How does chemistry shape evolution?
When people think of evolution, many reflect on the concept as an operation filled with endless random possibilities–a process that arrives at advantageous traits by chance. But is the course of evolution actually random? In A World from Dust: How the Periodic Table Shaped Life, Ben McFarland argues that an understanding of chemistry can both explain and predict the course of evolution. To understand what he means, we’ve created this slideshow to uncover how the rules of chemistry have influenced many of life’s fundamental processes, and in turn, the course of evolution.

Why chemistry is important when studying evolution
“All life, from a lakewater bacterium to the neurons firing in your brain as you read this, is hemmed in. It is free to randomly adapt to its surroundings with nearly infinite creativity, but its overall path is as constrained as if it were walking on the deck of a ship crossing the ocean. The ultimate movement, on the scale of billions of years, is shaped by chemical rules.” –Ben McFarland, A World from Dust.
Image Credit: Fishing boat, Public Domain via Takeopix.

Determining which elements play an important role in our lives
“A molecule needs three characteristics to be useful for building life’s structures:
1.) It must be available in water.
2.) It must have a fitting shape and charge for its purpose.
3.) Its bonds should last as long as they need to (and no longer).”
–Ben McFarland, A World from Dust.
Image Credit: Waves in water by bykst. CC0 via Pixabay.

Phosphorus
Phosphorus is the perfect chaining element in our cells because of its ability to establish medium-strength bonds with negatively charged oxygen atoms in water. These bonds allow phosphorus to complete the following functions in our cells:
• Phosphorus is used in the form of phosphate as a “signal molecule”—sending messages through its bonds such as the need for proteins to be made from DNA.
• Phosphorus is used in energy transfer, most importantly through the energy released to muscles by breaking the bonds of the phosphate group ATP.
• Phosphorus is used in maintaining the structure of DNA. Phosphate is an important component of DNA’s double helix backbone because each phosphate in the chain has a negative charge—thus keeping the DNA chain spread out and inhibiting it from tangling.
Image Credit: Red Phosphorus Powder by Hi-Res Images of Chemical Elements. CC BY 3.0 via Wikimedia Commons.

But elements don't act on their own, they react with each other
“Chemistry is the study of pairings.” –Ben McFarland, A World from Dust.
Image Credit: Beakers by usehung. CC BY 2.0 via Flickr.

Magnesium
Because of phosphorus’ prevalence within cells, magnesium’s positively charged bonds play a crucial role in balancing the negative charge of the bonds formed with phosphorus—thus balancing and neutralizing the net charge within our cells.
Image Credit: Magnesium crystals produced via the Pidgeon process by Warut Roonguthai. CC BY-SA 3.0 via Wikimedia Commons.
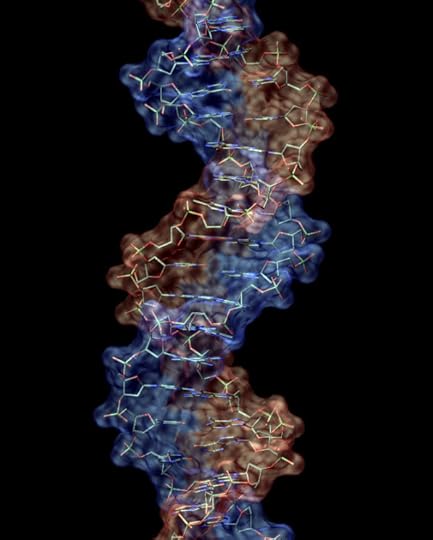
CHON: Carbon, Hydrogen, Oxygen, and Nitrogen
96% of your body weight is made up of carbon, hydrogen, oxygen, and nitrogen, because proteins and DNA are mostly made up of these four elements. As such, these four elements are often referred to as the building blocks of life.
Oxford University Press's Blog
- Oxford University Press's profile
- 238 followers



