. If you wonder why I say that, just visit there. Chemical-free chemistry could not be turn up in a more appropriately titled publication.

March 26, 2011
A Dazzle in the Bones
This is the last of a three-part "series" on the Radium Girls, the young workers who painted luminous watch faces during the 1920s – and unknowingly became some of the first human test subjects on the dangers of radiation exposure. I told my version of their story in my book, The Poisoner's Handbook, but it's worth revisiting here. It remains a cautionary tale of radioactive elements, the slow recognition of their danger, and the risks of scientific over-confidence – that rings remarkably true today.
The bones, removed in 1928 from a five-year-old grave, belonged to an Italian-American woman. Her name was Amelia Maggia and she'd died just after she'd turned 25.
Before her death, Maggia worked at the U.S. Radium Corporation for four years, faithfully painting watch faces with luminous paint, lip-pointing her brush to create the fine point needed for the work. In her last year at the factory, 1921, she'd started abruptly losing weight. Her joints started to ache; she found herself moving, she told her doctor, like a tired old woman.

Radium Illuminated Clock Face
The following year, her dentist discovered that Maggia's jaw was splintering apart; almost all of it was removed. But she then developed a horrifying anemia; she bled constantly from her mouth, and she'd died in September 1923. Her death certificate read: "ulcerative stomachitis."
Medical examiner Harrison S. Martland, of Essex County, N.J., had found Maggia on a list of former dial painters. He was deep into his investigation of radium as a possible poison and he suspected that the diagnosis was, well, completely wrong. The symptoms read like textbook radium sickness to him. He didn't blame the attending physician; he'd been shocked himself to realize how wicked the element could be. His first report on the dial painters was simply titled "Some Unrecognized Dangers in the Use and Handling of Radioactive Substances".
 But how deep did those dangers run? How deeply did the radium settle into the bones of these workers? How long did it stay there, spitting radiation? He had Maggia's body exhumed –to check his theory that she'd died of radium poisoning – and to get a better measure of the element's destructive power. For help, he contacted the New York City Medical Examiner's Office, asked if its brilliant toxicologist – Alexander Gettler – could figure out a way to find the rattle of alpha radiation in a dead woman's bones.
But how deep did those dangers run? How deeply did the radium settle into the bones of these workers? How long did it stay there, spitting radiation? He had Maggia's body exhumed –to check his theory that she'd died of radium poisoning – and to get a better measure of the element's destructive power. For help, he contacted the New York City Medical Examiner's Office, asked if its brilliant toxicologist – Alexander Gettler – could figure out a way to find the rattle of alpha radiation in a dead woman's bones.
Martland's red flag of a report had been published earlier, in 1925. That same year the U.S. Radium Corporation was sued by a small group of former dial painters. There were only five of the Radium Girls – as the press liked to call them – in that action. A few had already settled; more were afraid to take on a big corporation; sure that they'd lose the jobs they held now, that they'd lose in the courts anyway.
And the doubters were right about one thing. The company had no intention of making this easy. It took three years of legal wrangling to even get a trial date set in 1928. And coincidentally – and with what would turn out to be very bad timing for the U.S. Radium Corporation – that coincided with the decision by Martland to contact the New York City Medical Examiner's Office about those ragged bones.
The New York City scientists methodically set about figuring out how to test an aging skeleton for evidence of a little understood radioactive element. They scraped away the shreds of remaining tissue from the bones; they burned those scraps into ash. They boiled a selection of bones – skull, five cervical vertebrae, five slices of rib, both feet, femurs, the right tibia, the right fibula – for hours in a solution of washing soda. The bones were scrubbed, air-dried, the larger ones sawed into two inch pieces.
The prepared bones and the tissue ash were then taken into a darkroom and placedonto x-ray films wrapped in black photographic paper. Then, for comparison, they went through the same process with pieces of washed bone and tissue from a normal corpse. The bone, tissue and film packages were left to sit for ten days in a sealed darkroom with the idea that "If radioactive, the bones and the tissue ash would emit rays, and the beta and gamma rays would penetrate the black paper and affect the photographic film."
The published photographs – those of the dial painter's bones – showed a dazzle of pale spots, starred against a black background, as unmistakable as the glitter of a constellation on a dark night. By contrast, "Those (films) on which normal bones were placed are not shown, because they did not show any impression." But from Amelia Maggia's remains, "every piece of bone, as well as every tissue ash that we examined, showed radioactivity by the photographic method." And if a dead woman's bones still sparked with radiation, they had no doubt that the same could be said for the bones of the still living dial painters.

Cartoon published May 20, 1928, New York World
As the lawsuit dragged on, the Radium Girls became sicker and sicker.
Two of them, Quinta MacDonald and Albina Larice, were sisters of the dead woman whose bones had provided so much evidence in Gettler's laboratory. Both of Quinta's hips had fractured; Albina was bedridden, one of her legs was now four inches shorter than the other; Edna Hussman could barely shuffle across her room; oddly, years after leaving the factory, her hair still glowed in the dark; Grace Fryer now worked in a bank; with a metal brace from neck to hips to support her spine. Katherine Schaub's jaws were starting to break apart; as she told her lawyers, she hoped the money – they were asking $250,000 each – would pay for her funeral. "If I won my $250,000, mightn't I have lots of roses?"
Thirteen other dial workers, including Schaub's cousin, had died in the three years since the lawsuit was filed. And the company lawyers, even now in the spring of 1928, had found another argument for dismissing the complaint. This time they proposed that the statute of limitations had run out on the plaintiffs' injuries. The workers should have come to court when they were actually exposed to radium, not now, years later, when they no longer had jobs with the U.S. Radium Corporation.
It was true that several of them were unemployed because they could no longer walk or talk or had had most of their faces removed due to bone necrosis. And it was true that legal maneuvering had delayed proceedings but, the company asserted, the case had lost all validity. New Jersey law required court action within two years of an injury. Some of these workers had left the factory long before the 1925 filing of the lawsuit. And so much time had passed since then, the corporation lawyers argued, it was a matter of law that the Radium Girls' time had come and gone.
The response for the attorneys for the injured women came legal came right out of the research of Harrison Martland and Alexander Gettler.
The plaintiffs' lawyer replied that radium was a different kind of poison; like arsenic or a mercury compound, those old fashioned poisons with their simple, direct toxicity. This wasn't a matter of a one time exposure, but rather a permanent one. These women were still being poisoned; poisoned every day by a radioactive element that never left, simmered in the body, bubbled in the bones.

Published in Everyday Science and Mechanics, June 1932
Yes, the suit was three years old, and, yes, the women had left those dial painting jobs years earlier. But this minute, even, all five were still exhaling radon gas, and the radium in their bones was still killing them.
The judges in Newark's chancery court found the plaintiff's argument, the image of those irreversibly radioactive bones absolutely plausible. And appalling. The court dismissed the corporation's motion and set the trial, at last, for June 8 in federal district court in Manhattan.
The following day the company agreed to settle the case – $10,000 in cash for each woman, a $400 a year pension, and the guarantee of complete medical care, to be covered by the U.S. Radium Corporation and its insurers.
It was less than the dial painters had hoped for – but they had made their point, proved their poison, and started the country on a path to regulating radioactive materials (with both Martland and Gettler involved in that crusade). And the Radium Girls, well, they were glad to get the money while they were still alive to use it.

March 25, 2011
Life in the Undark
This is the second of a three-part "series" on the Radium Girls, the young workers who painted luminous watch faces during the 1920s – and unknowingly became some of the first human test subjects on the dangers of radiation exposure. I told my version of their story in my book, The Poisoner's Handbook, but it's worth revisiting here. It remains a cautionary tale of radioactive elements, the slow recognition of their danger, and the risks of scientific over-confidence – that rings remarkably true today.
How long does any scientific discovery remain completely untarnished? Radium raised that question and in a very specific way. How long does it take for a miracle cure – a trace element, a white-silver gleam in the rocks – to be viewed not as a savior but a killer?
Marie Curie, one of the Nobel Prize winning discoverers of radium, tended to defend the element as if it was a child under attack. She toured the United States, seeking money for radium research in 1921.As Curie told the crowds that gathered to see her, she did not fear her own discovery. She kept vials of radioactive isotopes in her skirt pockets, bringing them out to show off during her lectures. She liked to see them in the dark, she'd say, to sit back and watch their pretty blue-green light.

Radium in a Flask (American Institute of Physics)
But in other quarters, a certain scientific wariness was starting to surface regarding radium. There were rumors that Curie's husband, Pierre, killed in 1906 by a horse-drawn carriage, had stumbled in the street due to radiation-induced weakness. Several scientists from the European radium laboratories had developed disturbing leukemias. And when Curie finished her tour, American dignitaries presented her with a gram of radium as a gift from the U.S. – but carefully contained in a 110-pound lead box.
The occasional deaths of scientists across the Atlantic stirred little reaction in the United States, caused no easiness in the blue-collar factories of Orange, New Jersey. The worries about the element grew slowly, almost entirely driven by the health problems among the dial workers, the young women painting watch faces with luminous paint. The paint, called "Undark" by their employers at the U.S. Radium Corporation, gained its glow from the element radium. The workers had almost coincidentally began falling ill shortly after Curie's triumphant American tour. By 1924, as workers continued to die, managers at the U.S. Radium Company hired a team of scientists from Harvard University to investigate the accelerating reach of inexplicable death.
The Harvard scientists discovered that the plant was thick with radium dust, the  employees coated with it. In the dark, one researcher said, the dial-painters glowed like ghosts. The investigation concluded that the deaths were connected to the factory work. Still the scientists noted that radium had such a safe reputation, they were reluctant to blame it completely. Even this cautious assessment did not go over well with factory management. The U.S. Radium Corporation refused to allow the study to be published, saying the information was too sensitive to be released.
employees coated with it. In the dark, one researcher said, the dial-painters glowed like ghosts. The investigation concluded that the deaths were connected to the factory work. Still the scientists noted that radium had such a safe reputation, they were reluctant to blame it completely. Even this cautious assessment did not go over well with factory management. The U.S. Radium Corporation refused to allow the study to be published, saying the information was too sensitive to be released.
The same year, though, a team of less cooperative scientists also started pursuing the problem at U.S. Radium, running tests on many of the ailing workers, some still employed, others who had moved on to other jobs. The doctors from the Consumers League of New Jersey, already well known for its uncompromising positions on worker safety, did publish their findings, summing up with a declaration that the factory in Orange was incubating a new, strange and terrible occupational disease.
At this point, the chief medical examiner in Essex County, Harrison S. Martland decided to conduct his own investigation, one that would be uncolored by claims of pro-management or pro-worker bias on either. It didn't take him long to agree with both sides on one point: radium exposure was the problem. In his examination of the dial painters, he'd discovered a fact that made that impossible to dismiss:
The women were exhaling radon gas.
 That finding provided the first real clue as to what was happening to the dial painters. It was also a testament to the way radium worked, especially its naturally self-destructive nature.The element essentially existed in a state of perpetual breakdown, discarding excess parts as it decayed, subatomic particles fizzing away in all directions, leaving behind an even more crazily unbalanced chemical arrangement, prone to immediate further decay. As it fizzed apart, the resulting own breakdown products included the hyper-charged element polonium (sometimes called radium A) and radon gas.
That finding provided the first real clue as to what was happening to the dial painters. It was also a testament to the way radium worked, especially its naturally self-destructive nature.The element essentially existed in a state of perpetual breakdown, discarding excess parts as it decayed, subatomic particles fizzing away in all directions, leaving behind an even more crazily unbalanced chemical arrangement, prone to immediate further decay. As it fizzed apart, the resulting own breakdown products included the hyper-charged element polonium (sometimes called radium A) and radon gas.
Radium, then, was "radioactive" because it was constantly turning into something else, discarding unwanted parts as it did so in the form of energetic subatomic particles. The primary emissions from radium were called alpha particles; these were basically tight little bundles of protons and neutrons.
As alpha particles sped away, they took with them some of the energy-charged life
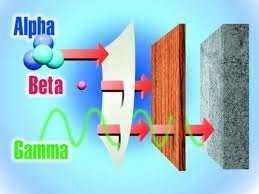
(deq.idaho.gov)
of the element. Thus thee flight of charged particles was often simply called alpha radiation. Radium emitted other forms of radiation but Martland calculated that more than 90 percent of the particles shooting out of radium came from alpha radiation. This wasn't all that bad: alpha particles were in their way rather wishy-washy bits of atomic energy. They could be stopped by a sheet of paper, a layer of clothing, even the upper layer of dead cells that overlay the skin. The other forms of radiation were actually more formidable. Beta radiation easily sliced through paper but could be stopped by a sheet of aluminum; the hurtle of gamma radiation could only be blocked by a dense material like lead.
But inside the body, as Martland would soon realize, alpha radiation created a precisely engineered internal poisoning. The radium dust noted by the Harvard team posed a definite hazard because it could be inhaled. But the reason that the hard-working dial painters were so much sicker than others in that dusty factory was their practice of lip-pointing the brushes. Every time a painter put a brush in her mouth to bring the bristles to a sharper point she ended up swallowing radium.
That would turn out to be the worst way to absorb the poison. Structurally, the element radium could be considered a close if crazed cousin of the element calcium. Both were alkaline earth metals, silvery white in color. Both were built by cubic crystalline structures. When a person swallowed radium, the body channeled it in a way similar to calcium – some was metabolized away, some went toward nerve and muscle function, most was deposited into the bones.
But where calcium, of course, strengthened and added to the mineral content of the

1928 newspaper cartoon about the Radium Girls (gvsu.edu)
skeleton, radium did the opposite – it bombarded skeletal material with alpha radiation, blasting it full of tiny holes, and then larger ones, and then larger. It irradiated the blood-forming marrow in the bone's center. No wonder that the dial painters' jaws literally rotted away, hips broke, ankles crumbled away, anemias and leukemias bubbled in the bone marrow. No wonder that, gradually, people were beginning to realize that radium – far from offering a radioactive sparkle of renewed health – glowed like a warning light, hinting at death.
In 1925, Martland had detailed these principles of radiation poisoning in the Journal of the American Medical Association. He'd learned many of those facts by studying the bodies of dial painters who had died; among those still living, he'd learned to derive a formula calculating the amount of radium in their bodies. That was based on the gas they exhaled. Radon gas was produced in the skeleton as the radium there decayed; the gas diffused into the bloodstream, was carried to the lungs, exhaled to drift away.
Until the next breath. Make that the next poisoned breath.
This series on The Radium Girls will conclude tomorrow.

March 24, 2011
The Radium Girls
As we analyze and worry over radiation seeping from Japan's earthquake-damaged nuclear plants, it seems a curiosity that less than a hundred years ago, many people still believed that radioactive elements were the stuff of wonder. Of course, that changed in the horrific aftermath of the nuclear bombs the U.S. dropped on Japan in World War II. But there were warnings beforehand, small ones, really, canaries in the radioactive mines, if you will. The story I find most haunting is that of the Radium Girls, the young painters of luminous watch dials in the 1920s. I told my version of their story in my book, The Poisoner's Handbook, but it's worth revisiting here. It remains a cautionary tale of radioactive elements, the slow recognition of their danger, and the risks of scientific over-confidence – that rings remarkably true today. Today's post is the first of three that I'll post in the next few days.

Dial painters working at the U.S. Radium Corporation (Argonne National Laboratory)
The bones were five years old, slightly yellowed, with scraps of decayed tissue clinging to them. But the New Jersey doctor who'd ordered the skeleton excavated thought they had a lethal story to tell, if he could only understand it. In 1928, he contacted the New York City Medical Examiner's office to find out if they could decipher the story in the bones. What he wondered – and no one had ever asked this before – was whether those aging bones might be radioactive?
To put that question in context, one had to look back some 30 years, to when scientists in France had announced a startling discovery, that the rocks of the Earth's crust were not all cold dead chunks of metal and mineral. Some were strangely alive; some sizzled with energy, even emitted radiation.
The French physicist Henri Becquerel reported the first such discovery in 1896. He'd found that the element uranium emitted atomic particles that could pass through metal foil, creating a spatter of light spots on photographic film. His work was taken up by newly married physicists, Pierre and Marie Curie, and two years later they reported their discovery of two new elements, both of which emitted particles at a greater rate than uranium. One they named polonium, after Marie Curie's native Poland. The second they simply named for radiation itself, calling it radium. They also proposed that elements like radium and polonium, with their peculiar atomic snap and sizzle, should be known as "radioactive" elements. All three scientists shared the 1903 Nobel Prize in Physics for this pioneering work.

Marie Curie (nobelprize.org)
It was radium – "my beautiful radium" as Marie called it – that seemed to embody the best, the most promising of these new materials. Polonium was too intensely active; it literally burned itself away within a year. Uranium was more stable but that was because it seemed less energized, leaking its radiation comparatively slowly. Radium, on the other hand, glowed with promise. It decayed slowly; its half- life was 1600 years, yet it spit and sparked with a steady release of energy. The Curies had measured radium's intensity at some 3,000 times that of uranium. It was rather like finding a tiny star buried in the dirt. A very tiny star – the Curies had isolated only 100 milligrams of pure radium from some three tons of uranium ore. But that only gave it the allure of something truly rare.
Within two years, physicians had learned that the application of radium salts to a tumor would shrink the cancer; "radium therapy" was introduced into hospitals shortly after the turn of the 20th century. Physicians reported what seemed to be miraculous healing effects, especially compared to the therapies of old. The newspapers compared radium's magic to the golden healthful rays of the sun. Everyone wanted to stand in what seemed to be a naturally healing light.
There were bottles of radium water (guaranteed to make the drinker sparkle with energy), radium soda, radium candy, radium-laced facial creams to rejuvenate the skin, radium-sprinkled face powder in four clearly labeled tints: white, natural, tan and African, soaps, pain-relieving liniments and lotions. Researchers discovered that the European hot springs, famed for their healing powers, contained radon, a gas created by an interaction of radium and water. Perhaps, scientists suggested, the health effects of the mineral hot springs came from radioactive elements in the ground around them; spas in upstate New York rushed to compete by dropping uranium ores into their swimming pools; a New Jersey company grew rich selling hundreds of thousands of bottles of "Radithor: Certified Radioactive Water" as a tonic that guaranteed new vigor and energy. Radiant Health, the ads proclaimed, beautiful skin, endless vigor, and eternal health – ingesting radium seemed the next best thing to drinking sunlight.
energy), radium soda, radium candy, radium-laced facial creams to rejuvenate the skin, radium-sprinkled face powder in four clearly labeled tints: white, natural, tan and African, soaps, pain-relieving liniments and lotions. Researchers discovered that the European hot springs, famed for their healing powers, contained radon, a gas created by an interaction of radium and water. Perhaps, scientists suggested, the health effects of the mineral hot springs came from radioactive elements in the ground around them; spas in upstate New York rushed to compete by dropping uranium ores into their swimming pools; a New Jersey company grew rich selling hundreds of thousands of bottles of "Radithor: Certified Radioactive Water" as a tonic that guaranteed new vigor and energy. Radiant Health, the ads proclaimed, beautiful skin, endless vigor, and eternal health – ingesting radium seemed the next best thing to drinking sunlight.
The New Jersey physician, Harrison Martland, chief medical examiner of Essex County, had a different, less inspirational idea about radium. Of course, his first encounter with involved a rather mysterious health crisis arising at the U.S. Radium Corporation in Orange, N.J.
The corporation's success story began with the new technological demands of the Great War. Soldiers, huddled in the muddy trenches of Europe, learned quickly that the pocket-watches they carried were totally unsuited to the battlefields. The timepieces fell out of pockets, were crushed by the next crawling soldier, and if the watches somehow weren't smashed, they were hopelessly unreadable at night. Driven by military need, watch companies began putting watches on straps, which could be safely buckled on, and began looking for a way to make watch faces glow in the dark.
Luckily, German scientists had developed a "self-luminous" paint some years before the war. This paint glowed due to a rather neat little cascade of chemical interactions: if radium salts were mixed with a zinc compound, particles emitted by the radium caused the zinc atoms to vibrate. The vibration created a buzz of energy, visible as a faint shiver of light. This pale greenish glow was easily outshone by daylight, but in the dark, it was just luminous enough to make an instrument readable without making it easily detectable by a watching enemy.
 After American troops joined the war in Europe, the factory in Orange, New Jersey won a contract to supply radium-dial instruments to the military. By the time the war ended, wristwatches with their glowing dials and handy wristbands were all the style. So were luminous-faced clocks, nicely dressed up in gold and ebony for elegant homes. The corporation's business was as healthy as ever, as healthy, you might say, as radium itself.
After American troops joined the war in Europe, the factory in Orange, New Jersey won a contract to supply radium-dial instruments to the military. By the time the war ended, wristwatches with their glowing dials and handy wristbands were all the style. So were luminous-faced clocks, nicely dressed up in gold and ebony for elegant homes. The corporation's business was as healthy as ever, as healthy, you might say, as radium itself.
There was not a thought worth mentioning that radium might not really be the golden child of the elements.
At the factory, the dial painters were taught to shape their brushes to a fine point with their lips, producing the sharp tip needed to paint the tiny numbers and lines of watch dials, the lacy designs of fashionable clocks. Each worker was expected to paint 250 dials a day, five and a half days a week. They earned about $20 a week for that work, at a rate of one and a half cents per completed dial.
The painters were teen-aged girls and young women who became friendly during the hours together and entertained themselves during by breaks by playing with the paint. They sprinkled the luminous liquid in their hair to make their curls twinkle in the dark. They brightened their fingernails with it. One girl covered her teeth to give herself a Cheshire cat smile when she went home at night.. None of them considered this risky behavior. Why would they when doctors were using the same material to cure people, when wealthy spa residents were paying good money to soak in the stuff, when the popular tonic Radithor was promoted by neighboring company? No one – certainly not the dial painters themselves – saw anything to worry about it.
Until, one by one, the dial painters began, mysteriously, to fall ill. Their teeth fell out, their mouths filled with sores, their jaws rotted, they wasted away, weakened by an apparently unstoppable anemia. By 1924, nine of the dial painters were dead. They were all young women in their 20s, formerly healthy, with little in common except for those hours they spent, sitting at their iron-and-wood desks at the factory, painting tiny bright numbers on delicate instruments.
Those bones, the ones that Harrison Martland had sent for a radioactivity check? To bring the story full forward from those heady days of discovery in France, those bones belonged to a dial painter from Orange, New Jersey.
Continued tomorrow.

March 16, 2011
The Tom Lehrer Cure for the Blues
I've spent the last week writing – and discarding – posts about poisonous happenings around the planet. Murders in Ohio, accidental carbon monoxide poisonings in Wisconsin (two men, two dogs), the increase in the killing of birds of prey in Britain by toxic means.
But there are times when it's fun and fascinating to explore the strangeness of life in our chemical world, and there are times when it just seems to add to the ambient darkness. This week – shadowed by worry and grief in Japan – just seemed like the wrong time to deepen the shadows.
But I do have a chemical cure for that problem. I've found myself, as a cure for the blues, playing The Elements (song) by Tom Lehrer for friends and family alike. You may wonder, of course, if all my friends and family are as geeky as I am or if that's just polite laughter ringing in my ears.
To that end, here are a few of my favorite versions (yes, versions) of The Elements found on YouTube. There's this one:
And this one:
And this one which focuses on phonetic pronunciation (although I can't make up my mind about the bobbing chemist who rather reminds me of a target in a shooting gallery):
And THIS one of actor Daniel Radcliffe of Harry Potter fame reciting the song on a television talk show.
My favorite part of it, of course, is when he tells the audience to shut up. Cheers me up every time. But a writer of poisonous stories can't stay cheerful too long. Expect me back soon with a cold little tale of murder.

March 4, 2011
The Amazing Exploding Classroom
Some years ago, my older son enrolled – with some reluctance – in a summer chemistry camp. On the second day, while conducting an experiment, he and his fellow students accidentally, um, set the building on fire. Just a tiny sizzle, really, but one that resulted in evacuations, firefighters, and screaming sirens. He came home goggle-eyed: "Chemistry is the best science ever!"
I remembered this moment during a visit to Mount Royal University in Calgary, Canada, where the chemistry department had invited me to talk about The Poisoner's Handbook as part of an International Year of Chemistry celebration. In fact, it came back to me at precisely the time that chemistry professor Nathan Ackroyd was telling me about a spectacular classroom demonstration that involved methanol, an empty jug, and a lighted match.
To prep for the demonstration, Ackroyd filled a five-gallon jug with methanol vapors (not a home project; careful handling by a careful chemist required). Even though he was sure of the effect, he was a little worried about the next part of the demonstration – the part where he dropped a lighted match into the vapor-filled jug. During a test run, he had just slightly charred the ceiling of his office.
"My hand was shaking a little and the first match didn't drop in. The students all thought that was pretty funny – of course, they didn't know why I was nervous." The second match went right to target. The vapors ignited with a roar. Flames shot upward nearly eight feet toward the ceiling. The classroom had a 20-foot-high ceiling but the shockwave rattled the ceiling tiles and blew a nearby projection screen backwards.
"Everyone jumped backward," Ackroyd continues.
Eventually, the flames died back as they consumed all the oxygen in the jug. But a fresh supply of oxygen rushed in and the fire made another spectacular leap. "It continued to pulse for two of three minutes." He did move a later demonstration outside to a campus green space; the fiery results led another faculty member, watching out a window, to summon the university police.
After the police determined that this was merely a chemistry professor and not a crazed arsonist, they requested that he please let them know in advance if he planned any further demonstrations.
"So what were you demonstrating?" I asked.
"I wanted to talk about the need for oxygen in a combustion. And I wanted to get at the concept of detonation velocity. In any combustion reaction (visualize an explosion moving outward) there's an expansion of heated gases. If Ackroyd had created a fast-moving expansion, there wouldn't even have been time for that flame to shoot upward from the jug's spout. The blast of pressure and heat would have just blown out the sides of the container. Comparatively, then, methanol combustion is relatively slow.
"And the third reason," Ackroyd continues. "It's fun!"
Truly, I love the way they teach chemistry today. When I was studying chemistry at college, the only exciting, flammable moments were provided by me. That is, I set my braid on fire in a bunsen burner. The post-doc overseeing the laboratory was unfazed, merely tapping me on the shoulder and asking me if I smelled smoke. (I hadn't, actually.) True, the students surrounding me were entertained by watching me smack the sparks out of my hair. But that was merely a side show courtesy of an absent-minded student.
Today we have chemistry professors like Bassam Shakhashiri at the University of Wisconsin-Madison running a whole program titled Science is Fun. Shakhashiri – the incoming president of the American Chemistry Society (ACS) – puts on an annual holiday show for the public, a festival of chemical fireworks, that packs in hundreds every December. (Note: I also teach at the University of Wisconsin and my family has been among the pack.)

Bassam Shakhashiri sprays metal salt solutions to color a flame
Could we use more Ackroyds and Shakhashiris? Absolutely. No scientist, I believe, puts on a better show than a chemist, can better take an abstract idea like combustion velocity and make it vividly real. Obviously, such showmanship also carries with it real risks and I don't mean to dismiss them here. The very properties that allow for a fiery demonstration also make this a profession demands great respect for the power of a chemical reaction.
So, I'm not advocating for a fire-department-on-call situation with chemistry lessons (despite the positive impression on my son). Rather, I'd like to express my appreciation – and yes, envy – of the kind a terrific show that a good chemist can create. To admire the way that such scientists work to make research accessible – and memorable. "Because science explains how the world works, we can talk about whatever we want," Ackroyd says. So we might as well talk about something fun."
As a further demonstration, I've put one of my favorite fiery chemistry videos here. It involves, I'm afraid, the sad, sad end of a gummy bear:
And you know that old saying – that we just don't do it like they used to. I don't think it holds true with chemistry classes. Today, we do them better.

February 23, 2011
The Chemist as Murderer
On January 14th, a 39-year-old computer engineer was admitted to Princeton University Hospital in New Jersey with nagging, flu-like symptoms. The man was nauseated, suffering from severe joint pains, wracked by a strange, convulsive trembling in his legs. Doctors at the hospital tried one treatment after another but Xiaoye Wang only became weaker.
 Finally, a nurse at the hospital stepped hesitantly forward. She remembered a 1995 case in China in which a student at Beijing University became mysteriously ill. The cause was eventually found to be poisoning by the extremely toxic element thallium. The young woman received a life-saving antidote although she suffered lingering disabilities from the attack.
Finally, a nurse at the hospital stepped hesitantly forward. She remembered a 1995 case in China in which a student at Beijing University became mysteriously ill. The cause was eventually found to be poisoning by the extremely toxic element thallium. The young woman received a life-saving antidote although she suffered lingering disabilities from the attack.
And – as the nurse recalled from the highly publicized case – the student's symptoms were eerily similar to Wang's. During the man's hospital stay, he'd developed new signs of worsening illness – he'd lost his hair; his skin had thickened; his hands and feet had gone numb.
The Princeton doctors were dubious about a fairly exotic poison use, but they were running out of ideas. They desperately wanted answers. So although but hey couldn't find an in-state laboratory to do the tests, they sent blood and urine samples out of state. And to their shock, the tests proved the nurse right. The lab had discovered a shockingly high level of thallium in Wang's body.
On January 25, as soon as the results were in, the hospital contacted the New Jersey Poison Control Center for help. The doctors had no experience with thallium poisoning. They needed to know how to save their patient.
As Steven Marcus, head of the poison control center, told the Newark Star-Ledger, his first reaction was that no one suffers an accidental thallium poisoning. Thallium is a dangerous and carefully regulated poison, mostly found in laboratories. "It's either attempted suicide or homicide," he said. Marcus added that he knew of only one good antidote for thallium poisoning, a medication called Prussian Blue.
 Rather ironically, the antidote's name derives from another famously lethal substance. Prussian Blue refers to cyanide (a component of the medication) which can be used to produce a royal blue pigment. Some cyanide formulas are lethal, notably hydrogen cyanide or potassium cyanide. But mixed into the tidy antidote formula (brand name Radiogardase) cyanide merely becomes part of a chemical chain that wraps itself around thallium, binding it up, and allowing the body to remove the poison.
Rather ironically, the antidote's name derives from another famously lethal substance. Prussian Blue refers to cyanide (a component of the medication) which can be used to produce a royal blue pigment. Some cyanide formulas are lethal, notably hydrogen cyanide or potassium cyanide. But mixed into the tidy antidote formula (brand name Radiogardase) cyanide merely becomes part of a chemical chain that wraps itself around thallium, binding it up, and allowing the body to remove the poison.
By the time, the New Jersey doctors were able to secure the antidote though, it was too late. He died on January 26 leaving doctors – and now criminal investigators – to answer the question raised by Steven Marcus. Was it suicide or was it murder?
I actually devoted a chapter of my book, The Poisoner's Handbook, to thallium because it's such a fascinating poison. Agatha Christie knew this long before I did – it's the star of her murder mystery story, The Pale Horse. A key to the Christie novel is that thallium appears to be a near perfect homicidal poison is that it is tasteless, odorless, mixes smoothly and easy into food and drink. A key to my non-fiction tale of five thallium deaths in 1930s New York is that this is also a highly detectable poison. Or as I wrote in the book:
" In the manner of other metallic poisons, such as arsenic, thallium stayed stubbornly in the body, permeating the tissues for weeks and even months after death. Any knowledgeable forensic toxicologist could find it.
It was, one might say, a chemist's poison."
Which was exactly what the authorities in New Jersey concluded as well.
There was no evidence that the dead man was suicidal. But further investigation found that Wang was involved in an angry divorce which included disagreements over property division and custody of a two-year-old son. His wife, Tianie "Heidi" Li was a research chemist at Bristol-Myers-Squibb, working in a laboratory that included access to thallium.
Tianie Li in Court/photo credit ChinaSmack.com
On February 8, Li, 40, was charged with murdering her husband. She has since pleaded not guilty and is also seeking to have her bail, currently set at more than $4 million reduced.
Let's acknowledge first that Li has not been convicted of murder. Still her arrest raises some intriguing questions on the subject of poison murders. For instance, does one need a chemistry degree to be a thallium killer?
Actually, the answer to that one is an easy no. The killer does need to have some specialized knowledge though. Thallium (unlike cyanide) is not a well-known toxic substance. So while the killer in my own book was a high school graduate, those murders occurred at a time when thallium was a widely available pesticide. The U.S. government removed it from household markets in 1972, though, because of the risks.
I'm aware of one other well-publicized thallium murder that involved a killer with chemical training and that occurred in Alturas, Florida in the late 1980s. In that case, a Mensa member and troubled former chemist became annoyed with his neighbors, who he perceived as noisy and inconsiderate.
The angry chemist, George Trepal, left them an anonymous gift of Coca Cola spiked with thallium. The poisoned sodas killed one neighbor and hospitalized two others for months. Trepal was not a scientist with a happy history. At the time of the deaths, he had a criminal record, having served time for working as chief chemist for a methamphetamine laboratory. In 1991, he was convicted of one count of first degree murder and six counts of attempted murder. He remains on Florida's death row today and is the subject of a book titled Poison Mind.
But investigators were never sure how Joann Curley of Wilkes-Barre, Pennsylvania, decided to kill her husband, Robert, by putting thallium in his iced-tea. Curley confessed to the murder in 1996, after a relentless five year investigation into his death. And Ann Perry of Long Island, sentenced in 2002 for killing her abusive boyfriend with thallium-laced milkshakes, had no special chemical knowledge either.
The acclaimed British author, John Emsley, who specializes in writing about chemistry in books such as The Elements of Murder has suggested that it's mystery writers like Christie who really brought thallium into public's mind as a murder weapon. Among the examples he gives is the case of Graham Young, a worker in a British photographic instrument company, who killed two of his co-workers in 1971 by mixing thallium into their coffee.
Image credit: Daily Mail
As news of Li's murder arrest has spread, increasingly the stories have been framed around chemist-as-killer. "Chemist killed her husband with radioactive poison to avoid going through a divorce" was the headline in Britain's Daily Mail. "NJ chemist pleads not guilty to poisoning husband," was a more stately lead in BusinessWeek. And, of course, some of this is just headline writing shorthand for an event.
But make no mistake. The history of thallium homicides mostly serves as a reminder that any of us can play at the poison murder game. Yes, Dr. Li had specialized knowledge and access to thallium but as a Joann Curley proves, that's not necessary for a determined killer. If Li is convicted, being a trained chemist won't have made her anything special. She'll just be another one of those over-confident murderers who ended up getting caught.
But I'd like to add this footnote – she'll also be another murderer caught by a chemist.

February 15, 2011
In Defense of Science Blogs (yes again)
Two days ago, the acclaimed British science journalist and blogger, Ed Yong, published a post titled I think you have all you need for a blog. This detailed an e-mail exchange with a public information officer who'd been approached for, surprisingly enough, information for a story.
The PIO was – let's say – reluctant to help. He explained that after 15 years as a journalist, he was able to judge who needed in-depth details and, apparently, it wasn't a blogger. The PIO in question – later identified as Aeron Haworth of the University of Manchester – went on to assert that Yong was only a "journalist wannabe." This latter – let's say – exercise in poor judgment appeared in the comment section of another blog post, this one from another notable journalist/blogger, Ivan Oransky, a health editor at Reuters, titled How to demonstrate you're not about transparency and piss off reporters – as a PIO.
As Oransky noted, Haworth was refusing to share information about a study that was, in fact, already widely available. That angle was picked up by another outstanding journalist/blogger, Maryn McKenna, in a post titled How Not to Publicize Science: A Sad and Cautionary Tale (Bring Popcorn). In fact, the Haworth debacle was promptly picked up as "please don't do this" example by David Harris, who writes the savvy blog, The Enlightened PIO.
Myself, I want to pick at another point, found in that remark: "I think you have all you need for a blog." Italics mine. Haworth critics have justly pointed out that his fumble began with not being clued in enough to know that Ed Yong, who writes award-winning blog, Not Exactly Rocket Science, for Discover, is justly regarded as one of best science writers working today. The other two science bloggers I cited are also at the top of the game. Oransky (an MD) is executive health editor for Reuters; McKenna is author of the influential book on emerging infections, Superbug. My point is not to emulate Who's Who here; my point is that the world of science blogging is populated with some of the best journalists I know.
And my particular complain is not that Haworth wasn't sharp enough to know who Ed Yong is - its that he wasn't sharp enough to recognize just how good – and how influential – the world of science blogging has become. Or that bloggers are starting to set new standards in excellence regarding how we share information about research.
"I was a journalist for 15 years, which included being a newspaper reporter and a magazine publisher" Mr. Haworth says, explaining why he knows that a blog isn't worth his time. Well, not to date myself too much, but I was a newspaper staff writer for 22 years, during which time I won a Pulitzer Prize and began president of the National Association of Science Writers (USA). So I also feel somewhat qualified to judge meaningful journalism.
And what I've come realize, despite my print background, despite my abiding love for the science journalism I practiced at a traditional newspaper, is that science blogs offer some of the best, most illuminating, most intelligent communication of science out there today. I'm not telling you that I admire all blogs any more than I would claim to admire all newspapers. I am telling you that it's a mistake to let a newspaper background blind one to the sometimes amazing work being done online.
I blog myself – obviously – at the network hosted by the Public Library of Science (PLoS) and I've done work I'm proud of here. But sometimes, seriously, I am humbled by my fellow bloggers (and I wish I could list all them) – the incredible reporting done by Emily Anthes in her piece, Real, Live Practice Babies, the almost physically beautiful writing of Steve Silberman in posts like this one; the wonderfully smart work of John Rennie. If I could be as smart and funny as John, I am positive I would have won that second Pulitzer that I've always coveted. (No, I am never satisfied.)
But I am smart enough to recognize a blaze of talent and good journalism when I see it. I acknowledge that we're still in an evolutionary period in journalism – painful for many of my generation. It's not only public information officers who dismiss – or angst about – bloggers. Last October, as you may remember, the editor of the journal Analytical Chemistry went off into a rant about blogs and the future of science education and communication.
I suspect these miscues and rants are simply part of the process of change. But they offer opportunity as well as irritation. As is now ongoing, we dissect the mistakes. And we use the moment to illuminate the increasing professionalism of science blogging. Eventually, I hope, this leads us to a time in which whether it's an Ed Yong or a Tim Oleson, one of my science journalism students at the University of Wisconsin, who blogs about geology, the response is the same.
And it goes like this: "So glad you contacted me. How can I help?"

February 7, 2011
Plants, platypuses, poisons and, oh yes, paperbacks
 Why do I feel this sudden urge to plant a poison garden? Oh, nothing on the scale of the one at Britain's Alnswick Castle (provocative gates pictured at left), but at least a leafy border full of foxglove and monkshood, maybe with a little jimsonweed and poison ivy thrown into the mix.
Why do I feel this sudden urge to plant a poison garden? Oh, nothing on the scale of the one at Britain's Alnswick Castle (provocative gates pictured at left), but at least a leafy border full of foxglove and monkshood, maybe with a little jimsonweed and poison ivy thrown into the mix.
I blame it on my paperback giveaway for The Poisoner's Handbook, actually. so many of the entries concerned lethal vegetation. "Plants that people do not think are poisonous, but actually ARE, such as Hemlock, Foxglove, Hellebore, Nightshade and Yew," wrote one commenter. And here's another: "Foxglove, clematis, bryony, bloodwort… the list is endless, it seems. Would be interesting to find out how these were used historically as medicines and poisons."
And a personal favorite: "I've been reading a lot of gardening catalogs lately, and notice plants marked "poisonous, keep seeds away from children and pets." Could someone (hypothetically of course) grow castor beans, serve them up to granny, and feign innocence when she gets poisoned?" The poison ricin, in case you wondered, is extracted from castor beans, and is most famous for its use in the 1978 umbrella assassination of Bulgarian dissident Georgi Markov.
I've always liked the subject of plant poisons because it raises a point that I think the often forget. We humans didn't invent toxic substances. The natural world was always fully armed and from the beginning, our planet was fully loaded and capable of generating lethal events without our help – think, as one comment pointed out, about limnic eruptions and the suffocating potential of carbon dioxide. Or consider venoms – from snakes, from bees, and even from those rather adorable looking Australian platypuses. In fact, the comments about platypuses produced a link to a Grant Jacobs post on the subject on his terrific blog, Code for Life.
But there were also ideas concerning less "natural" hazards. The industrial compounds included hydrofluoric acid (a remarkably poisonous compound used in many pharmaceutical preparations) and the suspected carcinogen acrylamide found notably in french fries and potato chips. And one astute comment cited a post that I've been meaning to write for literally months, concerning over-the-counter the drug, acetaminophen, and its troubling and poisonous side-effects.
 In fact, the ideas were so good that I wish I had a larger stockpile of paperbacks to give away. I do hope that if you're intrigued by the ideas raised here that you'll take a moment to go back to the comment list on the original giveway post – it's a great way to get an overview of the kinds of questions that some very smart people are asking about poisons.
In fact, the ideas were so good that I wish I had a larger stockpile of paperbacks to give away. I do hope that if you're intrigued by the ideas raised here that you'll take a moment to go back to the comment list on the original giveway post – it's a great way to get an overview of the kinds of questions that some very smart people are asking about poisons.
For those who entered the giveaway contest, if you see your idea specifically cited or quoted here – congratulations! I'll be contacting you directly for instructions on sending your autographed copy of the brand-new paperback. And thanks to everyone who entered – just a terrifically smart list of ideas. I wish I could do them all.
And about that poison garden? My husband, I'm afraid, has vetoed the idea. For some reason.

Deborah Blum's Blog
- Deborah Blum's profile
- 426 followers