Deborah Blum's Blog, page 3
November 20, 2011
About Pepper Spray
 One hundred years ago, an American pharmacist named Wilbur Scoville developed a scale to measure the intensity of a pepper's burn. The scale – as you can see on the widely used chart to the left – puts sweet bell peppers at the zero mark and the blistering habenero at up to 350,000 Scoville Units.
One hundred years ago, an American pharmacist named Wilbur Scoville developed a scale to measure the intensity of a pepper's burn. The scale – as you can see on the widely used chart to the left – puts sweet bell peppers at the zero mark and the blistering habenero at up to 350,000 Scoville Units.
I checked the Scoville Scale for something else yesterday. I was looking for a way to measure the intensity of pepper spray, the kind that police have been using on Occupy protestors including this week's shocking incident involving peacefully protesting students at the University of California-Davis.
As the chart makes clear, commercial grade pepper spray leaves even the most painful of natural peppers (the Himalayan ghost pepper) far behind. It's listed at between 2 million and 5.3 million Scoville units. The lower number refers to the kind of pepper spray that you and I might be able to purchase for self-protective uses. And the higher number? It's the kind of spray that police use, the super-high dose given in the orange-colored spray used at UC-Davis.

Photo courtesy: California Aggie
The reason pepper-spray ends up on the Scoville chart is that – you probably guessed this - it's literally derived from pepper chemistry, the compounds that make habaneros so much more formidable than the comparatively wimpy bells. Those compounds are called capsaicins and – in fact – pepper spray is more formally called Oleoresin Capsicum or OC Spray.
But we've taken to calling it pepper spray, I think, because that makes it sound so much more benign than it really is, like something just a grade or so above what we might mix up in a home kitchen. The description hints maybe at that eye-stinging effect that the cook occasionally experiences when making something like a jalapeno-based salsa, a little burn, nothing too serious.
Until you look it up on the Scoville scale and remember, as toxicologists love to point out, that the dose makes the poison. That we're not talking about cookery but a potent blast of chemistry. So that if OC spray is the U.S. police response of choice – and certainly, it's been used with dismaying enthusiasm during the Occupy protests nationwide, as documented in this excellent Atlantic roundup - it may be time to demand a more serious look at the risks involved.
My own purpose here is to focus on the dangers of a high level of capsaicin exposure. But as pointed out in the 2004 paper, Health Hazards of Pepper Spray, written by health researchers at the University of North Carolina and Duke University, the sprays contain other risky materials:
Depending on brand, an OC spray may contain water, alcohols, or organic solvents as liquid carriers; and nitrogen, carbon dioxide, or halogenated hydrocarbons (such as Freon, tetrachloroethylene, and methylene chloride) as propellants to discharge the canister contents.(3) Inhalation of high doses of some of these chemicals can produce adverse cardiac, respiratory, and neurologic effects, including arrhythmias and sudden death.
Their paper focuses mostly, though, on the dangerous associated with pepper-based compounds. In 1997, for instance, researchers at the University of California-San Francisco discovered that the "hot" sensation of habeneros and their ilk was caused by capsaicin binding directly to proteins in the membranes of pain and heat sensing neurons. Capsaicins can activate these neurons at below body temperature, leading to a startling sensation of heat. Repeated exposure can wear the system down, depleting neurotransmitters, reducing the sensation of the pain. This knowledge has led to a number of medical treatments using capsaicins to manage pain.
Its very mechanism, though, should remind us to be wary. As the North Carolina researchers point out, any compound that can influence nerve function is, by definition, risky. Research tells us that pepper spray acts as a potent inflammatory agent. It amplifies allergic sensitivities, it irritates and damages eyes, membranes, bronchial airways, the stomach lining – basically what it touches. It works by causing pain – and, as we know, pain is the body warning us of an injury.
In general, these are short term effects. Pepper spray, for instance, induces a burning sensation in the eyes in part by damaging cells in the outer layer of the cornea. Usually, the body repairs this kind of injury fairly neatly. But with repeated exposures, studies find, there can be permanent damage to the cornea.
The more worrisome effects have to do with inhalation – and by some reports, California university police officers deliberately put OC spray down protestors throats. Capsaicins inflame the airways, causing swelling and restriction. And this means that pepper sprays pose a genuine risk to people with asthma and other respiratory conditions.
And by genuine risk, I mean a known risk, a no-surprise any police department should know this risk, easy enough to find in the scientific literature. To cite just three examples here:
1) Pepper Spray Induced Respiratory Failure Treated with Extracorporeal Membrane Oxygenation
2) Assessing the incapacitative effects of pepper spray during resistive encounters with the police.
3) The Human Health Effects of Pepper Spray.
That second paper is from a law enforcement journal. And the summary for that last paper notes: Studies of the effects of capsaicin on human physiology, anecdotal experience with field use of pepper spray, and controlled exposure of correctional officers in training have shown adverse effects on the lungs, larynx, middle airway, protective reflexes, and skin. Behavioral and mental health effects also may occur if pepper spray is used abusively.
Pepper spray use has been suspected of contributing to a number of deaths that occurred in police custody. In mid-1990s, the U.S. Department of Justice cited nearly 70 fatalities linked to pepper-spray use, following on a 1995 report compiled by the American Civil Liberties Union of California. The ACLU report cited 26 suspicious deaths; it's important to note that most involved pre-existing conditions such as asthma. But it's also important to note a troubling pattern.
In fact, in 1999, the ACLU asked the California appeals court to declare the use of pepper spray to be dangerous and cruel. That request followed an action by northern California police officers against environmental protestors – the police were accused of dipping Q-tips into OC spray and applying them directly to the eyes of men and women engaged in an anti-logging protest.
"The ACLU believes that the use of pepper spray as a kind of chemical cattle prod on nonviolent demonstrators resisting arrest constitutes excessive force and violates the Constitution," wrote association attorneys some 13 years ago.
Today, the University of California-Davis announced that it was suspending two of the police officers who pepper-sprayed protesting students. Eleven of those students were treated by paramedics on scene and two were sent to a hospital in Sacramento for more intensive treatment.
Undoubtedly, these injuries will factor into another scientific study of pepper spray, another acknowledgement that top of the Scoville scale is dangerous territory. But my own preference is that we start learning from these mistakes without waiting another 13 years or more, without engaging in yet another cycle of abuse and injury.
Now would be good.

November 11, 2011
Bedtime Scoop and Easy Lay
 In the spring of last year, a 21-year-old college student from Wisconsin named Julia Sumnicht decided that she needed to thaw out after a Midwestern winter. She flew to Miami, Florida, dreaming, I imagine, like so many of her peers, of sun and sand and drinks all the colors of confetti. But not for long.
In the spring of last year, a 21-year-old college student from Wisconsin named Julia Sumnicht decided that she needed to thaw out after a Midwestern winter. She flew to Miami, Florida, dreaming, I imagine, like so many of her peers, of sun and sand and drinks all the colors of confetti. But not for long.
Sumnicht died in Miami of an overdose of GHB, the shorthand name for gamma-hydroxybutyric acid. There are plenty of other names for GHB, ugly ones like Bedtime Scoop, Easy Lay, Grievous Bodily Harm. Because GHB - colorless, odorless, slightly salty but easily disguised by a fruity cocktail, a potent sedative with memory-damaging side effects – is one of today's more popular date rape drugs.
According to an analysis done through the non-profit Project GHB, more than 20 people (mostly male) were killed every year between 1995 and 2005 by GHB overdoses. The researchers suggested, however, that their findings underestimated the problem because at many smaller hospitals the routine toxicology screen is not designed to detect the compound. At least it wasn't back then.
Today, medical examiners are giving GHB and its ilk (Rohypnol, Lorazepam, Ketamine) more poisoning priority because, as a newly released national study warns, the practice of mixing date-rape drugs into drinks seems to be on rise in the United States. In the single year of 2009, according to the Substance Abuse and Mental Health Services Administration (SAMHSA) 14,720 emergency room visits resulted from intentional poisonings and many resulted, apparently, from evenings at bars, clubs and parties.
"Approximately three in five (60 percent) drug-related ED visits attributed to intentional poisoning involved alcohol in combination with other drugs ," the report notes. In other words, the classic date-rape drug scenario, the drug slipped into that sweet-colored drink. Or as SAMSHA administrator Peter Delany told CBS News: "These are people that are being given drugs that they don't know about."
The report is the first such survey of intentional poisoning related emergency room visits from the Rockville, Maryland based agency. But unpublished data from the previous year, 2008, clocked only 7,609 such emergency room visits. The sharp increase suggests radically improved reporting measures, a rather alarming upward trend in criminal behavior, or most probably, a combination of these and other factors. A few more years of such reports are likely to give us a more accurate – and possibly even more depressing - picture of the problem.
But if you don't hear the siren-loud "beware" message already blaring from the SAMSHA report then you weren't really listening. Setting aside the body count for a minute, by some accounts, GHB and other such "club drugs" are linked to some 3 million rapes over the last few decades. And this isn't just about fun-and-sun spring breaks. In September, I gave a talk at the University of Oklahoma. Even at that middle of the country campus, on a cloudy fall day, students were talking about the use of the sedative Lorazepam as a date-rate drug.
The beware message is all about level-headed common sense. Don't take drinks from strangers. Don't leave your drink unwatched on a table. Don't be a target. Easy to say, I know, from the tidy safety of a government agency office. Hard to remember in the buzz of a really good party, the blur of yet another round of drinks all the colors of confetti.
But try, okay? Think of the warning message as simply as this: come home safe tonight.
As

November 7, 2011
Remembering Christina

Christina Scott, courtesy South African Science Journalists Association
Some half-dozen or so years ago, a friend volunteered me to work with the World Federation of Science Journalists. I had just stepped down as president of the National Association of Science Writers (USA) and I took on the responsibility with a slight feeling of resentment.
It didn't take me long to realize that I'd been an idiot. That it wasn't only that I was working with science journalists from Africa and Asia, Europe and Latin America but that I was learning from them. I learned how much I'd taken for granted in terms of resources and access to information in my own country. I was reminded of how much one journalist could accomplish with a threadbare budget, a shared office computer, and a passion to make a difference.
No one could get that point across better than Christina Scott, the managing editor of Research Africa, one of South Africa's most influential science journalists, who once illustrated it by walking onto a darkened conference stage holding up the faint glow of a cigarette lighter and reminding the audience that in the corners of Africa, some journalists did their jobs with no more than a spark of light or power in the room. And still did great work.
Christina was 49 years old when she died in an automobile accident just over a week ago. She was, typically, helping someone else at the time, teaching an intern how to drive, in the wrong place when the young woman lost control of the car. It was so sad for everyone concerned and it was such a shock.
You know those sparklers that children play with at Halloween? Tiny wands of fizzing light? Christina was just like that – energy and sizzle, humor and glow. She liked people and her first instinct was to help them. She was, in fact, the kind of person that you could hope would light things up for a very long time. You can get a sense of that on the Remembering Christina Facebook page established by the South African Science Journalists Association.
Among the things posted there are a series of tips she wrote for SciDev, the science news service for developing countries. They're tips to help scientists better communicate with journalists. And they're Christina to a perfect fit in their dancing humor and lovely use of language. But they're also thought-provoking and smart. Which is, I think, another wonderful way to remember Christina:
Christina Scott's 10 things for a scientist to do in an interview
1. Say "What's exciting about my research is …". Say "The single most important issue here is ….". Say "What I love about my work is ….". The people who write, edit and eventually read, listen or watch the media probably don't know much about your work. But we're all human, and we all understand the feeling conveyed by words such as 'exciting,' 'important' and 'love.' Such words seldom belong in peer-reviewed literature so it can be disconcerting for academics to try them out, rather like long-neglected muscles that ache when you return to gym. But these words are great for most mass media interviews. (Don't love your work? Take up another job.)
2. Use your hands as much as possible. Think Italian opera. Especially for television, there can never be too much movement. (Especially if you learn the great tv trick of waving your hands around at shoulder level. It feels really weird the first time you do it, as if you've somehow morphed into being a cricketing referee. But it looks great.) Vary your expressions as much as possible. Shake your head vigorously when you disagree. Nod your head when you agree. And alter your voice as much as possible – higher highs, lower lows. Speak loudly, because electronic recording equipment tends to cancel out lots of nuances and a lot of microphones pick up lots of background sounds, and electronic hissing and humming. Don't back away from the hand-held microphone. Lean towards it. Research suggests that a vast amount of information is absorbed via the body language and the visual and verbal responses of the person being interviewed. Imagine what it's like for someone who's slightly deaf, a little hazy in vision, a listener who's multi-tasking or a reporter who's so anxious that they're not following everything you say – you need to turn into an actor temporarily.
3. Make eye contact with the person asking the question. In an age of Blackberries, iPods, cellphones and laptops, face-to-face conversation is becoming a bit of a lost art. But you don't have the chance in an interview to correct any misunderstandings, so you want to closely watch the person asking the questions, to see if they understand you. If they frown or their eyes glaze over, it's not a good sign. Making eye contact is absolutely critical for television interviews, because if your eyes start shifting sideways to the cameraman, you look like a delinquent. And there's no point in staring at yourself in the monitor to see if your hair is good, or trying to wriggle around to see which camera is aimed at you, because you're going to make yourself look very, very strange.
4. Finish off a response with, "Does that make sense to you?" or "Does that answer your question?" Most people will be too shy to say they didn't understand you at all, so you often have to assess this by looking at a presenter's body language or listening to a caller's tone of voice. If it's a print interview, you may want to ask if the writer wants to read the quotation back to you, especially if you think you've baffled him or her completely. Sometimes people answer 'yes, it makes sense, you said ABC' and you realise that actually, no: you said 'XYZ' and you're able to correct the misinformation easily. But don't ask to listen to the tape, disc or read the notes – they're not your property and you're going to hate everything you say and do anyhow. By the way, don't end every statement with this question, or you're going to come across as needy and insecure. Save it for the most complex bits.
5. Anything involving the following words is good: the first, the best, the biggest, the most. Very few journalists are any good at numbers. But journalists – and their audience – understand a few concepts such as first, second and third. Big. Small.
6. Laughter. You're allowed to laugh in an interview. Most interviews, believe it or not, are fun. Laughter makes you sound less intimidating. And it relaxes your vocal chords so you sound less nervous. I tell people to smile when they're being introduced in a radio or television programme: it makes them sound (and look) better. (I also tell people not to cough or rattle papers when someone's asking a question, it makes for horrible sound and visuals. You shouldn't bring papers with anyhow. Bring props, if you can, but no papers.)
7. Use concrete words. If you refer to an organisation by the term 'it,' I may not know if you're talking about the University of Pretoria or SWEAT (Sex Worker Education and Advocacy Trust). If you're going to be edited, it helps to be as specific as possible. If someone's writing down your words, being specific is a big help as well.
Use specific pronouns. 'We ran a survey…'? Who's the 'we'? What about 'With the help of my colleagues in the department of economics, I ran a survey…' Words like 'they' and 'them' are other words which should be transformed into something more specific, like 'My first-year students' or 'Many AIDS researchers ….'
8. Use the reporter's first name, but only at the beginning of the sentence, where it can be edited out without damaging the sentence. Using first names is particularly effective in radio and television panel debates, where it establishes a sense of casual conversation and makes you sound in command of the situation.
9. Talk in a sequence of clear, short sentences. Avoid subordinate clauses because they don't make sense if the top or beginning of the sentence is chopped off (or forgotten, or misspelt). Avoid talking in the style where you pick up where the questioner left off: instead, add the question to the sentence so it is as explicit as possible. I've heard people asking a question, and the interviewee is thinking about an earlier question, and responding to that earlier question without making it clear, and chaos ensues. Perhaps the question is, 'What is the most important aspect of your work?' A stupid question, but one you'll get quite often from nervous junior reporters who have been thrown into the assignment without any time to do research by a cranky editor. In a real conversation, you might begin, "It ….' But I've already said 'it' is a bad idea. Try: "The most important aspect of my work is …." and fill in the blanks. If you need time to think, even if the interview is being broadcast live, you can always fall back on that time-honoured stand-by response: "Oooh, that's a good question!"
10. Finish your statement by dropping your voice. It's a universally-recognised symbol for 'I'm done, now it's your turn.' If it's a press conference or a print interview or a one-on-one interview, avoid speaking for more than ten minutes. After ten minutes, hand cramp sets in and batteries run out and your chances of getting misquoted zoom right up. Better to speak for no more than ten minutes total, including questions, and spend the next five minutes clarifying points made the first time around. Remember, most of the time, you're going to get pruned, slashed, edited, squeezed and chopped by editors. So you may as well do it yourself. Save your own time as well as theirs.

October 31, 2011
A Colorful Little Tale of Halloween Poison
 I grew up on a dead-end street in Baton Rouge, Louisiana, where remnants of swampy forest surrounded the old wood-frame homes. Live oaks lined the streets. Spanish moss dripped from their branches. Snakes coiled under the ancient azaleas that edged the yards.
I grew up on a dead-end street in Baton Rouge, Louisiana, where remnants of swampy forest surrounded the old wood-frame homes. Live oaks lined the streets. Spanish moss dripped from their branches. Snakes coiled under the ancient azaleas that edged the yards.
It was, in fact, the perfect setting for a haunted Halloween night. And there was this one house, you know, where the yard was so dense with bush and tree that it could barely be seen through its thicket of shadow. To trick-or-treat, you walked up the dark sidewalk toward a faint glow on the front porch, just the one lit window. The air hummed with passing insects and the porch creaked like Dracula's coffin under your feet, the slow, dry eek of old wood.
Reader, you had to beware on Halloween night. Just a block over lived a maniacal dentist who liked to dress up like a werewolf on October 31 and fill his front hall with clouds of drifting fog created by dropping dry ice (super-chilled chunks of carbon dioxide) into water. Bwa-ha-ha, he would chortle as he opened the door, as the chilly wisps of fog drifted out around him.
But this silent house, dressed in darkness, was so much scarier. We children would gather in front of the gate, unable to walk alone through those prowling shadows. The crowd would form on the sidewalk: tiny pillowcase ghosts and jeweled princesses, small pirates and glittery fairies. When someone decided we'd achieved a safe number, we'd start edging toward the green door at the top of the porch steps. Whispering about what the old man who lived there would hand out – what dangerous treats might wait for us there.
This was the 1960s and even then, people told stories, warned their children, about the psychopaths out there who might drop poisoned candy into one's hands. In the long history of the holiday, truthfully, this has almost never happened. But the very nature of Halloween – the witch at the door, the monster in the closet – lends itself to such ideas. Wasn't there a crazy woman on Long Island in 1964, after all, who handed out arsenic to trick-or-treaters she thought too old for the candy hunt?
It hardly mattered that as Snopes points out, she didn't kill anyone. And her deliberate poisoning attempt seems to be an odd exception to the general goodwill of the holiday. The psychopath at the door is an urban myth. Most of the poisonous Halloween stories turn out to be mistakes or far more personal tragedies. The worst is that of a Texas father who murdered his eight-year-old son in 1974 for insurance money.
He did so by putting cyanide into into the fruit-flavored sugar inside a Pixie Stick, one of the child's favorites. In an attempt to make the death seem like a random poisoning, the father – Ronald Clark O'Bryan – also gave cyanide-laced candy to his daughter and three other children in his Deer Park neighborhood. These other lethal treats were collected by police as (fortunately) the children hadn't touched them.
O'Bryan – nicknamed The Candyman by the Texas media – was executed by lethal injection ten years after his son's death. But people remembered. And they forgot that the worst outbreak of Halloween candy poisoning had nothing to do suspected killers. The biggest poison outbreak – linked to Halloween of 1950 – was simply caused by orange food coloring used by candy manufacturers.
Scores of children across the country fell ill with severe diarrhea and welting rashes [image error]after eating candy and popcorn balls tinted by the FDA approved Orange Dye No. 1 ( also known as FD&C Orange No. 1, Acid Orange 20, and Orange 1). The "FD&C" indicates that the dye is used in food, drugs and cosmetics. Throughout the first half of the 20th century, Orange 1 was used primarily in candy, cookies, cakes, carbonated beverages, and meat-products such as hot dogs.
As federal investigators would discover upon investigation, the dye was also a rash-inducing occupational health hazard. Orange 1 belonged to a group of seven dyes first approved by the federal government in the year 1906, the first year that this country began regulating food safety. All seven of these dyes were coal-tar dyes, derived originally from the hydrocarbon byproducts of processed coal. Orange 1, for instance, contained benzene, today one of our better known toxic compounds.
But at that Halloween moment in 1950, no one had thought much about colored food. In fact, officials at the U.S. Food and Drug Administration suddenly realized that no one had really taken a good look at these turn-of-the-century food dyes for almost 50 years. The FDA promptly launched an investigation that found that, yes, Orange 1 was definitely poisonous: an oral dose of one gram of the dye per one kilogram of food killed two out of five mice in a day. A 20-week-experiment mixing the dye into rat food killed three of eight test rats.
The researchers also found that manufacturers were tossing the dye into candy corn and sugary little pumpkins with surprising enthusiasm. According to a 1954 article in The New York Times, one piece of candy was 1,500 parts per million pure Orange 1. Two years later, in 1956, the FDA delisted Orange 1 as well as Orange 2 (used to deepen the color of oranges) and Red Dye No. 32. Twelve other food colorings have been delisted since that time. This doesn't reassure everyone; consumer advocates still worry over the health effects of food coloring.
But – take at least this reassurance: it's been a long time since we saw children falling ill across the country because they indulged in an extra handful of candy corn, not realizing that its cheerful orange was a signal for trouble. We're mostly smart enough to realize that regulating food safety offers more protection than worrying about the crazy man behind the door.
Which brings me back to my friends and I hesitating at that shadowy gate on a Halloween night in Louisiana. Let me tell you what happened, Halloween after Halloween. Slowly, we inched down the sidewalk, creaked up the steps, quavered at the door. Slowly, the door pulled open and the slightly tottering elderly man opened the screen to drop glossy red apples into our bags.
Every year it added an extra thrill to the night. But, reader, you had to beware on Halloween night. I'm almost positive they were just bright fall apples. But our parents wouldn't let us eat them.

October 26, 2011
A (slightly belated) science blog celebration
About a year ago, in this very blog, I wrote a response to a journal editor's complaint about science bloggers. My post was both a defense of science blogging and a critique of long-standing scientific reluctance to engage with the public.
This year, my post, "The Trouble with Scientists", was selected for the anthology, Best American Science Writing 2011, edited by Rebecca Skloot (author of the terrific best-seller The Immortal Life of Henrietta Lacks) and her father, Floyd Skloot, another acclaimed writer.
This isn't exactly news. The anthology was published in September. And yet is is news – or at least news that should be celebrated, because this was the first edition of that anthology to include blog posts, not only from me but from Ed Yong, of Not Exactly Rocket Science, and Carl Zimmer, of The Loom.*
And that probably makes the point as well as my original defense of science blogging. But in celebration, and in case you missed it, here's my post that made the anthology:
When I first started in journalism I worked as a general assignment reporter. After a few years, I decided to become a science journalist. I thought it made sense, to focus on a subject that fascinated me rather than continue to rattle around assorted news beats. But I still remember the look of frozen horror on my father's face when I announced the decision.
As you may deduce, my father is a scientist. He received his PhD in 1955 from the University of Illinois, where in addition to studying entomology he learned the essential lesson that "real" scientists shared their work only with each other and did not attempt to become "popularizers" because that would lead to "dumbing down" the research.
He emerged from paralysis to say: "I hope you're not planning to interview my friends." A science historian at the California Institute of Technology once told me that this disdain is rooted in the way we teach science. In particular, he said, K-12 science classes in the United States are essentially designed as a filtration system, separating whose fit for what he called "the priesthood of science" from the unfit rest of us.
"Why would I want to interview boring old entomologists?" I naturally replied. This conversation was in my parents' living room (father in armchair, daughter pacing) but variations on this theme occur any time, any place. Scientists won't talk to journalists; they don't want to waste their time "dumbing it down"; they don't see it as "making us smarter." So many of the good stories in science don't get covered at all. Or the stories get covered only for an already science-literate audience – explored in publications like Discover or Science News – rather than for that far larger group, the science disenfranchised.
Last week's editorial by Royce Murray, the editor of Analytical Chemistry, "Science Blogs and Caveat Emptor" brought home the point that while the medium may change, the dilemma remains the same. My PLoS colleague David Kroll, has done a brilliant job of blogger defense, pointing out that many are scientists (like Kroll himself) or award-winning science writers, emphasizing the rise of smart science blog networks. He demonstrates perfectly that Royce's broadbrush declaration "the current phenomenon of 'bloggers' should be of serious concern to scientists" shows that the editor failed to do his homework.
My first reaction to Murray's piece was to wonder if he belonged to my father's generation of scientists-who-hate-to-share. Sure enough, he received his PhD in 1960, reinforcing my feeling we'll really move forward in improving public understanding of science when we approach it through Kroll's kind of 21st century mindset.
For one thing, one of Kroll's remedies is to suggest that more scientists became bloggers – yes, public communicators of science – themselves. I've always thought that my own profession of science journalism grew to fill the void created by scientists who couldn't be bothered to "dumb down" their work. Since the mid-1950s, the National Association of Science Writers (and, yes, I'm a past-president so I like to mention it) has grown from several hundred members to nearly 3,000. At the same time, science journalism programs have sprung up at universities from UC-Berkeley to New York University.
Science writers, journalists, broadcasters and bloggers became the voice of science during a time during which too many scientists simply refused to engage. Scientists have ceded that position of power amazingly readily; ask yourselves how many research associations offer awards to journalists for communicating about science but none to their own members for doing the same. Ask yourself how the culture of science responds even today to researchers who become popular authors or bloggers, public figures. Whether young scientists are rewarding for spending time on public communication? And ask yourself how hypocritical this is, to complain that the general public doesn't understand science while refusing to participate in changing that problem?
As it turns out, the culture of the "real" scientist who exists somehow separate from the rest of us has not been a boon for public understanding or appreciation of science. So let me make a case that it's not too late for Prof. Murray and those who think like him to approach science communication differently. It doesn't hurt to remember that we in the science-literate section of the bleachers aren't the only ones who matter here. He writes that he's worried about the anti-science voices on the Internet; the best way to counter is probably not through an inner circle editorial in
Analytical Chemistry.
To end on a happy note, my father decided that he wouldn't disown me after all, that having a science journalist daughter wasn't quite as embarrassing as he'd anticipated. He started calling his friends to make sure they would talk to me. He went on the Today show and persuaded former host Bryant Gumbel to eat beetles on the air. Of course, he once gave an interview to the National Enquirer, under the impression that it was the National Observer. But as I keep telling him, he should congratulate himself on reaching a new audience.
* Carl Zimmer wrote (you'll see it in comments) to remind me that his anthology piece was a magazine article. Just me anticipating – The Loom is fantastic.

October 12, 2011
The Poisoner's Calender (No. 2)
DENVER, Colorado – October 10, 2011 – An 80-year-old woman was hospitalized Sunday after carbon monoxide seeped into her home from an old heater. Levels of the gas were so high that firefighters had to wear oxygen masks to rescue her.
So I know this makes me sound a little twisted – and I have noticed that some people do tend to sidle away at parties – but for almost two years now, I've been tracking Google alerts on the subject of poison and poisoning. It's a habit that began when my book, The Poisoner's Handbook, was published in 2010. As that story took place mostly in the 1920s, I found myself wondering about today – about the chemical web that we continue to navigate, about the poisons that we continue to use (and sometimes abuse). About how far we'd come, I suppose, in the 70-odd years since the the first forensic medicine program was established in the United States in 1934.
FRISCO, Texas – October 9, 2011 - Fifteen people from this small town north of Dallas were sent to the hospital with carbon monoxide poisoning Saturday after the gas seeped into a hair salon from a loose heater vent. Most of those sickened were high school girls visiting the salon to get ready for a Prom night dance.
We don't poison in the free-handed manner of the early 20th century – thankfully. We've moved on from the days of confident poison killers, sure they wouldn't get caught. We no longer have major American cities warning that "skillful poisoners can operate almost with impunity", as New York City's commissioner of accounts did in the winter of 1915. That doesn't mean that we've left the age of homicidal poisoners entirely behind.
COAL TOWNSHIP, Pa – October 4, 2011 - An elderly couple and their 43-year-old daughter were found dead in their apartment after co-workers began to worry when the younger woman failed to show up for work. Police said all three were killed by carbon monoxide seeping into their home from a faulty gas heater.
This week, for instance, a South African woman was arrested for killing her three-month-old daughter by mixing a pesticide into her cereal. Last week, a New York state man tried to kill both himself and his daughter with ammonium chloride, a toxic compound found in household fire-extinguishers. Last month, a Utah woman was charged with trying to kill a former roommate by stirring antifreeze (which contains the poison, ethylene glycol) into a fruit smoothie. We haven't entirely left behind our poison paranoia either. In August, a San Diego man stabbed his wife after deciding that she was trying to poison him, slashing his daughter and son-in-law as they rushed to the mother's defense.
CAPE CORAL, Fla – September 27, 2011 – A 76-six-year old woman died and her husband was hospitalized due to carbon monoxide poisoning, after she left the car running in the garage after she returned from some late evening errands.
More often than we poison each other, though, we poison each other's pets. Cats. More cats. More cats. Oh, and more cats, although these just represent a scatter of the cases that jam my mailbox. Not to mention dogs. More dogs. Way too many dogs. Not to mention wild birds, wild elephants, lions, horses…I keep promising myself I'll do a full post on this subject and but reading the stories always leads to the moment when I'm out the door and walking off a clouding case of depression.
CLEMENTS, California – September 20, 2011 – A grandfather and his 14-year-old granddaughter were found dead in their horse-trailer-camper this weekend, both killed by carbon monoxide poisoning. Rescuers said they had been cooking inside on a charcoal grill.
I don't mean to give you the impression that all poisonings are homicidal or deliberate. Most are accidental – an encounter with the wrong container, a malfunctioning piece of equipment, a genuine mistake. According to the Journal of Pediatrics, the accidental poisoning of children by prescription drugs rose 22 percent between 2001 and 2008. We use more of them is all; there's more opportunities for kids to pick up a container from a bedroom dresser or kitchen counter.
We worry so much about exotic chemical exposures that I can get 44 million hits on Google if I type in the phrase "chemical free", leading me down the path where dwells such improbable ideas as a "chemical-free bug repellent" or a "mattress that is free of chemicals" (although, um, containing latex among other materials.) I mention this in case you feel the need here for some comic relief.
But my point is that we far too often dwell on the risks that aren't and are careless with the risks that are. What do we make of two teenage boys who are poisoned by anti-freeze because a family member had stored some left-over amount in a whiskey bottle? But as you may have already guessed, my real focus here is on the most routinely lethal poison in our lives.
CLARKESVILLE, Tenn. – September 19, 2011 – Five bikers at a charity event for needy children died when carbon monoxide seeped from a faulty heater into their rented camper during the night. They were found dead in the morning by other friends gathered for the Bikers Who Care festival.
You'll notice by the title of this post that is the second Poisoner's Calendar post to appear on Speakeasy Science; the first appeared last fall. You'll also find that they have a remarkably – okay – identical structure. That's because of all the poisonous tales that come my way, carbon monoxide remains the perpetual murderous star of the story. The U.S. government estimates that it kills about 500 people in this country annually and puts at least another 15,000 or so into the hospital. We're hardly alone; a report released this week in the United Kingdom found a tripling of carbon monoxide related deaths over the last year.
I've picked a few random examples of carbon monoxide poisoings from the last few weeks, at the moment they seep in at about one or so a day. But that will change for worse as predictably as the weather chills. In winter, leaky furnaces and aging generators and the way snow seals off ventilation in houses combine to push the numbers up. Already you can find stories out there, with headlines like "Cold Weather Serves a Reminder to Check Gas-Fire Appliances." Or at least install a carbon monoxide detector or two (above your head, by the way. Carbon monoxide is light. It rises to the ceiling, fills a room from the top down.)
And if such standard reminders don't work, try this one:
MIAMI, Fla – January 8, 2011 – Services were held today for five teens killed when the poisonous gas carbon monoxide seeped into their hotel room, drifting from a car left running in the unit garage.
Don't make me write this again next year.

September 29, 2011
So, 268 chocolate chip cookies later…

Credit: verybestbaking.com
1 cup butter flavored shortening OR maybe 1 cup of butter. Or maybe, I'm thinking, one cup of each. Yeah, that would work.
"Wow, that's a lot of fat," a friend says to me after I confess to a baking marathon that featured one-and-half pounds of butter and one pound of butter-flavored Crisco.
"Well, the Crisco was trans-fat free," I retort snappily. Although how snappy can one be after spending three straight weekends in front of a cookie-packed oven? "And it was all in the interests of science."
She just looks at me. "Yeah, right," is written all over her face. I can read it clearly – it's written in capital letters.
Obviously, she doesn't appreciate chemistry at its finest.
3/4 cup white sugar and 3/4 cup brown sugar. Doubled, of course, so that the same amount of sugar goes into the butter recipe and the trans-fat Crisco recipe.
In late August, I was part of a panel about communicating chemistry at the national meeting of the American Chemical Society in Denver. I represented the poison part of the program. Popular Science columnist Theodore Gray showed videos of his Mad Science experiments, as an example, the flaming bacon lance, and don't miss the upcoming one in which he sets a tree on fire while trying to deep fry a turkey. especially liked the deep-fried turkey one that accidentally set a tree on fire. McGill University's Joe Schwartz gave a rapid-fire tour of everyday chemistry questions – can copper bracelets treat arthritis? why can't you use fresh pineapple to make Jell-O? – the answers to which can be found here. There were ten of us, in all, including the session organization, ACS President-elect Bassam Shakashiri, who is so passionate about public engagement with chemistry that his website is actually titled Science Is Fun.
The last speaker was biochemist, food scientist, and cookbook author Shirley Corriher. And as you've probably already guessed, she talked about chocolate chip cookies. If you listen to her here, on an earlier NPR interview on the same subject, you'll catch that same fizz of enthusiasm that sent me home, inspired to bake.
2 eggs (for each recipe). In my kitchen, these are the cage-free, humane-certified, yes, I like chickens, kind of eggs. Just to let you know.
And I'd been thinking about the mystery of the cookie for a while anyway. My late Kentucky grandmother made the best chocolate chip cookies known to humankind (yes, even better than your grandmother) and I'd never been able to recreate them. She claimed she just followed the recipe on the Toll House bag but I just knew there was some grandmotherly magic that she hadn't shared.
grandmother made the best chocolate chip cookies known to humankind (yes, even better than your grandmother) and I'd never been able to recreate them. She claimed she just followed the recipe on the Toll House bag but I just knew there was some grandmotherly magic that she hadn't shared.
Two teaspoons vanilla extract.
And Corriher raised three points that I thought it would be fun to explore as a kitchen chemist:
1) The chemistry of butter gives it a lower melting point than shortening. That means that cookies made with butter spread out more rapidly. They're flatter and crisper. "If you want soft, fluffy cookies," she said, "then you want to try shortening." I was on that one.
2) The amount of protein in flour affects the texture of the cookie. And all wheat flours contain protein. When exposed to moisture and heat, the proteins break down to form a protein-composite called gluten. The more gluten the more elasticity and strength you'll find in the dough. So if you want a chewier cookie, you want a higher protein flour. For delicate and crumbly, a lower protein flour. As a general rule, cake flours contain about 8 percent protein and all-purpose flours between 10 and 12 percent.
3) A chocolate chip cookie recipe has very little liquid in it. What's there comes from the eggs, the water in butter or shortening, and the vanilla. So the longer the dough is left to sit, the more the liquids absorb into the flour and sugars. The dough is drier as the liquid soaks up, easier to handle, and – both chemists and professional bakers tell us - the flavors absorb more richly as well. In that earlier NPR interview, Corriher recommended up to 36 hours after mixing the dough before baking. Talking to me – and I suspect recognizing that I wasn't nearly patient enough to wait that long - she suggested 24 hours instead.
2 1/4 cups flour (at Corriher's recommendation I used a bread flour which is in the medium range of protein content)

Credit: babblebacon.com
I set up my experiment as follows:
Every Saturday morning for three weekends in a row, I made two batches of chocolate chip cookies. One was made with butter, one with butter-flavored shortening. I split each batch in two. I baked half of the dough immediately, saved the other half in the refrigerator, baked it the next day.
1 teaspoon baking soda, 1 teaspoon salt
And here's where I discovered the natural limits of the home experiment. I needed a polished test kitchen surrounded by chemists with measuring cups. Instead, I had a 17-year-old son who hated dark chocolate (milk chocolate chips, Mom!) and a husband who couldn't believe I'd consider anything but dark chocolate. ("Don't you think they're better with that contrast to bitter chocolate?" And back to the son ("Mom! Don't listen to Dad!")
Two cups of milk chocolate chips.
My son hates nuts. My husband prefers them in his cookies. They're in my test kitchen discussing the merits of the additives. I burn a batch. Just a little. My husband likes his cookies extra crispy. My son likes them on the still doughy side. I agree to leave out the nuts since I've ruined this batch of cookies for the teenage control group.
The debate continues. I continue baking cookies. The butter version goes into the red tin. The Crisco version into the blue – a completely scientific separation. Although not for long. As I said, I have a 17-year-old son. I was only grateful that he didn't inhale the dough in his spare time.
But here's the other problem with home test kitchen. The cook has a hidden agenda, that of recreating her grandmother's cookies. And about 100 or so cookies into the experiment, she realizes that these are too sweet. The overnighting of the dough does create more uniformly golden batches, yeah. But they're too sweet, too white sugary, too lacking in the faint tinge of caramel that colors my memory.
So I try:
1 cup brown sugar, 1/2 cup white sugar.
And then I try:
1 1/2 cups brown sugar.
And in the butter version of these cookies that last one comes pretty damn close.
I'm not sure I've discovered either the science or the art – and it's both, as any cook knows - of a perfect chocolate chip cookie. But chemistry of a childhood memory? I stand by the oven, breathing the buttery chocolate air of my childhood summers.
As I said, chemistry at its finest.
Final Recipe
1 cup butter
1 1/2 cups brown sugar
2 eggs
2 teaspoons vanilla extract
2 1/4 cups all-purpose flour
1 teaspoon baking soda
1 teaspoon salt
2 cups milk chocolate chips
Directions
Preheat oven to 350 degrees F (175 degrees C). Grease cookie sheets.
In a large bowl, cream together the butter and brown sugar. Add the eggs one at a time, beating with each addition, then stir in the vanilla .Combine flour, baking soda and salt; gradually stir into the creamed mixture. Finally, fold in the chocolate chips. Drop by spoonfuls onto cookie sheets.
Bake for 8 to 10 minutes in the preheated oven. Remove cookies from hot sheets and allow to cool on a rack or board.

September 19, 2011
Dr. Oz and the Arsenic Thing
Let me get this out of the way first: I don't watch Dr. Mehmet Oz on television.
I did see a show the year before last while I was keeping an older relative company. I can't tell you what it was about, though, because we weren't that long into it before my relative suggested that that I take myself, my twitches, and my sarcastic mumbling to another part of the house.
Consider this a full disclosure of attitude toward Dr. Oz. Consider it also an explanation of why I didn't see his show last week on the (alleged) dangers of arsenic in apple juice. It was impossible to miss, of course, the backwash of the critical reaction that followed, my favorite being Steve Salzberg's wickedly smart take, "Dr. Oz Tries to be A Scientist" in Forbes. I also enjoyed Pharyngula's tale of the FDA's unsuccessful efforts to educate Dr. Oz about arsenic prior to his show. The theme of the news coverage throughout was, let's say, unsympathetic.
The primary criticism was that for a man with a medical degree, Dr. Oz didn't seem to know very much about arsenic. The FDA – rather testily, actually – had pointed out to him that he was testing for total arsenic load. Their objective was that this overstates risk by combining levels of both inorganic (bad, bad) and organic (not so very bad) arsenical compounds.
On average, inorganic arsenic is considered about 500 times more poisonous that organic arsenic. So a high test number that combined the two but was mostly organic would actually indicate less risk than a lower number that involved more inorganic arsenic. Unfortunately – for Dr. Oz and his viewers – he either didn't get this or considered it too complicated for the audience.
As Salzberg pointed out, those combined totals weren't necessarily reliable anyway. Dr. Oz didn't follow the standard test practice of sending his samples to multiple labs. Instead he relied on one testing facility. When the FDA sent juice samples from the same lots to other laboratories, the arsenic levels were a fraction of what Dr. Oz reported. All of which leads us to the essential criticism here, that Dr. Oz sensationalized a non-problem and by doing so irresponsibly frightened consumers of apple juice.
In a cranky, reluctant way, if you're me, you have to kind of admire the way Dr. Oz responded to this concerted hiss of dismay. He continued to maintain that arsenic exposure should always be considered a big, bad thing. And he managed to suggest that this big picture was more important than nitpicking whining about things like test accuracy and arsenic classification. He did this well enough that, for instance, U.S. Sen. Charles Schumer, D-NY, asked the FDA to take another look at arsenic levels in apple juice.
So I'm not going to dwell further on the problems with his broadcast; I'm hardly going to mention the issues with shoddy science and the sensationalism. Hardly at all. What I would like to mention, whine about, nitpick, however, is Dr. Oz's lost opportunity to r illuminate the actual risks. This is arsenic, after all, one of the world's most important – and fascinating – poisons.
He could have sifted out those organic and inorganic test results, for instance, and helped his viewers to understand what they meant. Arsenic (As) is, after all, a naturally occurring metallic element (sometimes called a metalloid). It's also one of those elements that likes to partner up, either with organic (carbon-based) compounds or with inorganic (which for these purposes pretty much means no carbon involved).
Fortunately for us, our bodies tend to break down and metabolize away most organic arsenic compounds fairly efficiently. In fact, many of these organic arsenic compounds (such as arsenobentaine, in case you wondered) form naturally in fish and shellfish. Fish-lovers thus receive get a steady low level exposure to organic arsenic, as far as we know, without reported health effects. A few years ago, there was a suggestion that kelp-based health supplements might contain an arsenic problem, but it foundered – just as Dr. Oz's apple juice case did – on the type-of-arsenic issue.
We humans – and, in fact, most living creatures, don't handle inorganic arsenic nearly as well. Arsenic trioxide (AsO3) or white arsenic is one of history's most famous homicidal poisons – so much so, that back in the 19th century, it was often referred to as the inheritance powder. By some estimates, inorganic arsenic can be fatal in the amount of 60,000 micrograms (about 1/50th the weight of a penny).
Why is it so dangerous? And don't we wish that Dr. Oz had used his moment to ask this very question? As it turns out, the answer lies in actually being nitpicky about the question. Inorganic arsenic toxicity has a lot to do with the number of valence bonds the compound possesses. My favorite, relatively simple, chemical definition of this explains that: A valence bond (or covalent bond) is a type of chemical bond where one or more atoms cling together because of an interaction between the electrons in their outer shells.
In other words, the higher the valence bond number, the grabbier the compound, the greater its ability to insinuate itself into a living system. The two grabbiest forms of inorganic arsenic are trivalent (three bonds) and pentavalent (five). Pentavalent arsenic can, in fact, do a perfectly lethal job of disrupting cellular metabolism. But toxicologists tend to worry more about trivalent arsenic forms, which are also nasty poisons, more persistent, and much harder to remove from drinking water supplies.
And naturally occurring, inorganic arsenic in drinking water around the world does real and physical harm. I've written about this myself regarding the poisoning of water supplies in countries like Bangladesh. But there's health risks to go around even in countries like the United States.
In other words, Dr. Oz could have used this arsenic moment to have picked out a real health risk, educated people about it, maybe even saved a few lives and there. And that's what I hold against him – the careless waste of opportunity - and that's why he makes me twitch. Even at this safe distance from a sofa in front of the television set.

September 2, 2011
Et tu, Science Magazine?
 Earlier this week, I gave a brilliantly titled talk – The Poisoner's Guide to Communicating Chemistry - at the national meeting of the American Chemistry Society in Denver.
Earlier this week, I gave a brilliantly titled talk – The Poisoner's Guide to Communicating Chemistry - at the national meeting of the American Chemistry Society in Denver.
My speech was part of a symposium on communicating chemistry to the public, organized by ACS President Elect Bassam Shakashiri, a passionate crusader for science literary.
Along the way, I mentioned my own small, personal crusade against the term "chemical-free." Yes, I know, it refers to the notion of something being toxic-chemical-free. But first of all, our ideas about toxicity exist on an ever-shifting path of knowledge. And second, as everything in world including the laptop I'm writing this on, the chair I sit on, and myself (as well as every other life form we know) is made of chemical compounds, the phrase chemical-free is at best ridiculous and at worst misleading. And it's the latter issue that troubles me more – the fact that our careless use of this wrong-headed phrase contributes to a general public misunderstanding about not only chemistry but its fascinating and fundamental role in the world around us.
Or words to that effect. After my talk, one of the attending scientists, David Gottfried, of Georgia Tech's Nanotechnology Research Center, came over to talk about the issue. I brought up my exasperated reaction when the usually excellent newspaper, The New York Times, had used the words "chemical free" and "mineral based" in the same sentence. Oh, he said, but he had an even worse example. This summer, the research journal, Science, had – incredibly – cited a chemical free process in its News and Comment section. Specifically, in describing a method for creating fibers out of milk proteins, the July 29 story's concluding paragraph noted: "The best part? The process uses no chemicals or pesticides….".
How, you may wonder and I certainly did, could this appear in a science magazine? How could the writer mention casein biopolymers in one sentence and declare the product free of chemicals in the other? How could an editor miss the illogical nature of the statement?
Because, I suspect, too many people have been conditioned to equate the words "chemical" and "toxic" so that too many people don't even register the contradiction. Do I worry about what it means for science literary when this kind of thinking even pervades science-focused publications. You bet I do.
"How do we change this?" Gottfried asked me. He'd hoped for more of an outraged reaction to the Science piece than actually occurred. My own feeling is that we're coming late to this issue, that we've got years of casual, chemical-free acceptance to overcome, years of chemistry literacy to build. But that's it's never too late to push back. "I don't know, except to keep calling attention, make an issue of the bad examples," I answered.
I've always liked this point made by the remarkable 18th century French physician René Laennec: Do not fear to repeat what has already been said. Men need (the truth) dinned into their ears many times and from all sides. the first rumor makes them prick up their ears, the second registers, and the third enters.
So, on that exalted note, here is a copy of the bad example in question. Let it register far and wide.

August 25, 2011
Of Dead Bodies and Dirty Streets
 In the fall of 1924, five bodies from New Jersey were delivered to the New York City Medical Examiner's Office. You might not expect that to cause the chief medical examiner to worry about the dirt blowing in city streets. But it did.
In the fall of 1924, five bodies from New Jersey were delivered to the New York City Medical Examiner's Office. You might not expect that to cause the chief medical examiner to worry about the dirt blowing in city streets. But it did.
To understand why you need to know the story of those five dead men, or at least the story of their exposure to a then mysterious industrial poison.
The five men worked at the Standard Oil Refinery in Bayway, New Jersey. All of them spent their days in what plant employees nicknamed "the loony gas building", a tidy brick structure where workers seemed to sicken as they handled a new gasoline additive. The additive's technical name was tetraethyl lead or, in industrial shorthand, TEL. It was developed by researchers at General Motors as an anti-knock formula.
But, as I wrote in a previous post, men working at the plant quickly gave it the "loony gas" tag because anyone who spent much time inside showed signs of mental deterioration, from stumbling memory loss to sudden twitchy bursts of rage. In October of 1924, workers in the TEL building began collapsing, going into convulsions, babbling deliriously. By the end of September, 32 of the 49 TEL workers were in the hospital; five of them died.
The problem, at that point, was that no one knew exactly why. Oh, they knew – or should have known – that tetraethyl lead was dangerous. As Charles Norris, chief medical examiner for New York City pointed out, the compound had been banned in Europe for years due to its toxic nature. But while U.S. corporations hurried TEL into production in the 1920s, they did not hurry to understand its medical or environmental effects.
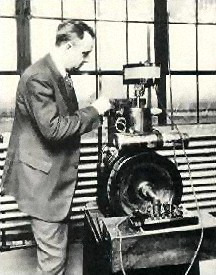
Thomas Midgley, Jr. in the Laboratory: damninteresting.com
Two years earlier, the U.S. Public Health Service had asked Thomas Midgley, Jr. – the developer of the leaded gasoline process – all research into the health consequences of tetraethyl lead (TEL).
Midgley, a scientist at General Motors, replied then that no such research existed. Two years later, he could gave the same answer. Although GM and Standard Oil had formed a joint company to manufacture leaded gasoline – the Ethyl Gasoline Corporation - its research had focused solely on improving the TEL formulas. The companies preferred to avoid the lead issue. They'd deliberately left the word out of their new company name to avoid its negative image.
In response to the worker health crisis at the Bayway plant, Standard Oil suggested that the problem might simply be overwork. Unimpressed, the state of New Jersey ordered a halt to TEL production. And then the compound was so poorly understood, state health officials asked the New York City Medical Examiner's Office to find out what had happened.
In 1924, New York had the best forensic toxicology department in the country; in fact, it had one of the few such programs period. The chief chemist was a dark, cigar-smoking, perfectionist named Alexander Gettler, a famously dogged researcher who would sit up late at night designing both experiments and apparatus as needed.
It took Gettler three obsessively focused weeks to figure out how much tetraethyl lead the Standard Oil workers had absorbed before they became ill, or crazy, or dead. "This is one of the most difficult of many difficult investigations of the kind which have been carried on at this laboratory," Norris said, when releasing the results. "This was the first work of its kind, as far as I know. Dr. Gettler had not only to do the work but to invent a considerable part of the method of doing it."
Working with the first four bodies, then checking his results against the body of the last  worker killed, who had died screaming in a straitjacket, Gettler discovered that TEL and its lead byproducts formed a recognizable distribution, concentrated in the lungs, the brain, and the bones. The highest levels were in the lungs suggesting that most of the poison had been inhaled; later tests showed that the types of masks used by Standard Oil did not filter out the lead in TEL vapors.
worker killed, who had died screaming in a straitjacket, Gettler discovered that TEL and its lead byproducts formed a recognizable distribution, concentrated in the lungs, the brain, and the bones. The highest levels were in the lungs suggesting that most of the poison had been inhaled; later tests showed that the types of masks used by Standard Oil did not filter out the lead in TEL vapors.
Rubber gloves did protect the hands but if TEL splattered and made any direct with skin, it absorbed alarmingly quickly. The result was intense poisoning with lead, a potent neurotoxin. The loony gas symptoms were, in fact, classic heavy lead toxicity.
After Norris released his office's report on tetraethyl lead, New York City banned its sale, and the sale of "any preparation containing lead or other deleterious substances" as an additive to gasoline. So did New Jersey. So did the city of Philadelphia.
Afraid that the trend would accelerate, that they would be forced to find another anti-knock compound, as well as losing considerable money, the manufacturing companies demanded that the federal government take over the investigation and develop its own regulations.
The manufacturers agreed to suspend TEL production and distribution until a federal investigation was completed. In May 1925, the U.S. Surgeon General called a national tetraethyl lead conference, to be followed by the formation of an investigative task force to study the problem. That same year, Midgley published his first health analysis of TEL, which acknowledged just a minor health risk: "compared with other chemical industries it is neither grave nor inescapable."
It was obvious in advance that the federal task force was going to reach that same conclusion. The panel only included selected industry scientists like Midgely. It had no place for Alexander Gettler or Charles Norris or, in fact, anyone from any city where sales of the gas had been banned, or any agency involved in the producing that first critical analysis of tetraethyl lead.
In January 1926, the public health service released its report which concluded that there was "no danger" posed by adding the compound to gasoline…"no reason to prohibit the sale of leaded gasoline" as long as workers were well protected during the manufacturing process.
 The task force focused on the risks associated with every day exposure by drivers, automobile attendants, gas station operators, and found that it was minimal. It was true that lead had turned up in dusty corners of garages and that all the drivers tested showed trace amounts of lead in their blood. But a low level of lead could be tolerated, the scientists concluded. After all, none of the test subjects showed the extreme behaviors and breakdowns associated with places like the looney gas building. And the worker problem could be handled with some protective gear.
The task force focused on the risks associated with every day exposure by drivers, automobile attendants, gas station operators, and found that it was minimal. It was true that lead had turned up in dusty corners of garages and that all the drivers tested showed trace amounts of lead in their blood. But a low level of lead could be tolerated, the scientists concluded. After all, none of the test subjects showed the extreme behaviors and breakdowns associated with places like the looney gas building. And the worker problem could be handled with some protective gear.
There were critics, even then, insisting that this was a biased panel, too deliberately underestimating the risks, too willing to introduce lead into the environment. There was one cautionary note, though. The federal panel warned that exposure levels would probably rise as more people took to the roads. Perhaps, at a later point, the scientists suggested, the research should be taken up again. It was always possible that leaded gasoline might "constitute a menace to the general public after prolonged use or other conditions not foreseen at this time."
But, of course, that would be another generation's problem. In 1926, citing evidence from the TEL report, the federal government revoked all bans on production and sale of leaded gasoline. The reaction of industry was jubilant; one Standard Oil spokesman likened the compound to a "gift of God," so great was its potential to improve automobile performance.
In New York City, at least, Charles Norris decided to prepare for the health and environmental problems to come. He suggested that the department scientists do a base-line measurement of lead levels in the dirt and debris blowing across city streets. People died, he pointed out to his staff; and everyone knew that heavy metals like lead tended to accumulate. The resulting comparison of street dirt in 1924 and 1934 found a 50 percent increase in lead levels – a warning, an indicator of damage to come, if anyone had been paying attention.
It was some fifty years later – in 1986 – that the United States formally banned lead as a gasoline additive. By that time, according to some estimates, so much lead had been deposited into soils, streets, building surfaces, that an estimated 68 million children would register toxic levels of lead absorption and some 5,000 American adults would die annually of lead-induced heart disease. As lead affects cognitive function, some neuroscientists also suggested that chronic lead exposure resulted in a measurable drop in IQ scores during the leaded gas era.
Or, if you prefer, our long – and preventable – loony gas era.
The second of a two part blog series on the early history of leaded gasoline. I discovered this while researching The Poisoner's Handbook and I've always considered it a fascinating and troubling part of our forgotten chemical history.

Deborah Blum's Blog
- Deborah Blum's profile
- 439 followers




