The structure of a diamond crystal is mathematically fascinating. But there’s a related form of carbon, sometimes called the triamond, that’s theoretically possible but never yet seen in nature. Here it is:
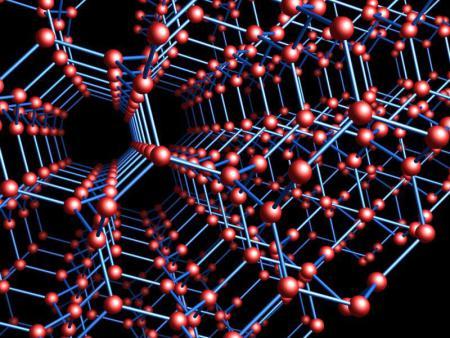
In the triamond, each carbon atom is bonded to three others at 120 angles, with one double bond and two single bonds. Its bonds lie in a plane, so we get a plane for each atom.
But here’s the tricky part: for any two neighboring atoms, these planes are different. In fact, if we draw t...
Published on April 11, 2016 13:43
