Tens of Thousands of Mothers Sue Makers of Tylenol for Pregnancy Use that Led to Babies Born with Autism
by Brian Shilhavy
Editor, Health Impact News
Tens of thousands of mothers are suing the makers of Tylenol for using the popular over-the-counter pain reliever during pregnancy, which resulted in them giving birth to babies diagnosed with autism.
Tens of thousands of mothers are suing the makers of Tylenol in a class-action lawsuit that claims its use during pregnancy led babies to be born with autism.
A study from the NIH found that pregnancy exposure to acetaminophen, the main ingredient in Tylenol, may increase a child’s risk for autism and ADHD.
Karleen DeGroodt is among the mothers in the lawsuit and discussed her use of Tylenol during pregnancy and her son’s autism during an appearance on NewsNation’s “Prime.” (Full article.)
Last October, a federal judicial panel consolidated dozens of these lawsuits alleging that acetaminophen, the active ingredient in Tylenol and generic versions of the drug, can cause autism spectrum disorder and attention deficit hyperactivity disorder.
TorHoerman Law is one of the law firms representing these mothers, and they have produced the following video explaining the lawsuit.
[The main defendant in the lawsuit is Kenvue, which is the former Johnson & Johnson’s consumer health unit that is now a “spin-off” of J&J. (Source)Co-defendants in the lawsuit are CVS Health, Rite Aid Corp, Safeway Inc, Target Corp and Walgreens Boots Alliance, which are being charged with failing to warn consumers about the risks of Tylenol.
Kenvue has suffered some setbacks in recent weeks in trying to get some of the lawsuits dismissed, with one of the reasons given to dismiss the lawsuits being that the FDA had approved the product and its labels.
Kenvue Inc cannot immediately appeal a federal judge’s order allowing lawsuits claiming that its popular over-the-counter painkiller Tylenol can cause autism in children of mothers who take it during pregnancy, the judge has ruled.
U.S. District Judge Denise Cote in Manhattan on Thursday ruled that Kenvue, formerly Johnson & Johnson’s consumer health unit, had not shown any basis for allowing the unusual step of an appeal to the 2nd U.S. Circuit Court of Appeals before final judgment in the case.
In April, Cote denied Kenvue’s motion to dismiss one of the lawsuits on the grounds that the U.S. Food and Drug Administration’s approval of Tylenol’s label preempted any state law claims. Had she ruled in the company’s favor, it would have ended the entire litigation. (Source.)
The amount of studies published in the medical journals linking Tylenol taken during pregnancy to babies born with autism is overwhelming.
Here are just a couple of the more recent studies published this year (2023).
Acetaminophen causes neurodevelopmental injury in susceptible babies and children: no valid rationale for controversyClinical and Experimental Pediatrics – June 14, 2023
Abstract
The use of acetaminophen in pregnancy: a double whammy
Despite worldwide acceptance of acetaminophen as a necessary medicine in the field of pediatrics, evidence that early life exposure to acetaminophen causes neurodevelopmental injury in susceptible babies and children has mounted for more than a decade.
Evidence is diverse, including extensive work with laboratory animals, otherwise unexplained associations, factors associated with the metabolism of acetaminophen, and some limited studies in humans. Although evidence has reached an overwhelming level and has been reviewed in detail recently, some controversy remains. In this narrative review, some of those controversies are evaluated.
Evidence from the prepartum and the postpartum period is considered, avoiding controversies raised by considering only the limited evidence pointing exclusively toward risks during the prepartum period. Among other issues, the associations through time between acetaminophen use and the prevalence of neurodevelopmental disorders are considered.
A systematic review reveals that the use of acetaminophen in the pediatric population was never tracked carefully, but historical events that affected use of the drug were documented and are sufficient to establish apparent correlations with changes in the prevalence of neurodevelopmental disorders.
In addition, problems with exclusive reliance on results from meta-analyses of large datasets and from studies involving small time frames of drug exposure are reviewed. Further, evidence demonstrating why some children are susceptible to acetaminophen-induced neurodevelopmental injury is examined.
It is concluded that, at least among the factors considered, there is no valid rationale for controversy regarding the conclusion that early life exposure to acetaminophen causes neurodevelopmental injury in susceptible babies and small children. (Source.)
Annals of Medicine and Surgery – March 14, 2023
Abstract
Acetaminophen is the most widely over the counter used analgesic in the world, and the World Health Organization advises using it as first-line treatment for pain issues (WHO).
However, various side effects have been documented with its use such as nausea, vomiting, constipation at low doses whereas in large doses, it might even result in hepatoxicity.
Recent literature suggests that the use of acetaminophen in pregnancy even in optimal doses could result infant being born with ADHD and autism, so in this short communication we talk about the prevalence of neurodevelopment disorders in infants as a result of its use, as well as shed light to the measures that should be adopted to minimize the adverse effects. (Source.)
We have been publishing articles exposing the dangers of Tylenol for over a decade now. A search on Health Impact News for this killer drug, which is available as an over-the-counter (OTC) drug that even minors can purchase in their local pharmacy or retail store will result in over 80 articles.
Pregnant women are the not the only ones who should immediately STOP using this killer drug. NOBODY should be using it.
Tylenol is a classic example of how corporate profits from the pharmaceutical drug companies are far more important than patient safety, as millions of lives are sacrificed to keep these drugs on the market.
It is also just another example of how the FDA works to protect pharmaceutical companies, and NOT consumers.
Tylenol has been on the market for 75 years bringing in annual revenues that exceed $300 million.
And the adverse effects from Tylenol have been widely published for over a decade now.
In 2013 ProPublica published one of the most comprehensive reviews of Tylenol’s toxic side effects:
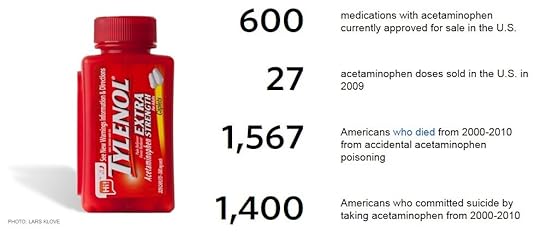
About 150 Americans a year die by accidentally taking too much acetaminophen, the active ingredient in Tylenol. The toll does not have to be so high.
During the last decade, more than 1,500 Americans died after accidentally taking too much of a drug renowned for its safety: acetaminophen, one of the nation’s most popular pain relievers.
Acetaminophen – the active ingredient in Tylenol – is considered safe when taken at recommended doses. Tens of millions of people use it weekly with no ill effect. But in larger amounts, especially in combination with alcohol, the drug can damage or even destroy the liver.
Davy Baumle, a slender 12-year-old who loved to ride his dirt bike through the woods of southern Illinois, died from acetaminophen poisoning. So did tiny five-month-old Brianna Hutto. So did Marcus Trunk, a strapping 23-year-old construction worker from Philadelphia.
The toll does not have to be so high.
The U.S. Food and Drug Administration has long been aware of studies showing the risks of acetaminophen – in particular, that the margin between the amount that helps and the amount that can cause serious harm is smaller than for other pain relievers. So, too, has McNeil Consumer Healthcare, the unit of Johnson & Johnson that has built Tylenol into a billion-dollar brand and the leader in acetaminophen sales.
Yet federal regulators have delayed or failed to adopt measures designed to reduce deaths and injuries from acetaminophen overdose, which the agency calls a “persistent, important public health problem.”
The FDA has repeatedly deferred decisions on consumer protections even when they were endorsed by the agency’s own advisory committees, records show. (Full article.)
[…]
The Most Revolutionary Act
- Stuart Jeanne Bramhall's profile
- 11 followers



