Potential Tactics for Defeating Cancer — A Toolkit in 1,000 Words

(Photo: Irina Souiki)
I’ve wanted to publish this post for years.
It will propose a few simple approaches for minimizing the occurrence of cancer.
With 19 billion capillaries in our bodies, on average, virtually 100% of us have microscopic cancers by the time we’re 70 years old, more than 40% of us by age 40. There’s a good chance you have pinhead-size cancers in your body right now. These “cancers without disease” aren’t typically a problem, as they can’t grow larger than 0.5 mm without a blood supply.
But if cancer cells gets constant blood and glucose? That’s when you can end up dead.
That’s not where I want to be, and it’s not where I want you to be.
A Little Backstory…
While at the annual TED Conference in 2010, I learned that two close friends had been diagnosed with cancer. The year before, another friend had died of pancreatic cancer in his early 30′s.
This all made me furious and sad. It also made me feel helpless.
As luck would have it, TED in 2010 was abuzz about someone named Dr. William Li. His 24-minute presentation had introduced the crowd to “anti-angiogenesis therapy”: in plain English, how to starve cancers of blood. Dr. Li specializes in inhibiting cancer-specific blood-vessel growth, which ostensibly keeps abnormal growth in check. The simplest “drug” he recommended was tea. Drinking a daily blend of white tea (specifically Dragon Pearl jasmine) and green tea (Japanese sencha).
I started drinking the cocktail immediately, but it was just a first step…
In clinical trials, you see, anti-angiogenesis has been largely been unsuccessful. The father of the field, Judah Folkman, was brilliant, but his brainchild (Avastin) has been a disappointment. For about $100,000 a year of Avastin, one might extend lifespan by a month or so.
So, while I kept drinking my tea, I realized it probably wasn’t enough by itself. That said, it pointed me to new research.
I, for one, believe there are systemic causes of cancer with systemic treatments. This belief began with metformin experimentation in college (not recommended without doctor supervision), followed by reading the work and references of Gary Taubes, all of which has been reinforced by conversations with oncologists over the last decade.
All trails have led back to blood and glucose.
It’s also important to realize that killing cancer cells isn’t hard. Doctors have known how to do this for 100+ years. The real questions is: how do you exploit a weakness in cancer that is NOT a weakness in normal cells? Killing cancer is easy. Killing cancer while not killing non-cancer has proven almost impossible.
The below guest post is written by Peter Attia, M.D.. It explores a simple theory of cancer growth, which simultaneously shows how you can minimize it.
Peter is the President of the Nutrition Science Initiative (NuSI). Peter spent five years at the Johns Hopkins Hospital as a general surgery resident, where he was the recipient of several prestigious awards and the author of a comprehensive review of general surgery. Peter also spent two years at the National Institutes of Health as a surgical oncology fellow at the National Cancer Institute under Dr. Steve Rosenberg, where his research focused on the role of regulatory T cells in cancer regression and other immune-based therapies for cancer. Peter earned his M.D. from Stanford University and holds a B.Sc. in mechanical engineering and applied mathematics from Queen’s University in Kingston, Ontario, Canada.
This post is designed to allow you to skim…or go deep. Here are the options:
The quickie (10-15 min) - Read the post but ignore footnotes. Definitely a good start if you’re in a rush.
The weekend warrior (30 minutes) – Read the post and footnotes, which provide an excellent intro to the science.
The semi-pro (60 minutes) – Read the post, footnotes, and at least one top-10 suggested articles. This will give you more of a plan and put you ahead of 90% of the people who discuss cancer.
Enter Pete
One night Tim and I were having dinner and the topic of cancer came up.
Personally and professionally, I have a great interest in cancer, so when Tim asked if I could write something about cancer that was: (i) interesting to a broad audience, (ii) not technically over the top, (iii) not my typical 5,000 word dissertation, (iv) yet nuanced enough for his readers, I agreed to give it a shot, in about 1,000 words.
(Before reading this post, you may find some value in first reading a previous post which sets up the context for this one.)
So here it is, in roughly 1,000 words…
###
In 1924 a scientist named Otto Warburg happened upon a counterintuitive finding.
Cancer1 cells, even in the presence of sufficient oxygen, underwent a type of metabolism2 cells reserved for rapid energy demand – anaerobic metabolism3. In fact, even when cancer cells were given additional oxygen, they still almost uniformly defaulted into using only glucose4 to make ATP via the anaerobic pathway. This is counterintuitive because this way of making ATP is typically a last resort for cells, not a default, due to the very poor yield of ATP.
This observation begs a logical question? Do cancer cells do this because it’s all they can do? Or do they deliberately ‘choose’ to do this?
The first place to look is at the mitochondria6 of the cancer cells. Though not uniformly the case, most cancers do indeed appear to have defects in their mitochondria that prevent them from carrying out oxidative phosphorylation7.
Explanation 1
Cancer cells, like any cells undergoing constant proliferation (recall: cancer cells don’t stop proliferating when told to do so), may be optimizing for something other than energy generation. They may be optimizing for abundant access to cellular building blocks necessary to support near-endless growth. In this scenario, a cancer would prefer to rapidly shuttle glucose through itself. In the process, it generates the energy it needs, but more importantly, it gains access to lots of carbon, hydrogen, and oxygen atoms (from the breakdown of glucose). The atoms serve as the necessary input to the rate-limiting step of their survival — growth. The selection of cancer cells is based on this ability to preferentially grow by accessing as much cellular substrate as possible.
Explanation 2
Cells become cancerous because they undergo some form of genetic insult. This insult – damage to their DNA8 – has been shown to result in the turning off of some genes9 (those that suppress tumor growth) and/or the activation of other genes (those that promote cell growth unresponsive to normal cell-signaling). Among other things, this damage to their DNA also damages their mitochondria, rendering cancer cells unable to carry out oxidative phosphorylation. So, to survive they must undergo anaerobic metabolism to make ATP.
Whichever of these is more accurate (a discussion beyond my word count), the end result appears the same – cancer cells almost exclusively utilize glucose to make ATP without the use of their mitochondria. The point is: cancer cells have a metabolic quirk. Regardless of how much oxygen and fatty acid10 they have access to, they preferentially use glucose to make ATP, and they do it without their mitochondria and oxygen.
So, can this be exploited to treat or even prevent cancer?
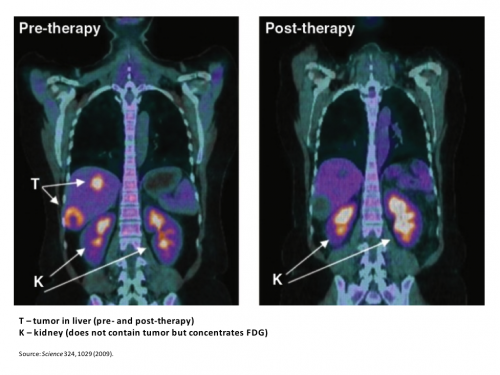
One way this quirk has been exploited for many years is in medical imaging. FDG-PET scans11 are a useful tool for non-invasively detecting cancer in people. By exploiting the obligate glucose consumption of cancer cells, the FDG-PET scan is a powerful way to locate cancer (see figure).
You can probably tell where I’m leading you. What happens if we reduce the amount of glucose in the body? Could such an intervention ‘starve’ cancer cells? An insight into this came relatively recently from an unlikely place – the study of patients with type 2 diabetes.
In the past few years, three retrospective studies of patients taking a drug called metformin have shown that diabetic patients who take metformin, even when adjusted for other factors such as body weight and other medications, appear to get less cancer.
And when they do get cancer, they appear to survive longer. Why? The answer may lie in what metformin does. Metformin does many things, to be clear, but chief among them is activating an enzyme called AMP kinase, which is important in suppressing the production of glucose in the liver (the liver manufactures glucose from protein and glycerol and releases it to the rest of the body). This drug is used in patients with diabetes to reduce glucose levels and thereby reduce insulin requirement.
So, the patients taking metformin may have better cancer outcomes because their glucose levels were lower, or because such patients needed less insulin. Insulin and insulin-like growth factor (IGF-1) also appear to play an integral role in cancer growth as recently demonstrated by the observation that people with defective IGF-1 receptors appear immune to cancer. Or, it may be that activation of AMP kinase in cancer cells harms them in some other way. We don’t actually know why, but we do know that where there is smoke there is often fire. And the ‘smoke’ in this case is that a relatively innocuous drug that alters glucose levels in the body appears to interfere with cancer.
This may also explain why most animal models show that caloric restriction improves cancer outcomes. Though historically, this observation has been interpreted through the lens of less ‘food’ for cancer. A more likely explanation is that caloric restriction is often synonymous with glucose reduction, and it may be the glucose restriction per se that is keeping the cancer at bay.
Fortunately this paradigm shift in oncology – exploiting the metabolic abnormality of cancer cells – is gaining traction, and doing so with many leaders in the field.
Over a dozen clinical trials are underway right now investigating this strategy in the cancers that appear most sensitive to this metabolic effect – breast, endometrial, cervical, prostate, pancreatic, colon, and others. Some of these trials are simply trying to reproduce the metformin effect in a prospective, blinded fashion. Other trials are looking at sophisticated ways to target cancer by exploiting this metabolic abnormality, such as targeting PI3K12 directly.
To date, no studies in humans are evaluating the therapeutic efficacy of glucose and/or insulin reduction via diet, though I suspect that will change in the coming year or two, pending outcomes of the metformin trials.
EDITOR’S NOTE:Though it might seem premature to some, let’s make this actionable. To reduce glucose, consider following a diet (way of eating, really) such as The Slow-Carb Diet, Paleo, or any diet that induces ketosis. Many of the most influential researchers in the US, in addition to following ketogenic diets, take slow-acting metformin as a preemptive measure. NOTE: This should NOT be done without medical supervision.
Influences
I’ve been absurdly blessed to study this topic at the feet of legends, and to be crystal clear, not a single thought represented here is original work emanating from my brain. I’m simply trying to reconstruct the story and make it more accessible to a broader audience. Though I trained in oncology, my research at NIH/NCI focused on the role of the immune system in combating cancer. My education in the metabolism of cancer has been formed by the writings of those below, and from frequent discussions with a subset of them who have been more than generous with their time, especially Lewis Cantley (who led the team that discovered PI3K) and Dominic D’Agostino.
• Otto Warburg
• Lewis Cantley
• Dominic D’Agostino
• Craig Thompson
• Thomas Seyfried
• Eugene Fine
• Richard Feinman (not to be confused with Richard Feynman)
• Rainer Klement
• Reuben Shaw
• Matthew Vander Heiden
• Valter Longo
Further Reading from Tim — A Top-10 List
There is a deluge of writing about cancer.
Below, I’ve suggested a top-10 list of articles as starting points. Some are for lay audiences, some are technical, but all are worth the time to read. Here you go:
Looking for articles to pass to your parents, or to read as a lay person? Read these, in this order:
1. Non-technical talk by Craig Thompson, Pres/CEO of Sloan-Kettering
2. Science piece written about cancer (for non-technical audience) by Gary Taubes
Have a little background and want the 80/20 analysis, the greatest bang for the buck? Read this:
3. Relatively non-technical review article on the Warburg Effect written by Vander Heiden, Thompson, and Cantley
Peaking on modafinil during a flight to Tokyo? Want to deep dive for a few hours? Here are three recommendations, in this order:
4. Detailed review article by Tom Seyfried
5. Review article on the role of carb restriction in the treatment and prevention of cancer
6. Talk given by author of above paper for those who prefer video
Want four bonus reads, all very good? As you wish:
7. Moderately technical review article by Shaw and Cantley
8. Clinical paper on the role of metformin in breast cancer by Ana Gonzalez-Angulo
9. Mouse study by Dom D’Agostino’s group examining role of ketogenic diet and hyperbaric oxygen on a very aggressive tumor model
10. Mechanistic study by Feinman and Fine assessing means by which acetoacetate (a ketone body) suppresses tumor growth in human cancer cell lines
Afterword by Tim
It’s my hope that this short article offers hope. Moreover, it’s intended to offer actionable directions for those dealing with cancer or fearful of it.
Note a few things:
I am not a doctor, nor do I play one on the Internet. Make medical decisions with medical supervision.
This is a 1,000-word primer and therefore simplified. It’s not incorrect, but it is not comprehensive either, as it would impossible to digest for most people. Be sure to read the “further reading” above if you’re serious.
Have you stumbled upon any novel science/treatments related to cancer? Please share in the comments below, if so, as I’d love this post to become a living resource.
Many thanks for reading this far.
###
Footnotes: