Ivermectin Antiparasitic Anticancer Wonder Drug
 Ivermectin Antiparasitic Anticancer
Ivermectin Antiparasitic Anticancer
Wonder Drug
by Jeffrey Dach MD
The 2015 Nobel Prize in Medicine went for discovery of Ivermectin, an “astonishingly safe” FDA approved anti-helminthic drug.
200 million people take the drug globally for prevention or treatment of parasitic disease.
Dr Sharmeen at the University of Toronto screened a library of 100 drugs for activity against a leukemic cell line, and reported Ivermectin as most promising, inducing leukemic cell death at low micromolar concentrations, while sparing normal cells. Ivermectin was also effective against leukemia mouse xenografts.
Left Image Ivermectin Chemical Structure courtesy of Stanford.EDU.
Ivermectin was patented in 2012 for treating hematological maligna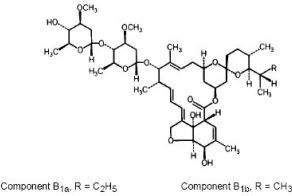 ncy.
ncy.
Dr Alice Melotti studied Ivermectin as an inhibitor of the WNT‐TCF pathway in cancer. Her report was published in 2014 EMBO molecular medicine. Dr Melotti used a transcriptional reporter assay for TCF activity driven by Beta-CATENIN to test a collection of 1,040 drugs and small molecules. Only one agent, Ivermectin, perfectly tracked the gene expression profile induced by blocking the TCF gene, and therefore blocks the WNT pathway. This has profound significance for anti-cancer stem cell therapy, because blocking the WNT pathway is the key to killing cancer stem cells.
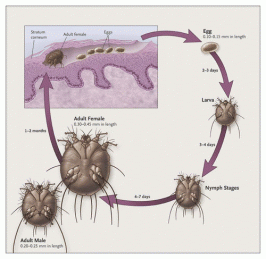 Left Image From NEJM showing life cycle of scabies parasite involving skin. Treatment with Ivermectin.
Left Image From NEJM showing life cycle of scabies parasite involving skin. Treatment with Ivermectin.
Ivermectin is a highly effective anti-parasitic drug, FDA approved for pediatric scabies (see left image) . Ivermectin has extensive veterinary use as an anti-parasitic drug for pets, horses and farm animals (See HeartGuard header image)
The anti-cancer effect of Ivermectin was an unexpected clinical benefit. Will Ivermectin revolutionize leukemia and cancer treatment, making chemotherapy and stem cell transplant obsolete relics for the medical museum?
 Left Image Medical Museum courtesy of Army.
Left Image Medical Museum courtesy of Army.
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Fl 33314
954-792-4663
Links and references
Ivermectin inhibits WNT-TCF pathway
Ivermectin – WNT pathway
 1) Melotti, Alice, et al. “The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT‐TCF pathway responses in human cancer.” EMBO molecular medicine (2014): e201404084.
1) Melotti, Alice, et al. “The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT‐TCF pathway responses in human cancer.” EMBO molecular medicine (2014): e201404084.
Ivermectin used as a therapeutic WNT-TCF pathway response blocker to treat WNT-TCF-dependent diseases including multiple cancers.
We find that macrocyclic lactones of the Avermectin family have specific anti-WNT-TCF response activity in human cancer cells and that the clinically approved compound Ivermectin (EMEA- and FDA-approved) is a specific WNT-TCF response blocker at low micromolar concentrations.
cancer stem cells
Pre-treatment with Ivermectin and Selamectin inhibits colon cancer stem cell self-renewal in clonogenic spheroid assays. These results suggest an action on both the bulk of the tumor and its cancer stem cells.
Moreover, they might also be useful as routine prophylactic agents, for instance against nascent TCF-dependent intestinal tumors in patients with familial polyposis and against nascent sporadic colon tumors in the general aging population.
Constitutive activation of canonical WNT-TCF signaling is implicated in multiple diseases, including intestine and lung cancers, but there are no WNT-TCF antagonists in clinical use. We have performed a repositioning screen for WNT-TCF response blockers aiming to recapitulate the genetic blockade afforded by dominant-negative TCF. We report that Ivermectin inhibits the expression of WNT-TCF targets, mimicking dnTCF, and that its low concentration effects are rescued by direct activation by TCFVP16. Ivermectin inhibits the proliferation and increases apoptosis of various human cancer types. It represses the levels of C-terminal ß-CATENIN phosphoforms and of CYCLIN D1 in an okadaic acid-sensitive manner, indicating its action involves protein phosphatases.In vivo, Ivermectin selectively inhibits TCF-dependent, but not TCF-independent, xenograft growth without obvious side effects. Analysis of single semi-synthetic derivatives highlights Selamectin, urging its clinical testing and the exploration of the macrocyclic lactone chemical space. Given that Ivermectin is a safe anti-parasitic agent used by > 200 million people against river blindness, our results suggest its additional use as a therapeutic WNT-TCF pathway response blocker to treat WNT-TCF-dependent diseases including multiple cancers.
Wingless/integrase-1 (WNT) signaling. The name Wnt was a portmanteau of int and Wg and stands for “Wingless-related integration site. Other cancers also show an active canonical WNT pathway; these include carcinomas of the lung, stomach, cervix, endometrium, and lung as well as melanomas and gliomas
We have used a transcriptional reporter assay for TCF activity driven by APC-insensitive N’?ß-CATENIN, to test a collection of clinical-trial tested small molecules (Microsource 1040 library). Of the 4 putative antagonists, only one, 4B5 (Avermectin B1), perfectly tracked the gene expression profile induced by dnTCF4. anti-helmintic agent Avermectin B1, which belongs to the 16-membered Avermectin macrocyclic lactone family derived fromStreptomyces avermitilis.
The drug is used in humans against insect and worm infections, including river blindness caused by Onchocerca volvulus. The dominant negative forms of TCF (dn-TCF) that can be used to block Wnt signaling in the nucleus. as a therapeutic WNT-TCF pathway response blocker to treat WNT-TCF-dependent diseases including multiple cancers.
We find that macrocyclic lactones of the Avermectin family have specific anti-WNT-TCF response activity in human cancer cells and that the clinically approved compound Ivermectin (EMEA- and FDA-approved) is a specific WNT-TCF response blocker at low micromolar concentrations.
Ivermectin Inhibits cancer stem cells
Pre-treatment with Ivermectin and Selamectin inhibits colon cancer stem cell self-renewal in clonogenic spheroid assays
These results suggest an action on both the bulk of the tumor and its cancer stem cells.
Moreover, they might also be useful as routine prophylactic agents, for instance against nascent TCF-dependent intestinal tumors in patients with familial polyposis and against nascent sporadic colon tumors in the general aging population.
commercial form from the pharmacy, Stromectol™,
Selamectin, which scored as toxic in the primary screen at 10 µM, was ˜ tenfold more potent than ivermectin.
Here we report that Ivermectin (Campbellet al, 1983), an off-patent drug approved for human use, and related macrocyclic lactones, have WNT-TCF pathway response blocking and anti-cancer activities. Whereas the exact molecular target for Ivermectin and Selamectin that affects WNT-TCF responses remains to be identified, the present findings show that these drugs block WNT-TCF pathway responses, likely acting at the level of ß-CATENIN/TCF function, affecting ß-CATENIN phosphorylation status.
Cell toxicity appears at doses greater (> 10 µM for 12 h or longer or > 5 µM for 48 h or longer for Ivermectin) than those required to block TCF responses and induce apoptosis.
This drug does not cross the blood–brain barrier.
Indications may include treatment for incurable ß-CATENIN/TCF-dependent advanced and metastatic human tumors of the lung, colon, endometrium, and other organs.
Moreover, they might also be useful as routine prophylactic agents, for instance against nascent TCF-dependent intestinal tumors in patients with familial polyposis and against nascent sporadic colon tumors in the general aging population.
————————————————————————-
2) NEW ZEALAND DATA SHEET STROMECTOL ivermectin 3 mg tablet 2011
3) Chhaiya, Sunita B., et al. “IJBCP International Journal of Basic & Clinical Pharmacology.” International Journal 2.6 (2013): 799. Chhaiya, Sunita. Ivermectin pharmacology and therapeutic applications Sunita Chhaiya 2012
4) The Pharmacokinetics and Interactions of Ivermectin in Humans. Canga, Aránzazu González, et al.”The pharmacokinetics and interactions of ivermectin in humans—a mini-review.” The AAPS journal 10.1 (2008): 42-46.
Ivermectin is exceptionally potent, with effective dosages
levels that are unusually low. In the treatment of onchocerciasis,
the optimal dose of ivermectin is 150 µg/kg, but the
frequency of administration is still controversial, ranging from
150 µg/kg once to three times yearly. The optimal duration of
treatment has not been established (6).
It is effective in most patients with scabies after a single oral dose of 200 µg/kg, but often the regimen involves two or three repeated doses, separated by interval of 1 or 2 weeks (7).
prolonged prothrombin ratios were observed in 148 subjects given ivermectin orally. Although no patients suffered bleeding complications, factor II and VII levels were reduced in most of them, suggesting interference with vitamin K
metabolism.
———————————————————–
Take Ivermectin with FOod every 4 days.
5) J Clin Pharmacol. 2002 Oct;42(10):1122-33.
Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. Guzzo CA1, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC.
Safety and pharmacokinetics (PK) of the antiparasitic drug ivermectin, administered in higher and/or more frequent doses than currently approved for human use, were evaluated in a double-blind, placebo-controlled, dose escalation study. Subjects (n = 68) were assigned to one of four panels (3:1, ivermectin/placebo): 30 or 60 mg (three times a week) or 90 or 120 mg (single dose). The 30 mg panel (range: 34 7-594 microg/kg) also received a single dose with food after a 1-week washout. Safety assessments addressed both known ivermectin CNS effects and general toxicity. The primary safety endpoint was mydriasis, accurately quantitated by pupillometry. Ivermectin was generally well tolerated, with no indication of associated CNS toxicity for doses up to 10 times the highest FDA-approved dose of 200 microg/kg. All dose regimens had a mydriatic effect similar to placebo. Adverse experiences were similar between ivermectin and placebo and did not increase with dose. Following single doses of 30 to 120 mg, AUC and Cmax were generally dose proportional, with t(max) approximately 4 hours and t1/2 approximately 18 hours. The geometric mean AUC of 30 mg ivermectin was 2.6 times higher when administered with food. Geometric mean AUC ratios (day 7/day 1) were 1.24 and 1.40 for the 30 and 60 mg doses, respectively, indicating that the accumulation of ivermectin given every fourth day is minimal. This study demonstrated that ivermectin is generally well tolerated at these higher doses and more frequent regimens.
————————————————————–
6) Editorial Commentary: Ivermectin as a Complementary Strategy to Kill Mosquitoes and Stop Malaria Transmission? Richard W. Steketee1 and Feiko O. ter Kuile2
Repeated doses of up to 800 µg/kg have been used in the treatment of onchocerciasis [8–10]. Furthermore, earlier dose-escalation studies with ivermectin have shown that doses up to 2000 µg/kg (ie, 5 times the highest US Food and Drug Administration–approved dose) are well tolerated with no indication of central nervous system or general toxicity [11]. Additional dosing during the third day of the ACT treatment (as done in this trial) or at day 7 (and perhaps at day 14)
—————————————————–
Ivermectin safely given incombination with Artemisinin Derivative Artemether
7) Efficacy and Safety of the Mosquitocidal Drug Ivermectin to Prevent Malaria Transmission After Treatment: A Double-Blind, Randomized, Clinical Trial
André Lin Ouédraogo1,a, Guido J. H. Bastiaens2,a, Alfred B. Tiono1, Wamdaogo M. Guelbéogo1, Kevin C. Kobylinski3,4, Alphonse Ouédraogo1, Aïssata Barry1, Edith C. Bougouma1, Issa Nebie1, Maurice San Ouattara1, Kjerstin H. W. Lanke2, Lawrence Fleckenstein5, Robert W. Sauerwein2, Hannah C. Slater6, Thomas S. Churcher6, Sodiomon B. Sirima1, Chris Drakeley7, and Teun Bousema2,7
Background. Artemisinin combination therapy effectively clears asexual malaria parasites and immature gametocytes but does not prevent posttreatment malaria transmission. Ivermectin (IVM) may reduce malaria transmission by killing mosquitoes that take blood meals from IVM-treated humans.
Methods. In this double-blind, placebo-controlled trial, 120 asymptomatic Plasmodium falciparum parasite carriers were randomized to receive artemether-lumefantrine (AL) plus placebo or AL plus a single or repeated dose (200 µg/kg) of ivermectin (AL-IVM1 and AL-IVM2, respectively). Mosquito membrane feeding was performed 1, 3, and 7 days after initiation of treatment to determine Anopheles gambiae and Anopheles funestus survival and infection rates.
Results. The AL-IVM combination was well tolerated. IVM resulted in a 4- to 7-fold increased mortality in mosquitoes feeding 1 day after IVM (P < .001). Day 7 IVM plasma levels were positively associated with body mass index (r = 0.57, P < .001) and were higher in female participants (P = .003), for whom An. gambiae mosquito mortality was increased until 7 days after a single dose of IVM (hazard rate ratio, 1.34 [95% confidence interval, 1.07–1.69]; P = .012). Although we found no evidence that IVM reduced Plasmodium infection rates among surviving mosquitoes, the mosquitocidal effect of AL-IVM1 and AL-IVM2 resulted in 27% and 35% reductions, respectively, in estimated malaria transmission potential during the first week after initiation of treatment.
Conclusions. We conclude that IVM can be safely given in combination with AL and can reduce the likelihood of malaria transmission by reducing the life span of feeding mosquitoes.
8) Chaccour, Carlos J., et al. “Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination.” Malar J 12.153 (2013): 10-1186.
Recent publications have highlighted the likely benefit of combining ivermectin with drugs such as artemisinin combination therapy (ACT). ACT is
highly effective in most malaria-endemic settings but does not prevent malaria-transmission in the first weeks after treatment [53,54].
=====================================================
87) Ivermectin Use in Scabies ROBERT S. FAWCETT, M.D., M.S., York Hospital Family Practice Residency, York, Pennsylvania. Am Fam Physician. 2003 Sep 15;68(6):1089-1092.
—————————————-
ivermectin cancer cell death
2015
9) Draganov, Dobrin, et al. “Modulation of P2X4/P2X7/pannexin-1 sensitivity to extracellular ATP via ivermectin induces a non-apoptotic and inflammatory form of cancer cell death.” Scientific reports 5 (2015).
We found that Ivermectin kills mouse and human triple-negative breast cancer (TNBC) cells through augmented P2X7-dependent purinergic signaling associated with caspase-1 and caspase-3 activation.
FIg 7 Model of P2X4/P2X7/Pannexin-1-induced cancer cell death.
Ivermectin induces P2X4/P2X7-dependent activation of Pannexin-1 channels and release of ATP. The release of ATP might be transiently protective, but only in cell types that are highly sensitive to Ivermectin-induces cell swelling when ATP and Ca2+ signaling are essential for control of cell volume. In cancer cells where no cell size changes can be observed (for example human TNBC MDA-MB-231 cells), high concentrations of ATP (1–3?mM) immediately enhance Ivermectin cytotoxicity. Potentiated P2X7 receptor signaling drives a fast progressing necrotic/pyroptotic mechanism driven by NADPH oxidases-generated ROS, cytosolic Ca2+/CaMKII activation, and MPTP, and characterized by caspase-1 cleavage, due to possible NLRP3 inflammasome activation. Necrotic killing is followed by a slower progressing apoptotic cell death program mediated by caspase-3 activation. The failure of the default apoptotic pathway might be attributed to faster activation of caspase-1, inadequate autophagic control of mitochondrial MPTP, collapse of cellular energy metabolism, resulting in rapid progression of necrotic cell death. Damage to mitochondria and ER stress as well as potential depletion of cellular ATP reserves simultaneously promote autophagy that might render even the slower apoptotic pathway immunogenic.
——————————–
2004
10) Drinyaev, Victor A., et al. “Antitumor effect of avermectins.” European journal of pharmacology 501.1 (2004): 19-23.
11) Searching for Ivermectin Deficiency Syndrome by Dr Simon Yu author of Accidental Cure.
12) 2012 Patent for Ivermectin as treatment for hematologic malignancy (including mantle cell lymphoma.) Use of synergistic combinations of an avermectin and an antineoplastic compounds for the treatment of hematological malignancies EP 2498785 A1 (text from WO2011054103A1)
13) Sharmeen, Sumaiya, et al. “The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells.” Blood 116.18 (2010): 3593-3603.
To identify known drugs with previously unrecognized anticancer activity, we compiled and screened a library of such compounds to identify agents cytotoxic to leukemia cells. From these screens, we identified ivermectin, a derivative of avermectin B1 that is licensed for the treatment of the parasitic infections, strongyloidiasis and onchocerciasis, but is also effective against other worm infestations. As a potential antileukemic agent, ivermectin induced cell death at low micromolar concentrations in acute myeloid leukemia cell lines and primary patient samples preferentially over normal hematopoietic cells. Ivermectin also delayed tumor growth in 3 independent mouse models of leukemia at concentrations that appear pharmacologically achievable. As an antiparasitic, ivermectin binds and activates chloride ion channels in nematodes, so we tested the effects of ivermectin on chloride flux in leukemia cells. Ivermectin increased intracellular chloride ion concentrations and cell size in leukemia cells. Chloride influx was accompanied by plasma membrane hyperpolarization, but did not change mitochondrial membrane potential. Ivermectin also increased reactive oxygen species generation that was functionally important for ivermectin-induced cell death. Finally, ivermectin synergized with cytarabine and daunorubicin that also increase reactive oxygen species production. Thus, given its known toxicology and pharmacology, ivermectin could be rapidly advanced into clinical trial for leukemia.
Articles With Related Interest:
Targeting Cancer Stem Cells With Nontoxic Therapies
Artemesinin Our Best AntiCancer Weapon
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
http://www.drdach.com
http://www.naturalmedicine101.com
http://www.bioidenticalhormones101.com
http://www.truemedmd.com
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2016 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation.
The post Ivermectin Antiparasitic Anticancer Wonder Drug appeared first on Jeffrey Dach MD .
Jeffrey Dach's Blog
- Jeffrey Dach's profile
- 3 followers



