Did We Really Save the Ozone Layer?
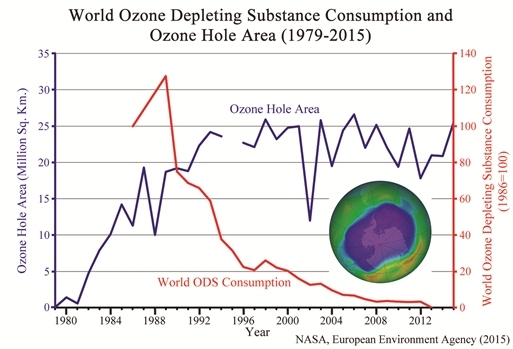
Ozone hole size and worldwide use of ozone depleting substances (click for credit)
A reader sent me this article and asked for my thoughts on it. It discusses the fact that the ozone “hole” over Antarctica grew 22% from 2014 to 2015. It presents the graph shown above, which demonstrates that despite the fact that the worldwide use of chemicals known to destroy ozone has dropped to nearly zero, the size of the ozone hole has not really decreased. It points out two studies that claim the ozone hole will shrink in size by either 2020 or 2040, and it concludes with this sentence:
But the longer the hole persists, the greater the likelihood that the ozone layer is dominated by natural factors, not human CFC emissions.
So what’s the story? By banning the use of CFCs, which we know can destroy ozone in the ozone layer, did we really fix the ozone “hole” problem? Or did we, as this story seems to imply, try to fix something that is probably the result of earth’s natural variability?
The first thing you need to know is that the ozone “hole” isn’t really a hole. It is a reduction in the amount of ozone that exists within the ozone layer, a portion of earth’s atmosphere that is roughly 15-35 kilometers above the surface of the earth. While all portions of the atmosphere have some ozone in them, this portion has the highest levels. Ozone’s molecular structure allows it to absorb some of the ultraviolet light that comes from the sun. That’s good for us, because ultraviolet light is energetic enough to kill living tissue. You can therefore think of the ozone layer as a “shield” that protects us from most of the sun’s ultraviolet light.
The amount of ozone in the ozone layer is measured using Dobson Units (DU). The larger the number of Dobson Units, the more ozone there is in the ozone layer. Globally, the average amount of ozone in the ozone layer is about 300 DU, but in Antarctica, that number fluctuates significantly with the seasons. While there are times the amount of ozone in the ozone layer above Antarctica is 300 DU and higher, there are also times it is significantly lower. The lowest recorded level of ozone in the ozone layer above Antarctica was in September of 1994, when there were only 74 DU of ozone. That reduction of ozone is what scientists refer to as the ozone “hole.”
Why does the amount of ozone in the ozone layer above Antarctica change with the seasons? Because during Antarctica’s winter, the polar vortex develops. This large ring of wind blows above the continent, and it produces a series of events that eventually lead to ozone destruction. When the polar vortex breaks up (usually in October), ozone destruction stops, and the level of ozone in the ozone layer above Antarctica rises again. So when we talk about the ozone “hole,” we are talking about a seasonal drop in ozone levels in the ozone layer above Antarctica. (There is a similar “hole” above the Artic, but it is small compared to the one above Antarctica.)
Now that you have all that information under your belt, you are ready for what I think is the most illustrative graph related to the ozone hole. Every year, NASA records the maximum size of the ozone hole as well as the minimum level of ozone found there. The following graph shows you both. The maximum size of the ozone hole in square kilometers is given in blue, and the minimum level of ozone in the hole is given in red. Please note that to make the graph easier to read, I divided the level of ozone by 2:
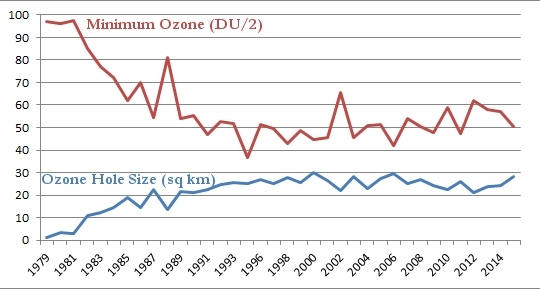
As was shown in the graph at the top of this post, the size of the ozone hole grew in a shaky but steady fashion until about 1998, and it has remained roughly the same since. Thus, while the ozone hole is no longer growing, it isn’t shrinking, either. Look, however, at the minimum level of ozone in the hole. It declined in a shaky but steady fashion until 1994, but it now seems to be growing in a shaky but steady fashion. The growth is very slow, but it seems unmistakable. So while the size of the ozone hole isn’t decreasing, the level of ozone in the ozone hole is rising.
My interpretation of the data, then, is that the ozone hole is recovering, but very slowly. Why is the recovery so slow? Based on the chemistry of CFCs, this is exactly what you would expect. One of the reasons CFCs became so popular is that they are fairly inert. They don’t like to react with much of anything on the surface of the earth. As a result, they are not toxic. However, because of that, they also don’t decay very quickly. This means that even though we have stopped using CFCs, there are still a lot of them in the environment, and it will take a long time for them to decay away. Until they do, they will continue to destroy ozone when the polar vortex forms.
In the end, then, I do think the data tell us that the reduction in CFC use has helped the ozone layer. However, due to the chemical nature of CFCs, it will take a while for this to have a strong effect on the depth and size of the ozone hole.
Jay L. Wile's Blog
- Jay L. Wile's profile
- 31 followers



