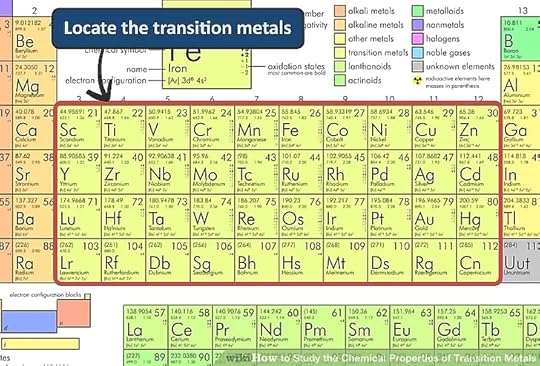
The transition metals are more complicated than lighter elements.
Why?
Because they’re the first whose electron wavefunctions are described by quadratic functions of 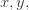 and
and  — not just linear or constant. These are called ‘d orbitals’, and they look sort of like this:
— not just linear or constant. These are called ‘d orbitals’, and they look sort of like this:

More precisely: the wavefunctions of electrons in atoms depend on the distance  from the nucleus and also the angles
from the nucleus and also the angles 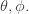 The angular dependence is described by ‘spherical harmonics’, certain functions on the sphere. These ar...
The angular dependence is described by ‘spherical harmonics’, certain functions on the sphere. These ar...
Published on December 09, 2021 14:53
