Does anyone know a real-world example of a cycle like this:
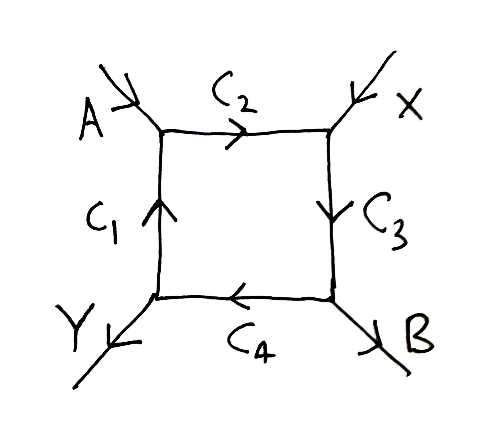
or in other words, this:
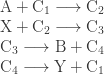
where the reaction
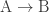
is exergonic (i.e., involves a decrease in free energy) while
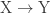
is endergonic (i.e., involves a free energy increase)?
The idea is that the above cycle, presumably catalyzed so that all the reactions go fairly fast under normal conditions, ‘couples’ the exergonic reaction, which ‘wants to happen’, to the endergonic reaction, which doesn’t… thus driving the endergonic one.
I would...
Published on June 26, 2018 12:17
