Oxygen Bleach an Introduction
 Heather says:
Heather says:
Welcome to the second installment of the Series on Common Household Chemicals.
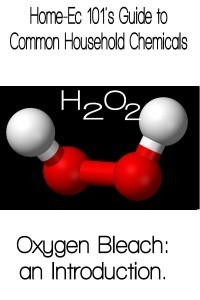
I think I was a kid when Billy Mays first showed up on my radar. He pitched Oxyclean late into the night and I’d sit there fascinated watching the red swirl away and magically disappear. Oxyclean is just a brand name for oxygen bleach or sodium percarbonate. When Na2CO3·1.5H2O2 is added to water the H2O2 is released. H2O2 should look familiar to you, if you didn’t sleep through your entire high school chem class. It’s the same stuff you buy in the little brown bottle and store in the medicine cabinet. H2O2 is hydrogen peroxide. It’s essentially a water molecule with an extra oxygen atom. This isn’t a very stable molecule, things like: light, heat, and agitation, can all break that weak bond leaving behind plain old water.
 Sodium percarbonate is made from natural soda ash or borax that has been treated with hydrogen peroxide.
Sodium percarbonate is made from natural soda ash or borax that has been treated with hydrogen peroxide.
Since hydrogen peroxide is so unstable, this powdered form is much better for shipping and storage.
As a regular consumer you most likely will find oxygen bleach in the following forms: ultra, concentrated, and as an added ingredient to things like laundry detergent, and liquid.
When you purchase oxygen bleach, you are going to get the sodium percarbonate you’re after and other filler ingredients. Sometimes it’s a detergent or surfactant, other times it’s just filler. Experiment with different brands and find the one you find most effective with your water.
Always use in accordance with the manufacturer’s directions and do not use with silk or wool.
Typical applications for oxygen bleach:
mold and mildew stain remover bleach & clean decks and siding color safe stain remover laundry disinfectant
When it comes to laundry oxygen bleach isn’t particularly good at brightening whites, but if used consistently it can help prevent the dulling that occurs over time.
In general oxygen bleach products break down into borax and water, which makes it an environmentally friendly choice.
Oxygen bleach is safe for septic systems, when used properly. Don’t go flushing pounds of sodium percarbonate down the toilet.
Since when we talk about sodium percarbonate we are essentially talking about hydrogen peroxide, it’s time to ask:
What makes hydrogen peroxide an effective cleaning agent?The extra oxygen molecule in the hydrogen peroxide molecule is essentially a scavenger just looking for weak bonds to break. These weaker single bonds are often found in organic molecules.
When material is dyed the pigments are typically set, rendering the item colorfast. This simply means the colors don’t bleed. Hydrogen peroxide, in low concentrations, can be a color safe bleach and works by breaking some of the single bonds in the pigments of a stain. Once these weak bonds are broken, you don’t see the color.
In higher concentrations, hydrogen peroxide will bleach more than stains. Follow the label directions for proper dilution.
As a disinfectant, hydrogen peroxide acts as an oxidizer. Those rogue -totally not a technical term, but you get what I’m saying- oxygen molecules can oxidize the molecules that make up the structure of bacterial cell walls. When this happens the cell walls break, killing the bacteria.
It is important to note that there is a big difference between the 3% hydrogen peroxide most people keep in their medicine cabinets and the 35% food grade hydrogen peroxide. 35% food grade peroxide is typically diluted to 6% strength to sanitize food preparation areas. You cannot get 6% hydrogen peroxide from 3% dilution, that busy little H2O2 molecule is just too unstable.
In case you weren’t aware, you’re made of organic molecules, the same ones those rogue oxygen atoms like to attack. Yes, at the proper dilution hydrogen peroxide is a fantastic disinfectant. However it is not shelf stable, you’re paying for the shipment of water, and in higher concentrations hydrogen peroxide is a strong irritant. 3% is the only strength approved for contact with skin. Use gloves if you use a 6% solution to sanitize your kitchen and follow the instructions carefully. Just because H2O2 breaks down into water and oxygen doesn’t mean it can’t do damage on the way.
There are a lot of snake oil websites out there touting hydrogen peroxide as a magic cure all. Some even want to dupe people into believing that hydrogen peroxide is an effective cancer treatment. Please read what the Cancer Institute has to say about oxygen therapy. On a personal note, I think it’s cruel to try to sell a sham to people in pain, who are in need of hope.
Use your common sense. If you find yourself short on that, default to the instructions on the label.
Send your questions to helpme@home-ec101.com .

Copyright Home-Ec101.com 2007-2014







 CommentsAs a chemist, those Oxyclean commercials used to drive me nuts! ... by Charles G.Wonderful article! We will be linking to this great article on ... by naprawa sterowników pompthis may sound out in left field but have you thought to maybe ... by articfire69I'm pretty picky about what I will put on my horses, so if I ... by LucyAh, it has to be wet, then. Fooey! The "doctor's advice" is ... by LucyPlus 11 more...
CommentsAs a chemist, those Oxyclean commercials used to drive me nuts! ... by Charles G.Wonderful article! We will be linking to this great article on ... by naprawa sterowników pompthis may sound out in left field but have you thought to maybe ... by articfire69I'm pretty picky about what I will put on my horses, so if I ... by LucyAh, it has to be wet, then. Fooey! The "doctor's advice" is ... by LucyPlus 11 more...

Heather Solos's Blog
- Heather Solos's profile
- 12 followers



