Mary Nash Stoddard's Blog, page 22
February 22, 2012
PART #2 - Aspartame Industry Coverup Exposed
A new, five-member internal FDA task force analyzed three of these studies, and Universities Associated for Research and Education in Pathology, Inc., a consortium formed by 15 universities, was contracted to look at another dozen. Much like the earlier team, the five-member FDA task force, headed by veteran Chicago inspector Jerome Bressler, assailed the quality of animal tests into whether the substance might cause birth defects and tumors.
The report said Searle laboratory employee Raymond Schroeder, who worked on related research, first told investigators the feed in a study of the aspartame breakdown product DKP was so inadequately mixed it appeared the rats could "discriminate" and avoid eating the SKP.
Schroeder, who has worked for another company since 1975, later backed off his statement. he told UPI, "I just didn't feel qualified to speak on something I didn't work on ... There's no one twisting my arm."
Bressler criticized the company's "sloppiness" on all three studies.
"The question you've got to ask yourself," he said in an interview, "is: Because of the importance of this study, why wasn't greater care taken? The study is highly questionable because of our findings. Why didn't Searle, with their scientists, not closely evaluate this, knowing fully well that the whole society, from the youngest to the elderly, from the sick to the unsick ... will have access to this product?"
Howard Roberts, acting director of FDA's Bureau of Foods, appointed a five-person task force to review the Bressler team's findings pending a decision on whether to throw out the three tumor and birth-defect studies.
Jacqueline Verrett, the senior scientist on the review team, said members were barred from stating opinions about the research quality.
"It was pretty obvious that somewhere along that line they (bureau officials) were working up to a whitewash," she said. "I seriously thought of just walking off of that task force."
Verrett, now a private consultant, said that she and other members wanted to "just come out and say that this whole experiment was a disaster and should be disregarded."
But on Sept. 28, 1977, the panel reported that deviations between Searle's raw data and its FDA submissions were "not of such magnitude" as to alter its conclusions.
Verrett said the bureau's intent seemed to be "to tone down what was really found." She noted the bureau felt pressure because safety concerns also had been raised about cyclamate, another alternative for the cancer- linked sugar substitute saccharin.
(In October, 1978, a year after ordering the review that helped get Searle's petition back on track, Roberts quit to become a vice president at the national Soft Drink Association. The NSDA's members later marketed a stream of NutraSweet-flavored diet soft drink products.
(Reached at NSDA, Roberts dismissed Verrett's criticism, asserting the task force report "really was of no importance." he said he had no concerns about the appearance of his taking an industry job, stressing he does not represent NSDA before the FDA.
("I sleep well at night," he said.)
Negotiations for an additional, outside review of Searle's studies had begun with an Aug. 4, 1976 meeting between Searle and 1O FDA officials. During the meeting, Searle officials said they desired to help pick the consultant to perform the review, an internal FDA memo said.
Agency memos show the FDA soon was negotiating with the Universities Associated for Research and Education in Pathology for a half-million dollar, company-funded "validation" of a dozen Searle studies.
The pathology organization's review concluded that Searle's studies were authentic and the discrepancies largely inconsequential.
Adrian Gross, an investigative consultant to the 1975 task force, later said the 16-month review was "at best irrelevant" because the group was limited to analyzing "whether Searle lied about the data in its tests."
"It was not our task to challenge the validity of the experimental methods, since the FDA had itself already accepted the methodolgoy," the group's executive director, Kenneth Endicott, said.
Jere Goyan, who was FDA commissioner in 198O, said he would have put less weight on the review than on the findings of FDA's task forces. Goyan also suggested that, after approving aspartame in 1974, the FDA's Bureau of Foods may have "felt they had to keep their previous position."
Regardless, the pathology group's findings carried major weight in the final approval decision. The chairman of the 198O Public Board of Inquiry, Dr. Walle Nauta of the Massachusetts Institute of Techanology, said the board had to rely on those findings because it was denied access to the task force reports by FDA officials.
"There was absolutely no way in which we could decide who was right here," Nauta said. "We simply had to accept the data as they stood."
Nauta was joined on the panel by Drs. Vernon Young of MIT and Peter Lampert of the University of California at San Diego.
Before voting 3-O to ban NutraSweet on the narrow cancer issue, the board itself was drawn into allegations of bias because two of the three members cane from MIT _ as did Bureau of Foods chief Miller and several scientists involved in the controversy.
Between 1979 and 1982, four more FDA officials who participated in the approval process took jobs linked to the NutraSweet industry: Pape; acting FDA commissioner Sherwin Gardner; Albert Kolbye, who was associate director of the Bureau of Foods for toxicology, and Mike Taylor, an FDA lawyer who represented the bureau before the Board of Inquiry. All four denied any conflict of interest.
After the Board of Inquiry ruled against NutraSweet on Sept. 3O, 198O, Searle waited until jan. 21, 1981, the day after President Reagan's inauguration, to press for a reversal of the FDA commissioner assuring the new administration would decide the issue.
Jere Goyan, Hayes' predecessor as commissioner, said he found the delay curious because, after eight years of legal battles, financially struggling Searle "obviously was most anxious to have this thing approved."
Robert Dormer, a lawyer for the NutraSweet Co., said there was nothing special about the Jan. 21 date or the papers filed that day.
But with Reagan's election, it was virtually assured that a Republican-appointed commissioner would replace Goyan and decide the appeal _ and Searle had strong GOP connections with Rumsfeld at the helm.
Goyan had set up a five-member "commissioner's team" of scientists with no prior involvement in the issue to review the board's ruling.
On May 18 and 19, 1981, a month after Hayes took office, scientists Satya Dubey, Douglas Park and Robert Condon each laid out concerns about the sweetener's safety in memos to team lawyer Joseph levitt.
Dubey not only expressed researvations about the reported incidence of brain tumors in one key Searle rat study, but also said key data in another study appeared to have been altered. Dubey, who still works at FDA, refuses to discuss the matter.
Condon, another statistician on the team, and Park, staff science adviser in the agency's Office of Health Affairs, each said the available evidence failed to prove NutraSweet's safety or lack of safety.
Park said that Levitt hurried the panel to decide the issue. "They wanted to have the results yeaterday," he said. "We really didn't have the time to do the in-depth review we wanted to do."
Park said levitt met frequently with Hayes and "was obviously getting the pressure to get a resolution and a decision made."
Sources have said the office of Sen. Howard Metzenbaum, D- Ohio, has received allegations of political influence in Hayes' final decision-making process.
In a letter written after the FDA cleared NutraSweet, one former Searle saleswoman, Patty Wood-Allott, asserted that Rumsfeld told his sales force shortly after Reagan took office that, if necessary, "he would call in all his markers and that no matter what he would see to it that aspartame would be approved that year." Rumsfeld declined to return phone calls.
With three of five scientists on the commissioner's team opposing approval, it was decided to bring in a toxicologist for his opinion on isolated issues.
Goyan said if the decision were his, he never would have enlarged the team. While the panel did not vote, it ended up split 3-3.
Levitt, who normally would have been expected to draft an options paper spelling out scientific evidence on key issues, took an unusual tack. He circulated an approval recommendation _ and only backed off when Dubey, park and Condon objected, team members said.
Levitt said he was not directed to draft the approval memo, but did so as a "tactical" step to break the team's weeks-long impasse by forcing each scientist to state his views.
"It worked, didn't it?" said levitt, who later was promoted to a post as an executive assistant to the FDA commissioner.
One team member said that during discussions, Hayes, appeared to be abandoning the agency's traditional standard of "reasonable" proof of safety and looking for "proof of hazard."
Hayes' July 1981 approval decision came in the face of a Searle threat to file a suit challenging the regulatory delays.
His ruling relied in part on a late rat study of brain tumors submitted by Ajinomoto, a Japanese company that manufactures aspartame for Searle. That study, however, tested Wistar rats, a strain that some scientists said is more tumor resistant than the Sprague-Dawley rats used in earlier research.
In his decision, hayes wrote: "Few compounds have withstood such detailed testing and the repeated close scrutiny and the process through which aspartame has gone should provide the public with confidence of its safety."
In late 1982, Searle petitioned for FDA approval to use the sweetener in diet soft drinks and children's vitamins. On a day when Hayes was away, Novitch aprpoved the petition, increasing the acceptasble daily intake level for humans by nearly half, from 34 to 5O milligrams per kilogram of body weight.
Novitch, now in private industry, said he and Hayes had worked together on the matter, but declined to say why he was left to sign the approval.
Just weeks later, Hayes resigned under the cloud of an internal Department of Health and Human Services investigation into his acceptance of gratuities from FDA-regulated companies _ including free rides aboard jets owned by a major NutraSweet user, the General Foods Corp.
Shortly after being named dean of the New York Medical School, Hayes also became a consultant to the New York-based public relations firm of Burson marsteller, which represents the NutraSweet Co. and several major users.
Hayes` former top spokesman, Wayne Pines, who previously had joined the firm, said he approached Hayes because he thought him "an added value" to clients.
Hayes, now president of the E.M. Pharmaceutical Co. in Hawthorne, N.Y., declined comment for this series of articles. He has in the past denied any impropriety in his consulting role, which sources said paid him more than $1,OOO per day.
Burson marsteller vice president Buck Buchwald stressed that Hayes was not involved in NutraSweet issues and worked but 1O to 15 days a year. But a former Burson Marsteller employee, who requested anonymity, said that Hayes was hired precisely because of his decision on NutraSweet and other issues affecting company clients.
Metzenbaum said it was "at the very least ... unbecoming, at the very most it probably was inappropriate" for Hayes to accept the position.
In July 1986, Anthony Brunetti, a consumer product officer who drafted the 1983 notice approving NutraSweet use in soft drinks, also took an industry job, joining the soft drink association as a science adviser. Brunetti said he cleared the move with the FDA's ethics officer.
"My situation," he said, "is no different than many, many people ... that go through the revolving door. It can be made to look like there is some duplicity going on. In terms of my own conscience, I have no problem."
Ron Lorentzen, an FDA toxicologist who was asked by current Bureau of Foods chief Sanford Miller to perform a separate, internal review of the agency's hadling of aspartame, described it as a "tortured" story.
But despite the myriad questions and revolving door issues, he asserted the FDA responded to each issue "in a way perfectly reasonable."
Other questions have arisen over the company and industry's funding of researchers who have invariably supported NutraSweet's safety _ with the exception of people with the rare disease phenylketonuria. Independent studies have often raised health concerns.
Metzenbaum said, "If it is a fact that no questions were raised and more than a million dolalrs was spent, you have to wonder whether their job was done as thoroughly as it should be done."
Stegink's longtime research collaborator, Dr. Jack Filer, serves as executive director of ILSI, the Washington foundation that funds aspartame research.
Filer said he see no conflict in his dual roles as ILSI's executive director and a company researcher, but declined to disclose his ILSI consulting fees. he said all the Iowa research money had gone to Stegink.
Filer said he had been "maligned over the years for taking money from corporations," but that the funding source never has influenced his findings.
Dr. David Hunninghake of the University of Minnesota was picked to study aspartame's effect on the liver by former Searle research director Daniel Azarnoff, once Hunninghake's mentor at the University of Kansas, a Hunninghake associate said. he said Searle helped design the study.
Susan Schiffman, named to head a Searle-funded Duke University Medical School study into NutraSweet's link to headaches, is a former General Foods and Searle consultant. Her research at Duke, where the medical school has a new Searle Center, has fallen under the office of university vice president William Anlyan, a former Searle director. Schiffman said Anylan had no role in Searle's promise to cover all costs of the study, expected to cost "hundreds of thousands of dollars." She said she took no salary for her work.
Another industry-backed researcher has been Ann Reynolds, now chancellor of California State University at Long Beach. Olney asserted that in a 1971 study, Reynolds confirmed his findings that the sweetener destroyed nerve cells in infant mice, burt Searle did not notify the FDA until 1975 or 1976 _ after the agency's initial review. Dr. Daniel Azarnoff, Searle's former science director, and other Searle officials have denied withholding any studies from the government.
Reynolds also co-authored a Searle monkey study that contradicted earlier aspartame research leading to seizures in mondeys. Olney alleged that Reynolds, who did not return phone calls, and several other company-funded researchers "have a pattern of avoiding" scientific peer review. Industry spokesman contend that few studies by scientific critics of NutraSweet have undergone peer review. But few such clinical studies have been completed because of a funding shortage.
George Liepa, a nutrition professor at Texas Women's University, said he was required to discuss his findings with Searle before reporting that NutraSweet is safe for diabetics on hemodialysis. Dr. David Horwitz, as associate professor of medicine at the University of Illinois who studied NutraSweet and diabetics, said the company did not influence the outcome but, "The results were favorable ... Obviously, that is perhaps why Searle was eager to fund an additional study of ours."
Dr. Lewis Stegink, a pediatrics professor at the University of Iowa who repeatedly had produced studies
that he says support aspartame's safety, has received more than $1.3 million in research grants and gifts _
including lab equipment _ from the company since the early 197Os, limited university records show.
Filer also said the company paid him and Stegink "$2,OOO to $3,OOO" to edit a book, "Aspartame,"
about research on the sweetener and another $1,OOO or $1,5OO to each of 23 contributors, including
researchers whose studies helped the company win FDA approval. The book states that "the extensive
research program carried out to demonstrate aspartame safety may serve as a new standard for the study of
food additives."
Next: Sweet corporate victories UPI investigative report: Maverick scientist at center of NutraSweet controversy
By GREGORY GORDON - WASHINGTON (UPI) _ Dr. Richard Wurtman was an ardent defender of NutraSweet's safety at public
hearings six years ago. Now he is one of the artificial sweetener's harshest critics. "I think the likelihood is very strong that NutraSweet does produce serious and potentially damaging brain
effects in a number of people," the nationally known neuroscientist from the Massachusetts Institute of Technology said in a recent series of interviews.
Wurtman's seemingly enigmatic flip-flop from a position as a G.D. Searle Co. consultant to a role as a foe urging restrictions on marketing of the firm's best-selling product appears to be much at the center of the controversy over NutraSweet's safety.
Wurtman says his views simply changed with the evolution of his scientific studies and his growing skepticism of industry's attitude toward research.
His sometimes stormy relationships with the company and an industry-funded foundation, the International Life Sciences Institute, provide a glimpse of the maneuverings surrounding research into a major food additive.
Wurtman, a brash-talking, hard-driving head of a major research laboratory, said he unilaterally severed his consulting relationship with Searle in 1985 after he grew concerned about NutraSweet's effects and the company's inaction. He said he rejected several approaches by the firm _ called the NutraSweet Co. since its sale that year to the Monsanto Corp. _ to rekindle the arrangement.
Wurtman accuses NutraSweet Co. officials of "misrepresenting" the nature of company-financed studies into links between the sweetener, generically known as aspartame, and epileptic seizures, of sidestepping key safety issues and of threatening to veto his grant application to ILSI's aspartame committee.
A spokesman for the company described Wurtman's public attacks as a "political issue," but declined to elaborate.
Wurtman`s relationship with Searle, The NutraSweet Co. and many of the companies that sell NutraSweet- flavored products dates to 1978. Beginning that year, according to public records, ILSI provided more than $2OO,OOO to finance his research on caffeine, a common beverage ingredient that was under FDA scrutiny.
Wurtman said he found no ill health effects during his caffeine research, and his relationship was "excellent" with ILSI _ a spinoff of the National Soft Drink Association.
During the same period in 1978, he said, he rejected a Searle offer of financial support for research on amino acids. Phenylalanine and aspartic acid, two such amino acids, are the main components of NutraSweet.
He said Dr. Sanford Miller, chief of the FDA's bureau of foods, later sought his testimony before a 198O Public Board of Inquiry because he had openly stated his belief that neither glutamate nor aspartic acid, a similar compound to that in NutraSweet, would not cause brain damage. Wurtman strongly defended aspartame at the hearing.
He said he did not focus on phenylalanine until about 1983 when he learned the FDA was considering expanding use of the low- calorie sweetener _ approved two years earlier for dry foods _ to include carbonated soft drinks.
From his caffeine research, Wurtman said, he was aware of the exploding soft drink market and concluded "that the use of aspartame was going to go up considerably."
"I was genuinely concerned that there might be an increase in brain phenylalanine levels."
Wurtman said that, while phenylalanine is vital to the brain, it can serve as a barrier to 2O other amino acids that provide protein.
At a meeting in July, 1983, Wurtman said he told National Soft Drink Association officials that "if you put large amounts of aspartame in soft drinks and people drink as much as I think they will, there are going to be problems."
Wurtman said that after the industry accepted his idea for combining NutraSweet with saccharin to cut the danger level, he accepted a Searle offer to serve as a consultant and relations were "all very friendly and chummy."
He said he became convinced that "these people really want to know the extent to which their product may be a real problem."
Shortly after he took the consulting job, he began getting letters from seizure victims who believed their problems stemmed from NutraSweet.
Wurtman said when he advised Dr. Gerald Gaull, Searle vice president for nutrition and medical affairs, in the spring of 1985 that he thought there was a link, "there was a very rapid souring of the relationship."
During a visit to his MIT laboratory, Wurtman said, Gaull asked to review a proposal for a seizure study by him and his collaborator, Harvard University neurologist Donald Schomer. He charged that when he advised Gaull the pair would seek funding from ILSI, Gaull "got very angry and said, `We, meaning Searle, are active members of ILSI and we will veto your study.'"
"I was incredulous that he would say it to me, and I was dumbfounded that he would say it in front of witnesses," Wurtman said.
Schomer said he did not recall the comment. Gaull said, "There is no way that I can veto anything at ILSI," because Searle has only one of 12 votes on the ILSI aspartame committee. He did not deny making the threat.
Wurtman charged that Gaull later advised ILSI that two company-funded seizure studies already were under way, and the foundation declined to approve the grant.
In July of 1985, Wurtman said, he and three other scientists who had expressed concerns about NutraSweet were among a group invited to Gaull's home in Northeast Harbor, Maine, for a two-day conference.
"I left there with the conclusion there was no way these people were going to do an honest job in assessing the possibility that aspartame contributed to seizures," Wurtman said.
He said he also was skeptical because, as a company consultant, Searle had asked him to chair its scientific advisory committee _ a role in which the company could use his name to defend the integrity of its own research. But, he said, Searle refused to let him see protocols and data from its studies.
"They wanted the name, but not the reality," he said.
Frustated by these developments, Wurtman said he wrote a letter to Robert Shapiro, president of Searle and later of The NutraSweet Co.
"Dear Bob," the letter said, "I know you'll agree that my value to Searle ... derives in part from my telling the company some things that it would rather not hear ... and then from helping the company to deal with those things.
"One such thing is that some consumers may develop significant medical symptoms after consuming very large amounts of aspartame, particularly if they happen concurrently to be on low-calorie, low-protein weight-reducing diets.... If Searle- supported studies are going to contribute to our understanding of these people and their symptoms, then the studies have to include them _ and not be restricted to people who have a can or two of soda per day."
He said Shapiro never answered the letter. Wurtman said he resigned his consulting role a short time later and rejected company efforts in the ensuing months to reinstate the arrangement.
(Editor`s note: UPI investigative reporter Gregory Gordon spent eight months examining industry research into the popular artificial sweetener NutraSweet and the Food and Drup Administration's handling of the product permeating the diet food and drink markets. Here is the last in his three-part report.)
UPI Investigative report NutraSweet: Questions swirl
Part 3: Sweet corporate victories By GREGORY GORDON
WASHINGTON(UPI) In October 1982, Sen. Howell Heflin, D-Ala., proposed an obscure amendment altering the laws covering U.S. patent extensions _ a move affecting only one company and one product, the artificial sweetner aspartame.
Without mentioning aspartame, which is sold under the name NutraSweet, the Senate passed the amendment to the Orphan Drug Act_ extending the G.D. Searle Co.'s domestic monopoly on aspartame sales for another five years, 10 months and 17 days.
"We think it is an excellent amendment," remarked Sen. Orrin Hatch, R-Utah, wrapping up a five-minute discussion on the Senate floor.
When the House approved the same language a month later, it all but cinched another $3.5 billion to $4 billion in revenues for the Chicago-based company.
It helped Searle's stockholders sell the company's assets, including its lucrative NutraSweet division and the two domestic use patents, for $2.7 billion to the Monsanto Co. in the summer of 1985.
Sponsors of the mearsure found their campaign committees enriched.
Heflin's 1984 reelection committee received contributions totaling at least $9,000 from Searle's top officers and its political action committee, more than any others among a long list of Searle beneficiaries in Congress, Federal Election Commission records show.
Hatch`s committee received at least $3,000, the records show.
Heflin defended his sponsorship of the measure, saying Searle had been victimized by regulatory delays that ate up most of its 17-year patent.
But a spokesman for the U.S. Patent Office said Heflin's legislation marked one of only a handful of instances in the last three decades in which a company's patent has been extended by a private bill in Congress.
It also provided a glimpse of the adeptness with which Searle, Monsanto and their lobbyists have guided the artificial sweetener through the obstacles of government regulatory bureaucracies to capture big financial rewards.
Headed by Donald Rumsfeld, a former Ford White House chief of staff, Searle repeatedly demonstrated its political acumen on other fronts, too, in the years prior to the sale to Monsanto.
In 1981, the company overcame a controversy-snarled, eight- year review process to win Food and Drug Administration approval for NutraSweet.
In 1984, Searle parried an assault on the sweetener's safety from Arizona food scientist Woodrow Monte after hiring Gov. Bruce Babbitt's former chief of staff as a lobbyist. Searle officers passed along campaign contributions of $2,000 to a key lawmaker, and the company soon had won passage of legislation crushing Monte's efforts to force tough state restrictions on the sweetener.
"I don't know of any company that has apparently covered all of its bases as well as has Searle," said Sen. Howard Metzenbaum, D-Ohio. "Whether it has to do with the scientists or lawyers, or non-profit institutions, or universities, or whatever; in every instance, I have found that they have expended their dollars very carefully and very wisely, but without apparent restraint as to the amount."
Indeed, besides Searle's hiring of up to a dozen lobbysists, UPI traced nearly $200,000 in federal campaign contributions between 1979 and 1986 from its officers and political action committee.
The political intervention in the patent process drew the ire of several small companies seeking to enter the aspartame market, triggering charges that a corporate giant benefited from unjustified or preferential treatment.
I think it's obvious they (Searle officials) used political muscle," Alan Kligerman, president of Lactaid, Inc., a New Jersey diet food manufacturer, said of the patent extension. He said his firm had been interested in manufacturing aspartame until the patent was extended, but "Searle was well wired in."
"It is posssible that they (the Senate) did not know what they were passing," he said. "I don't know how they got that through, except with the right phone calls."
"I would not hesitate to say," Metzenbaum said, "that the manner in which that five-year extension of the patent rights was put through on the floor of the U.S. Senate was totally inappropropriate.
"It should not have been done without the entire body being adivsed that that issue was going to be on the floor of the Senate."
Metzenbaum said that the Senate has an "alert" system under which all legislation is cleared with individual senators before it is brought to the floor, but the system was bypassed.
Jerry Ray, a spokesman for Heflin, asserted the offices of key senators _ including an aide to Metzenbaum _ approved the measure before it went to the floor. But Ray offered no explanation for the failure to fully disclose the contents and impact of the measure.
Ray quoted Heflin, chairman of the Senate ethics committee, as saying Searle representatives never mentioned campaign contributions in asking him to sponsor the amendment.
Heflin said he has "supported all patent restoration bills" because regulatory delays have created " a chronic problem" in which companies get so little use out of their 17-year patents they are reluctant to put money into research.
Hefling said he in Searle's case, "almost 35 percent of the patent term had been used on a long series of administrative hearings, trials and appeals (in) which, in the end, the corporation finally prevailed. To not restore some of the patent term lost would unfairly penalize them."
G.D. Searle sought an extension of its patent on grounds that the Food and Drug Administration's handling of its aspartame approval petition was "an unparelleled instance of unnecessary regulatory delay which worked a great injustice to Searle."
Critics argue that, to the contrary, the FDA suspended its 1974 approval allowing Searle to market the sweetener because of evidence the company's animal studies were flawed and the results were misrepresented to the FDA in the early 1970's.
The evidence prompted FDA chief counsel Richard Merrill to ask the U.S. attorney's office in Chicago to open a grand jury investigation into possible fraud by the company.
While a grand jury investigated similar allegations related to Searle drug products, no such inquiry ever was begun into the aspartame testing.
But the agency was concerned enough about Searle's research to appoint two task forces, a university research group and a Public Board of Inquiry to review various studies.
In 1981, shortly after taking office, FDA Commissioner Arthur Hull Hayes Jr. overturned the three-man Board of Inquiry and approved sale of NutraSweet in dry foods. Two years later, Hayes' deputy, Mark Novitch, approved the use of aspartame in soft drinks.
Kligerman dismissed as "crap" Searle's contention it had been victimized by the FDA bureaucracy, which delayed a decision from 1975 to 1981.
"The FDA had reason for doing this," Kligerman said of the intense review process. "It was not an unnecessary delay. It was Searle's fault that this happened."
For Purification Enigeering, Inc., of Columbia, Md., which raised money from private investors and built a plant solely to manufacture aspartame for Searle, the congressional action ultimately turned out to be devastating.
Gary Calton, senior vice president for Purification Engineering, said that on Jan. 4, 1985, Searle notified the firm its contract would not be renewed. Seven months later, the firm was sold to Rhone-Roulenc Co., a French firm.
My company would have been worth a great deal more if it had not been for that (patent) extension," Calton said.
Calling the action unfair, he said, "I don't think Congress should go around passing laws making G.D. Searle rich any more than they should go around making me rich."
Searle officials declined to discuss the patent extension, but a company lobbyist, former Ford White House official William Timmons, said the company "felt there was an injustice" in the delays following aspartame's 1974 approval. He said the company "took an advocacy role by talking to a lot of members" of Congress.
In May of 1984, FEC records show, Heflin's reelection committee received maximum $1,000 donations from Daniel Searle, the chief executive officer of the giant pharmaceutical company; his wife, Dain; William Searle, Searle's brother who was a company director; William Searle's wife, Sally; Suzanne Searle Dixon, a sister of the Searles, and her husband Wesley Dixon, who also was a company director.
Heflin also received $1,000 from William Searle prior to the general election, and $2,000 in Searle PAC contributions, the FEC records show.
On Nov. 9, 1982, a week after his reelection and a month after praising the amendment in the Senate chambers, Hatch's committee received $2,000 in contributions from top Searle officers, the records show.
Sen. Robert Byrd, D-W. Va., who brought the amendment up for a vote on Heflin's behalf, also received a $1,000 campaign contribution from Daniel Searle on Sept. 25, 1981.
Hatch received contributions of $1,000 each from Daniel Searle, Wesley Dixon and William Searle on Nov. 11, 1982, days after he was reelected to a second term in which he continued as chairman of the Labor and Human Resources Committee that oversees the FDA.
As chairman of the panel until last January, Hatch repeatedly blocked Metzenbaum's calls for new hearings into the safety of NutraSweet.
Prior to his reelection, Hatch also received $2,500 in contributions from the soft drink PAC.
Rep. Henry Waxman, D-Calif., who sponsored the Orphan Drug Act covering research for treating rare diseases and who carried Heflin's patent amendment to the bill in the House, received $1,500 in campaign contributions from the soft drink PAC, including $500 two days before the measure's introduction in the House.
Like Heflin, Waxman made no mention of aspartame in describing the Senate amendments to the drug act on the House floor.
Searle also flashed its political prowess after Arizona food scientist Monte stirred up a furor in 1984 by publicly assailing NutraSweet's safety.
The ensuing events, Monte charged, "reflected exactly what Searle has been doing all along. They've been buying their way into the hearts and minds of America. They've been using their financial acumen to get their way."
Within months, legislative rules were swept aside one day in early 1985 and, in a swift, subtle maneuver without notice to the public, Monte's campaign for state regulations on the sweetener was sidetracked.
Monte said he was convinced in 1983, when the FDA okayed use of NutraSweet in carbonated beverages, that the sweetener would break down into poisonous quantities of methyl alcohol in diet sodas left in the Southwest sun.
Monte, director of the Food Science and Nutrition Laboratories at Arizona State, and two consumer groups petitioned at the Arizona Department of Health Services to ban the sweetener.
Monte said his rat studies had shown that chronic ingestion of methyl alcohol causes brain damage similar to that in humans suffering from multiple sclerosis _ including seizures, amnesia, optic neuritis, numbness and dizziness. In the desert heat, Monte said, methanol degrades faster into toxic methyl alcohol.
Searle and FDA officials have argued that aspartame contains too little methanol to pose a health hazard.
could have opened the door for regulatory actions in other states. When he and the consumer groups pressed their legal challenge for more than a year, Searle flexed its
muscle: _The company dispatched a coterie of lobbyists to the state capitol, among them Andrew Hurwitz, Gov.
Babbitt's former chief of staff; prominent Arizona lobbyist Charles Pine; company lawyer Roger Thies, and another company official, David West. Between August 23, 1984, and Sept. 21, 1984, company officers Daniel Searle and his brother-in-law, Wesley Dixon, each contributed $1,000 to the campaign of state House Majority Leader Burton Barr, later a GOP candidate for governor, reports to the Arizona secretary of state's office show.
Campaign disclosure forms revealed that, during the same period, several House Republicans received contributions from the Committee to Re-elect Barr _ including state Reps. Don Aldridge, Karen Mills and Jan Brewer, all among Health Committee members who voted 13-0 to pass the measure affecting
NutraSweet. The trio received $1,500, $1,000 and $750 respectively from Barr, who for years has enhanced his influence by donating to colleagues' campaigns.
_Barr and Arizona State University regent William Reilly contacted the school's president, J. Russell Nelson, and Academic Vice President Jack Kinsinger to inquire into Monte's public attacks on NutraSweet, published reports said. Kinsinger insisted that the issue caused no delay in his decision to grant Monte tenure. Barr did not return phone calls.
When Monte's first petition was rejected, and he filed for reconsideration, Hurwitz wrote a letter offering legal advice to the DHS about its response and sent copies to Barr and aides to Babbitt.
In April of 1985, about the same time Monte and his associates finally were to be granted a hearing before the state agency on their petition, they learned that the Arizona legislature had used a rare maneuver to change the law, without public notice, to bar state regulation of FDA-approved food additives. The measure passed under the misleading title of a toxic waste bill.
Monte's campaign to ban NutraSweet in Arizona prompted the state Department of Health Services to conduct a study to determine how much NutraSweet soft drinks degraded in high- temperature conditions. The study, completed in July 1984, found that methanol levels were highest, 9.4 parts per million, in Diet 7- Up samples stored the longest time in the warmest temperature, 99-degree heat.
Present and former Arizona state officials have told UPI that the study concerned DHS officials enough that they discussed a NutraSweet ban.
But Norman Peterson, manager of the DHS's Office of Chronic Disease and Environmental health Services, said that the agency concluded that "the FDA had addressed the methyl alcohol question and had all sorts of supporting data. We had no basis for saying that the data they had presented in support was not correct and adequate."
Another source said Peterson was distressed enough that, during a meeting attended by DHS director Donald Mathis, he proposed being allowed to recommend that pregnant women and children limit their consumption of NutraSweet.
Peterson would not confirm the episode, but recalled that he "was upset about the fact that there were so many unanswered questions."
Mathis, who since has left the agency, said he was satisfied that it "wouldn't be humanly possible" to ingest levels of NutraSweet that would produce a toxic reaction.
In September 1984, Monte and his associates filed suit to force the DHS to impose storage and labeling requirements or ban NutraSweet altogether. But a proposed settlement under which the agency would hold a public hearing was scuttled because it lacked the approval of Mathis's successor, Lloyd Novick.
After more negotiations, the DHS agreed again to hold a hearing. But before it could take place, the issue was killed by the legislative change.
House Speaker, James Sossaman later admitted that the GOP- controlled House violated its own rules in passing a so-called "strike-all" amendment. Chairman Bart Baker of the Health Committee engineered the action, in which an existing bill was stripped, replaced with the NutraSweet language and brought to a vote without the required 24 hours public notice.
The success of the Searle family business, founded 8O years ago, is all the more astounding when compared to the company's predicament in 1977 when it plucked Rumsfeld as its president. Facing a company mired in debt, Rumsfeld, a native Chicagoan and former Illinois congressman, quickly hired three other outgoing Ford administration officials to join him.
As executive vice president, he named John Robson, a former partner in the Chicago law firm of Sidley & Austin who had served as President Ford's chairman of the Civil Aeronautics Board. Robert Shapiro, Robson's special assistant at the Transportation Department, was tapped as general counsel. Rumsfeld also hired William Greener Sr., who had been a spokesman in the Ford White House and Rumsfeld's chief spokesman at the Pentagon.
The pharmaceutical company suddenly was being run by lawyers and politicians.
Stomaching a $28 million net loss in his first year, Rumsfeld slashed Searle's operations, selling off more than 3O subsidiaries worth more than $4OO million.
Before Rumsfeld could mount a full-scale effort to lift an FDA freeze on the sale of NutraSweet, Searle was hit with serious new problems. Suits filed on behalf of 78O women alleged the company's Copper 7 intrauterine device had caused them to develop pelvic inflammatory disease, an infection of the reproductive tract that can lead to sterility, even death.
Beofre the suits could be settled, Searle sold out to Monsanto.
The huge, St. Louis-based chemical company and its officers promptly were met with stockholder suits alleging they had failed to explore potential safety problems with Searle's biggest moneymakers _ the Copper 7 IUD and NutraSweet.
Rejecting criticism of the acquisition, Earl Harbison Jr., executive vice president of Monsanto and chairman of the board of its Searle pharmaceutical subsidiary, said in October 1985 that Monsanto "studied this situation (the Copper 7 litigation) very closely prior to acquiring Searle, including consultations with independent physicians."
"We satisfied ourselves with the safety and efficacy of the product," he said.
Since then, the Copper 7 has been pulled off the market. Some lawyers likened the resulting legal morass to the failure of the Dalkon Shield that drove the Richmond-based A.H. Robins Co. into Chapter 11 bankruptcy protection.
But a former Monsanto official, who requested anonymity, said that as part of the sale agreement, Searle set aside reserves to cover the IUD lawsuits.
Thanks to NutraSweet, Searle family members Daniel and William Searle and their sister, Suzanne Searle Dixon, to date appear to have walked away unscathed from all the crises and legal battles.
And even if NutraSweet were proved hazardous, the purchase aggreement provided "no escrow, reserve or holdback for liability stemming from the potential health hazards attributed to the NutraSweet product line," says one lawsuit filed by Chicago lawyer Robert Holstein on behalf of a Monsanto stockholder.
And Rumsfeld emerged from his nine years with the company in solid financial condition. Securities and Exchange Commission records show that for his guiding the sweeping turnaround, he earned more than $2 million in salaries and more than $1.5 million in bonuses between 1979 and 1984.
UPI investigative report: Putting on the blitz: The selling of a sweetenre
By GREGORY GORDON - WASHINGTON (UPI) _ "Banana plants don't make NutraSweet," the television announcer noted wryly,
and the image of an exotic bird perched in a jungle tree filled the screen. "Neither do cows," said the voice, as the camera cut to a robust-looking heifer wagging its tail. "But they
might as well. If you've had bananas and milk, you've eaten what's in NutraSweet." True _ bananas, milk and NutraSweet all contain phenylalanine, one of 21 amino acids that form the
"building blocks" of protein. But that doesn't tell the whole story. Dr. Richard Wurtman, a neuroscientist at the massachusetts Institute of Technology says that because
NutraSweet lacks other important amino acids normally found in foods, the brain absorbs unusually high levels of phenylalanine that could increase the likelihood of epileptic seizures.
Referring to an ad proclaiming that the body treats the ingredients of the artificial sweetener "no differently than if they came from a peach or a string bean or a glass of milk," Wurtman said, "That's not true."
Dr. Louis Elsas, director of medical genetics at Emory University, groans at industry arguments that eating or drinking NutraSweet, known generically as aspartame, is just like eating a hamburger.
"Phenylalanine is a knwon toxin to the brain," Elsas said. "Aspartame is phenylalanine and drinking aspartame is like drinking phenylalanine as an individual amino acid."
A spokeswoman at the New York offices of Ogilvy and Mather, the lead ad agency on the sweetener account for the Chicago-based NutraSweet Co., declined comment on the allegations.
The drumbeat of NutraSweet advertisdments has been steady. Beverage Industry, a trade publication, labeled the NutraSweet blitz "probably the largest advertising campaign ever designed around a product ingredient."
Industry sources say that since 1984, The NutraSweet Co. alone has spent $3O million to $4O million a year on advertising, and ads by diet soft drink manufacturers and other companies whose products carry the swirl trademark of the sugar-free sweetener would easily send the figure past $1OO million a year.
The campaign has worked to make NutraSweet a household word.
Football stars Joe Montana and Dan Marino and boxer marvin hagler have pitched products containing the artificial sweetener on television. Former Democratic vice presidential candidate Geraldine Ferraro has appeared in advertisements endorsing a product containing NutraSweet, as have numerous celebrities, including Bill Cosby, Raquel Welch and Billy Crystal.
Chidlren, who some scientists say may be aprticularly susceptible to ill health effects linked to NutraSweet, are a primary target of the NutraSweet hype. In one ad, for a NutraSweet-flavored vitamin, a curious child asked his mother, "Why don't they put NutraSweet in broccoli?"
Although not in broccoli, the sweetener flavors scores of products ranging from coffee, cereal, chewing gum, cocoa mix, diet sodas and iced tea to gelatins, puddings, whipped toppings and vitamins.
The campaign to sell NutraSweet marked the first time a brand-name ingredient, rather than a rpoduct itself, has been so extensively advertised, industry observers said.
The NutraSweet campaign began with a highly successful theme of "why some things taste better than others," touting NutraSweet's flavor. But in 1985, after the first serious scientific concerns were raised, the thrust of Searle's afvertising shifted abruptly to a controversial new theme boasting that the sweetener is as safe as naturally grown foods.
Today, NutraSweet ads often appear during commercial breaks in TV fitness programs.
Aspartame Industry Coverup Exposed
(Methanol researchers received millions since early 1970’s to help prove Aspartame Safe see pp 12) United Press International Investigative Report of Aspartame
(Editor's note: UPI investigative reporter Gregory Gordon spent eight months examining industry research into the popular artificial sweetner NutraSweet and the Food and Drug Administration's handling of the product permeating the diet food and drink markets. here is the three-part report.)
Part 1: Did Searle ignore early warning signs?
By GREGORY GORDON WASHINGTON (UPI) _ A University of Illinois scientist says he warned the G.D. Searle Co. Years before
NutraSweet swept the diet food and soft drink markets that the company's new artificial sweetener could heighten risks of brain damage in fetuses and small children.
Dr. Reuben Matalon, a pediatrician and geneticist, said that between 1976 and 1984, he prodded Searle officials several times to do more research on the issue but Searle never performed the studies he suggested. The Chicago-based company did, however, pursue U.S. government approval for the low-calorie sugar
substitute _ and got it in a controversial ruling in 1981. Today, tens of millions of Americans guzzle diet soft drinks stamped with the NutraSweet Swirl, dump
packets of the NutraSweet tabletop sweetener Equal in their coffee and consume NutraSweet-flavored cereal, puddings, gelatins, cheesecake, chewing gum or vitamin tablets.
The Food and Drug Administration, despite receiving more than 3,6OO consumer complaints, is so confident of the sweetener's safety that it recently expanded uses to frozen and chilled fruit juices.
Matalon, however, has remained skeptical. In May, he reported that his initial, federally funded tests on 51 adults suggest heavy NutraSweet consumption may increase blood levels of a key amino acid enough to affect attention span, memory and concentration in some people, particularly small children. Pregnant women who are sensitive to the sweetener's main component, the amino acid phenylalanine, also may face a heightened risk that their infants will have birth defects, Matalon said.
More than a dozen other scientists, some of whom are conducting clinical studies, also say they suspect that subtle effects of the sweet powder could pose a major health problem. They believe NutraSweet _ known generically as aspartame _ is linked to brain damage, epileptic seizures, eyesight problems, allergic reactions, headaches or dizziness.
The likelihood is very strong that aspartame does produce serious and potentially damaging brain effects in a number of people,"" said Richard Wurtman, a neuroscientist at the Massachusetts Institute of Technology who is studying scores of people who suffered seizures after using NutraSweet.
Facing continuing controversy, The NutraSweet Co., the name adopted by Searle's NutraSweet division following its 1985 sale to the giant Monsanto Co., vouches for the sweetener.
The firm's president, Robert Shapiro, rejects the criticism voiced by matalon and others, saying, "The fact is that the world scientific community has considered these very specific allegations repeatedly, and has come to the same conclusion as the FDA."
An eight-month United Press International investigation not only turned up scientific concerns, but also raised questions about the way the product was approved, about the independence and depth of industry- funded research efforts into its safety, and about "revolving door" relationships between FDA officials _ including former commissioner Arthur Hull Hayes Jr. _ and the food and drink industries.
Shapiro, who obtained an advance copy of this UPI report, said, "Taken as a whole, the effect of the article is likely to be a thoroughly misleading impression of the state of knowledge of the subject." Company spokesman Thym Smith said the firm is contemplating litigation. Sen. Howard metzenbaum, D-Ohio, a leading skeptic of the FDA's approval who plans to hold a hearing on NutraSweet in the next few weeks, said, "I don't have hard evidence that the product is not safe. But I'm convinced that there's no hard evidence ... that the product is safe."
FDA officials stress they have yet to see hard data disproving the sweetener's safety. For that reason, the agency last year rejected a consumer group's petition to ban it on grounds that 14O users suffered seizures and eye problems.
NutraSweet has been at the center of intense controversy almost since July 18, 1981, the day Hayes approved its use in dry foods. Indeed, in rendering his decision, Hayes overrode six of the nine scientists on two agency review panels who felt studies on its possible link to brain tumors in rats had been inadequate.
Since then, some independent scientists have become unusually outspoken. Drs. Louis Elsas of Emory University and William Pardridge of the UCLA Medical School charged that the diet food and drink industry has engaged in a "whitewash" by rejecting health concerns, manipulating research studies and wining and dining scientific critics.
These and other researchers describe a world of subtle, high- stakes strategy in which the availability of corporate funds and the design of research protocols may have influenced the course of a multibillion-dollar industry and potentially affected the safety of millions of people.
The NutraSweet Co. and a non-profit industry group reject these allegations, asserting they have commissioned scores of studies to test the product's safety and that decisions on research funding are made solely on merit. Company spokesman Smith said NutraSweet's "phenomenal safety record is the result of the well known nature of the product rather than the manipulations of management."
Consumer complaints about NutraSweet surged in 1983, after Hayes' deputy, Mark Novitch, with the commissioner's support, approved its use in soft drinks such as "Diet Coke" and "Crystal Light," sending consumption soaring.
UCLA's Pardridge noted in a letter to the American medical Association Journal last year that, with aspartame, the food industry now is adding about five million pounds of phenylalanine _ "a known neurotoxin" to the food supply every year.
Roy Burry, an analyst with Kidder-Peadbody, Inc., said the exploding diet market now accounts for 24 percent of soft drink sales, compared with 1O percent in the late 197Os, and is growing at 2O to 25 percent a year.
The NutraSweet Co.'s sales are no longer public, but last year revenues were believed to have exceeded previously stated levels of $7OO million.
So intense has been the NutraSweet advertising campaign that the Professional Figure Skating Championship."
"Taking good care of oneself makes life a little better and NutraSweet makes it a little sweeter!" boasted one ad during a TV fitness program.
The NutraSweet Co. also has paid up to $3 million a year for a 1OO-person public relations effort by the Chicago offices of Burson Marsteller, a former employee of the New York PR firm said. The employee said Burson Marsteller has hired numerous scientists and physicians, often at $1,OOO a day, to defend the sweetner in media interviews and other public forums. Burson marsteller declines to discuss such matters.
Dismissing safety fears, The NutraSweet Co. stresses that its product, which in raw form is 18O times sweeter than sugar, has been endorsed by the AMA and other scientific bodies worldwide. Actually, the AMA's Council of Scientific Affairs gave a qualified endorsement based on "available evidence", including company-funded studies that were challenged by FDA task forces during investigations of the firm's laboratory practices in the 197Os.
Of 69 scientists who responded to a recent General Accounting Office survey, 28 said they felt more research was needed on NutraSweet and a dozen of those questioned considered it a major health problem.
An "aspartame victims" group has formed, a consumer group has pressed legal challenges and the company faces at least three personal injury suits. In one suit, Jim Stoddard, 32, a diabetic in Grand Rapids, Mich., charged that his heavy NutraSweet consumption triggered a dozen seizures _ the last one so violent he dislocated his shoulder and fractured his collar bone. Stoddard's lawyer, his sister, Cynthia, alleged he suffered brain damage and now has trouble understanding words because he consumed a product inadequately tested by Searle. She said she withdrew the suit recently for tactical reasons but would refile it early next year. The company denies the allegations.
Wurtman, who quit his job as a Searle consultant and became a vocal NutraSweet opponent, said he has been contacted by more than 2OO persons who suspect they suffered seizures as a result of NutraSweet use.
he said Dr. Gerald Gaull, a Searle vice president, visited his laboratory in 1985 and threatened to veto funding by ILSI, the Washington-based tax-exempt foundation, for his planned study into whether NutraSweet changes brain chemistry, lowering some humans' seizure threshholds.
Gaull said "there's no way" Searle, with one of 12 votes on the ILSI panel, could veto a grant decision, but he did not deny making the threat.
ILSI ultimately turned away Wurtman on grounds that Searle already had arranged for seizure studies at Yale University and New York's Mount Sinai Hospital _ studies that have drawn criticism because human volunteers were given aspartame only once or twice.
Wurtman said he now is tapping his laboratory's budget, which is extremely limited, slowing progress on his own studies. "Aspartame may be a serious health hazard," he said. "It's critically important that high quality research now be done to assess this hazard," he said. "It's critically important that high quality research now be done to assess this hazard." In his letter to the AMA Journal, pardridge said no one has fully researched the degree to which aspartame raises phenylalanine levels in the brain and, if so, what the possible effects are. he said in an interview that after he raised questions about the sweetener's effects on children, ILSI rejected his two grant proposals in 1985. Last year, he said, Gaull pressed him at a conference in Colorado to prove that phenylalanine _ one of 21 amino acids _ causes brain damage.
"It was incredible for his to ask that," Pardridge said. "That was the basis for my ILSI grant (proposal)."
"There's an internal conflict of interest," he said, "when a company which has profit at the bottom line is charged with finding out the true safety of its product."
Elsas, who publicly assailed NutraSweet in 1985, said he was put off for a year before ILSI rejected his proposal without stating a reason.
ILSI's executive director, Jack Filer, asserted research proposals were rejected because they cost too much or lacked scientific merit.
While denying funding for these aspartame skeptics, the company and ILSI have financed researchers with whom they have long-running relationships. A number of industry-funded scientists acknowledged that company and ILSI officials originated ideas for their studies or participated in the research design. These studies generally have reported the sweetener is safe.
Consumer lawyer Turner said, "The notion that an industrial company would take large sums of money and parcel it out to scientific consulting firms and university departments ... who they consider to be personal and commercial allies and friends is an unconscionable way to insure the safety of the American food supply."
He said the NutraSweet experience shows that "the entire system of the way scientific research is done needs to be carefully investigated, evaluated and revamped."
Food industry officials also said most studies financed by Searle or The NutraSweet Co. have been arranged as contracts, rather than grants. Smith said the company often uses contracts "to accomplish a specific research task."
James Scala, former director of health sciences for the General Foods Corp., a major NutraSweet user, said that a scientist working under contract became "more of an arm of the Searle research group than a grantee."
Scala, now with the Shaklee Corp., also said that most early NutraSweet research consisted of short-term studies that ignored possible "subtle," long-term effects.
Matalon said, "Let us say cigarettes were invented today, and you give 2O people two packs a day and after six weeks, no one has cancer, would you say that it is safe? That's what they did with NutraSweet."
Dr. Martha Freeman, who was a medical officer at the FDA's bureau of drugs in the early 197Os, argued in 1973 that the substance was "a new chemical ... that doesn't occur naturally" and should only be approved after long-term clinical studies, as if it were a new drug. Her arguments were rejected.
Despite these complaints, The NutraSweet Co. has insisted that company-funded studies prove that except for people with the rare disease phenylketonuria, the human body processes phenylalanine in aspartame just like any other food.
Thomas Stenzel, a spokesman for the International Food Information Council, a public relations arm for NutraSweet's manufacturers and biggest customers, contended scientific adversaries comprise a small minority.
He said he found it "very important that the leading professional health organizations" have found NutraSweet to be safe.
For example, the American Academy of Pediatrics concluded in 1985 that studies on people given massive aspartame doses showed no dangerous rise in blood phenyalanine levels; the Epilepsy Institute has reported the sweetener "to be safe for people with epilepsy."
Filer, executive director of the industry's main organ, the International Life Sciences Institute, suggested that problems blamed on aspartame may stem from "water load" on the brain resulting from over- consumption of liquids.
Maj. Michael Collings, who was an Air Force F-16 pilot in top physical condition, said he often drank up to a gallon of aspartame-sweetened products when he finished his daily, five- to eight-mile jogs in Nevada's desert heat. After noticing slight trembling in his hand over several weeks, he collapsed unconscious with a seizure on Oct. 4, 1985, a lawyer for Collings said.
Because of the seizure, Collings is grounded as a pilot for life, is on medication and was ordered transfered to Maxwell Air Force Base Alabama at a $4OO-a-month pay reduction, said attorney Bryan Gould, who charged in a state court suit last year that NutraSweet caused the seizure.
"He tells me that there's no way to describe the feeling of flight," Gould said. "He loves to fly and now he can't." The NutraSweet Co. denies any link between the sweetener and Gould's medical problems.
FDA officials, while publicly endorsing aspartame, are watching the situation closely. In late 1985, the agency took the unusual step of asking doctors nationwide to report adverse reactions to NutraSweet and another food additive, sulfites _ a move normally reserved for drugs. Sulfites since have been banned from the market. An FDA spokesman said about 25 doctors filed reports suggesting aspartame links to varying health problems.
The FDA approved NutraSweet products on the condition they carry a compulsory warning to phenylketonurics, individuals sensitive to its phenylalanine component. But Matalon, Elsas and others worry about millions of "carriers" of the disease who are unaware of their sensitivity. They say NutraSweet could damage fetuses of pregnant women whose bodies have trouble processing the amino acid.
Matalon, on releasing his new study, urged that products be labeled with the amount of NutraSweet they contain so consumers can monitor their intake. In Canada, aspartame is the only food additive for which such quantity labeling is required.
With comsumption soaring, Sanford Miller, chief of the FDA's bureau of foods, has acknowledged considering a labeling requirement in this country.
Dr. Gary Flamm, the FDA's top toxicologist overseeing food additives, said that beyond labeling, once a food additive such as NutraSweet has won approval, it is far more difficult to restrict its marketing.
"If ... our approval of it was a mistake, we couldn't rectify that without data showing that aspartame was unsafe," said Flamm, an aspartame defender.
Even then, he said, the agency would face a new regulatory thicket unless it could be shown NutraSweet posed "an imminent hazard." Consumer lawyer James Turner, who has campaigned for more than a decade for a NutraSweet ban, assailed the FDA's treatment of such safety issues. "Once a product is on the market, whether there by nefarious or honest means," he said, "it is impossible to get it off the market until it has caused severe, undeniable damage that has probably lasted over many years."
Several independent scientists have alleged that the industry has steered research money to allies in the scientific community, while denying funding to those who have raised health concerns.
A number of scientists who pressed for more studies into possible brain damage told UPI they were turned away by Searle and the International Life Sciences Institute, a tax-exempt industry foundation supported by the company, its Japanese aspartame-manufacturing partner and 1O sellers of NutraSweet- flavored products.
In interviews, Drs. Matalon, Wurtman, Elsas, Pardridge and John Olney of Washington University in St. Louis charged that the industry has paid millions of dollars for studies that have skirted the real issues about NutraSweet.
"There are virtually no studies," Turner said, "that have been done by individuals using resources other than the industry's that have given a clean bill of health to aspartame."
University of Illinois researcher Matalon recalled that he couldn't persuade Searle to do the kind of research necessary to put to rest lingering health concerns, neither on his first approach in 1976 nor when he submitted specific grant proposals to four more company officials beginning in late 198O.
After NutraSweet won FDA approval and began changing the dietary habits of millions of Americans, matalon said he lost patience in 1984 with the usual encouragement from Searle officials about prospects for future funding. "I felt they were just stringing me along," said Matalon, who obtained a $18O,OOO grant from the National Institutes of Health.
Comapny spokesman Smith said the NutraSweet manufacturer has "not discouraged Dr. Matalon's work _ nor anyone else's." While declining to comment on the decision not to fund Matalon's study, Smith said the company spends "between $3O (million) and $35 million annually on research."
"We do make decisions based on how we understand a study will be conducted and ... reasonable scientists may disagree on study designs," he said. The company has alleged that a number of its critics are seeking to pressure the industry to fund their laboratories.
Faced with sharply differing opinions on the sweetener's safety, the FDA and the national Institutes of Health, the government's chief funding mechanism for private research, have financed few studies on its effects. One former ranking NIH official, Artemis Simopoulos, argued the agency "should have a very extensive program on aspartame so people would know" whether it is safe.
Yet some NIH scientists have served as consultants to the ILSI foundation, helping decide the awards of $5OO,OOO in annual NutraSweet research grants in recent years. Even Simopoulos was a non-paid member of the foundation's board.
But ILSI's "aspartame technical committee," consisting of the NutraSweet Co. and 11 other manufacutrers and users of the sweetener, has been accused of discriminating against NutraSweet critics in granting awards.
Represented on the ILSI committee are General Foods, the Coca Cola Co., Pepsico, Inc., the Royal Crown Cola Co. and Seven-Up, Inc. ILSI insists that the NutraSweet Co. carries no special weight despite its U.S. monopoly on the sweetener. "The NutraSweet Co. is one of our members," said ILSI administrator Sharon Senzik. "Committees operate by Robert's Rules of Order."
Filer pledged that, despite his past ties to the company, as ILSI's head he would "let the chips fall where they may" on research results. Samuel Molinary, co-chairman of ILSI's panel, is Searle's former director of scientific affairs and now Pepsico`s research director. Molinary insists that ILSI is not "a lackey and tool" of The NutraSweet Co.
Peter Dews, a Harvard University phychobiology professor named to ILSI`s original board of trustees in 1978, has served as an ILSI consultant since then. Dews recently took the trouble to write and promote an article declaring that, based on scientific presentations at an ILSI aspartame conference in Spain last year, "There is now a mass of evidence" that NutraSweet is safe if consumed at FDA-recommended levels.
Dews declined to discuss his ILSI consulting fees, except to say it is "not enough to make any difference in my life." ILSI's 1984 return filed with the Internal Revenue Service showed payments to Dews that year of $31,OOO.
A lawyer for ILSI pledged to the IRS, in obtaining tax-exempt status for the foundation in 1983, that the organization "does not have any plans to engage in commercially sponsored scientific research." Attorney Roger Middlekauff advised the IRS that ILSI would "direct the research toward benefiting the public" and would release all research results.
But Elsas charged that ILSI "is definitely a front organization to try to make the public believe that there is some non-directed, non-biased research going on," when ILSI studies actually are likely to support NutraSweet's safety.
The industry has invited scientific critics for paid visits to company laboratories _ sometimes offering courtesy "honorariums," an industry source said.
Filer collaborated for several years on NutraSweet research with a colleague at the University of Iowa, Dr.
Lewis Stegink.
The NutraSweet Co. also has hosted critics at conferences in resort settings. Matalon briefed ILSI on his research at a meeting in the Costa del Sol region on Spain's southern coast. In the summer of 1985, the firm flew Wurtman, Elsas, Matalon, Pardridge, several of their wives and other NutraSweet critics to a two-day meeting at a luxurious home in Northeast Harbor, Maine. An afternoon was spent on a yacht, participants said. "This was industry wooing the concerned to shut up," Elsas said.
Pardridge said he was the only strong aspartame critic to accept an invitation in June 1986 to a heavily attended Searle- sponsored conference at a picturesque ski resort in Keystone, Colo. Pardridge said when he tried during the conference to raise his concerns about phenylalanine, the discussion was cut off. "It was just another typical industry whitewash," he said.
Next: An approval marred by controversy. Seizure, blindness victims point to NutraSweet.
By GREGORY GORDON - WASHINGTON (UPI) _ Susan Yarmey, a free-lance writer from Quincy, Mass., woke on a hot July
morning in 1984 with a large bump on her head and bruises all over her body. "I had no recollection of what had happened. There were marks on the wall, two wooden steps were
broken and there was a nice gash in the wall where my head had hit," she said. Yarmey's doctors diagnosed her injuries as resulting from a "classic" epileptic seizure. She and
Massachusetts Institute of Technology neuroscientist Richard Wurtman believe the incident may be connected to her consumption of the artificial sweetener NutraSweet, known generically as aspartame.
"A friend in New York directed me to the possible effects of NutraSweet consumption ... I was probably, at that particular time period, doing a liter and a half to two liters (of diet soda with NutraSweet) a day," said Yarmey, who said when she stopped taking NutraSweet her problems disappeared.
Yarmey is not alone. Many NutraSweet consumers _ particularly heavy users _ who have suffered headaches, tremors, blindness, allergic reactions and seizures blame NutraSweet for their ailments.
Wurtman says he personally is aware of more than 2OO cases in which he suspects NutraSweet has caused health problems such as headaches, dizziness and seizures.
Wurtman says the problem might be solved simply by stiffening the labeling requirements for NutraSweet products so that certain identified groups can monitor their intake.
"The groups I would identify are pregnant ladies, small children, people with a history of seizures and people who are taking certain drugs that interact with phenylalanine," an amino acid in the sweetener, Wurtman said.
Another former NutraSweet consumer, Shannon Roth, a mother of two who works as a goldsmith in Ocala, Fla., organized Aspartame Victims and their Friends, Inc. after suffering blindness in one eye. She said the group now has about 7OO members.
"I got up in the morning and had two pack (of Equal, the NutraSweet tabletop version) in each cup of coffee ... three or four cups of coffee before noon. Then I'd switch to the iced tea with it," Roth said.
In the summer of 1984, Roth said, she began to experience headaches, sleep and memory loss and irritability. After getting out of bed one morning, she discovered that she couldn't see when she closed her right eye, Roth said.
"I culd see like through a black veil. It was like a centralized, almond-shaped black spot," she said.
Doctors' laboratory tests failed to trace the cause of her partial blindness, she said, and one doctor told her not to expect vision to return to her eye.
Roth said she suspected NutraSweet as the cause after learning of a similar case that was allegedly linked to the sweetener _ and after about four weeks without NutraSweet, her headaches and other problems ceased. Her sight began to return a few weeks later, she said.
Joyce Wilson, a real estate agent in Stockbridge, Ga., said she began suffering from high blood pressure, dizziness and other ill health effects in 1982 after using Equal in her coffee and eating NutraSweet-flavored puddings. She said that in 1984 and 1985, she lost some vision.
"I'm not blaming this all on NutraSweet," Wilson said. "I'm just saying it's a strange coincidence that when I started using it, I started falling apart."
Dr. Morgan Raiford, an ophthalmologist at Emory University, examined both Roth and Wilson and believes their problems stem from consumption of the methyl alcohol in NutraSweet.
Dorris Bookhart, 43, a legal secretary in Lodge, S.C., started having what were later diagnosed as temporal lobe seizures in August of 1984. At the time, she said, she was drinking four 16-ounce bottles of Diet Coke a day, as well as diet lemonade. Both contained NutraSweet.
In January of 1985, after six months of problems, she suffered a grand mal seizure, a convulsive eqisode in which the victim loses consciousness, she said. Her doctors were mystified by the seizures, but they ruled otu epilepsy, Bookhart said.
She said she suspected NutraSweet as the culprit when, at her husband's suggestion, she stopped drinking Diet Coke and the problems ended.
"I've cried a lot of times thinking these people have destroyed my life and there isn't a damn thing I can do," she said.
Another heavy user of the artificial sweetener, Larry Taylor of Arlington, Texas, said he was hospitalized for five or six days to undergo a battery of tests after suffering a grand mal seizure in 1985. He also was a citim of migraine headaches that became more frequent between 1982 and 1984. After his seizures, Taylor, an anesthetist, was not allowed to work until January of this year, a disability he said left him "financially devastated."
What the critics say about NutraSweet.
By GREGORY GORDON - WASHINGTON (UPI) _ Despite The NutraSweet Co.'s insistence that scores of company studies have
proved the sweetneer is harmless, here's a sampling of the concerns from a hard core of scientific critics: Dr. Reuben Matalon of the University of Illinois has reported that heavy consumption of NutraSweet's main component _ the amino acid phenylalanine _ may cause neurological problems such as loss of memory and concentration. Matalon and Dr. Louis Elsas of Emory University say they fear aspartame consumption by some pregnant women can cause irreversible brain damage in fetuses. They worry most about women among an estimated 4 million to 2O million Americans who are carriers of the genetic disease phenylketonuria _ characterized by the liver`s inability to process phenylalanine. While there are an estimated 2O,OOO to 3O,OOO PKU victims nationwide who are warned not to take NutraSweet, carriers or heterozygotes _ do not have the disease and generally are unaware of their sensitivity, they said. The comapny has said that the Food and Drug Administration concluded "NutraSweet did not present any
additional health risk to pregnant women." Dr. Paul Spiers, a clinical neuropsychologist at Boston's Beth Israel Hospital, found in a recent pilot study
that, after consuming NutraSweet, some subjects with no previous problems failed to show the usual improvement in performance on cognitive tests. He plans further research. But Dr. Harris Lieberman of the Massachusetts Institute of Technology, who has received industry funding for NutraSweet research in the past, said his study of 2O adult males indicates that aspartame "has no readily measurable effect on mood and performance in normal humans."
In St. Louis, Washington University allergist Dr. Anthony Kulczycki found that wo women given NutraSweet capsules and a placebo suffered allergic reactions to NutraSweet. The women had reported hives and other skin reactions after using the sweetener.
Dr. Donald Johns, a neurology resident at Massachusetts General Hospital, reported last year that a "double-blind" study of a woman suffering migraine headaches showed her problems were aggravated by consumption of NutraSweet. NutraSweet, known generically as aspartame, consists of phenylalanine and another amino acid, aspartic acid, linked to a small quantity of methyl alcohol. Scientific critics seem to worry most about phenylalanine.
Dr. Richard Wurtman, a Massachusetts Institute of Technology neuroscientist, says heavy NutraSweet consumption may so flood the bloodstream with phenylalanine that other essential amino acids are blocked from reaching the brain, causing chemical changes that can affect behavior and lower the threshhold at which many people suffer epileptic seizures. Wurtman and Dr. Donald Schomer of Harvard University are testing seizure victims who used NutraSweet, particularly some whose bodies may have trouble processing phenylalanine. The NutraSweet Co. concedes aspartame raises brain phenlalanine levels, but says no harm results and that consuming the amino acids in NutraSweet "is just like eating other foods containing the same protein components."
Another Wurtman protege, Dr. Timothy Maher of Massachusetts General Hospital, supported his mentor by reporting that mice given a seizure-inducing drug and NutraSweet suffered more seizures than those receiving the drug alone. Dr. henry haigler, a scientist in a NutraSweet Co. Sister firm, said his similar study showed "no effect on seizure threshholds."
Dr. William pardridge of the UCLA Medical School, who also has done phenylalanine research, said he most fears the sweetener's effect on children, who he says are more likely to approach the FDA's acceptable daily intake level of 5O milligrams per kilogram of body weight. "If you're a child, seven to 12 years of age, the chances are good you'll have five servings per day" _ close to the acceptable level, he said. But Dr. Harvey Levy, head of the PKU clinic at Boston's Children's Hospital, wrote the Journal of the American Medical Association that Pardridge made an "inaccurate interpretation" of their data in predicting brain damage effects on fetuses from aspartame. Any danger level, they said, "would seem to be considerable higher" than levels from NutraSweet consumption.
Dr. Woodrow Monte, an Arizona State University food scientist, and Dr. Morgan Raiford, an ophthalmology professor at Emory, worry that a NutraSweet breakdown product, methyl alcohol, could produce severe eye damage. last year, Raiford examined more than a half dozen persons who said they suffered eye problems after consuming NutraSweet heavily. He said he diagnosed some cases of optic nerve damage and suspects NutraSweet's methyl alcohol is the culprit. The company denies any conn ection between NutraSweet and eye problems and has offered free exams to consumers who complain of such problems.
Dr. Sidney Wolfe, executive director of the Washington-based Health Research Group, said, "The thing that's really worrisome is that it clearly affects brain metabolism in animals, and anyone who disputes that is irresponsible."
Dr. John Olney of Washington University expresses fears about brain tumors _ a problem he and other scientists say would not show up in humans for 2O years and would be difficult to trace to NutraSweet. Olney said Searle rat studies have shown conflicting brain tumor data. As early as 1971, Olney reported that aspartic acid in aspartame killed cells in the brain's hypothalamus region, which regulates glandualr and hormonal functions.
Company responds to UPI series WASHINGTON (UPI) _ In response to the United Press International series of articles on NutraSweet,
The NutraSweet Co. issued the following statement: A series of articles to be released this week by UPI seriously misrepresents the vast body of scientific
evidence which estalbishes the safety of aspartame. Contrary to the impression created by these articles, the scientific record has been carefully reviewed by
independent and official scientific and regulatory agencies around the world. Without exception, each of these agencies has concluded that aspartame is a safe sweetner which can be
used as a normal part of the daily diet. The following quotations are representative of expert scientific and medical opinion around the world.
U.S. Food and Drug Administration: "The data and information supporting the safety of aspartame are extensive. It is likely that no food product has ever been so closely examined for safety ... Few compounds have withstood such detailed testing and repeated close scrutiny, and the process through which aspartame has gone should provide the public with additional confidence of its safety."
American Medical Association Council on scientific Affairs: "Consumption of aspartame by normal humans is safe ..."
American Diabetes Association: "Aspartame has been determined to be safe for the general population as well as for people with diabetes."
Government of Canada (Health Protection Branch): "(Aspartame) is one of the most extensively studied chemicals permitted for use in food. ... Based on the available data it has been concluded that aspartame would not pose any hazard to health when used in accordance with the current provisions of the Canadian food and drug regulations."
Government of Denmark (Danish Food Institute): "Research published in the scientific literature and/or studied in detail by governments and independent scientific committees maintains that the use of aspartame as an additive does not bear any health risk at all. ... There is therefore no toxicological basis for believing the intake of aspartame in soft drinks and food products should give rise to harmful effects in children or adults, even people with a high level of useage."
Government of Great Britain (UK Committee on Toxicity of Chemicals and Food): "Following detailed consideration of all toxicological data, we see no objection to the use of aspartame in food."
Other scientific agencies that have reviewed the evidence and confirmed the safety of aspartame include the World Health Organization of the United nations; the Scientific committee on Foods of the European common market; the Epilepsy Institute; and the American Academy of Pediatrics.
Aspartame has been reviewed and approved as a safe sweetener by the official food regulatory authorities in all the leading nations of the world, including many which forbid or restrict the useage of other sweeteners.
A recent article by Harvard Medical School Prof. Peter Dews reviewed the "massive evidence" that establishes the safety of aspartame. Dr. Dews concluded: "Many articles of everyday consumption that are known to be safe might not survive the scrutiny of such intensive and continued investigation.~
The respected consumer publication Consumer Reports summarized its conclusions this way: "An objective weighing of the evidence suggests that aspartame is the artificial sweetner to be preferred on safety grounds.:
The UPI articles also seek to discredit the process by which aspartame was reviewed and approved by the FDA. These charges have been conclusively rebutted by both the FDA itself and by the General Accounting Office, the investigative agency of the Congress. A full GAO report on the approval process concluded that the FDA had properly followed the appropriate procedures and had adequately addressed the scientific issues.
The UPI series is replete with misstatements and distortions which convey a totally misleading impression of the scientific facts.
Any concern or anxiety by consumers who read these articles is absolutely unwarranted. Aspartame is safe as approved by FDA and regulatory authorities around the world. Any contrary impression created by the UPi articles is a serious disservice to the public.
UPI investigative report: NUtraSweet: Questions swirl
(Editor's note: UPI investigative reporter Gregory Gordon spent eight months examining industry research into the popular artificial sweetener NutraSweet and the Food and Drug Administration's handling of the product permeating the diet food and drink markets. here is the second in his three-part report.)
Part 2: NutraSweet approval marred by controversy
By GREGORY GORDON- WASHINGTON (UPI) _ Poring over laboratory rat studies in the spring of 1981 in the government's final
safety review of a new artificial sweetener, senior statistician Satya Dubey of the Food and Drug Administration was troubled.
Dubey, a member of a special FDA "commissioner's team" formed to help decide the fate of the product to be known as NutraSweet, wrote in an internal memo that brain tumor data from rat tests was so "worrisome" he could not recommend approval.
Two Two other statisticians on the six-member team agreed with Dubey that the Chicago-based G.D. Serle Co. had not proved with "reasonable certainty" the safety of the sweetener, known generically as aspartame. A 198O Public Board of Inquiry had voted 3-O to ban aspartame because of similar fears.
But a few weeks later, on July 18, 1981, new FDA Commissioner Arthur Hull Hayes Jr., a pharmacologist who had been in office lessthan three months and had little background in food additives, overturned the board and approved the use of aspartame in dry foods.
The ruling, one of the first regulatory actions of the Reagan presidency, came at a time of growing concern that the most widely used lwo-calorie sweetener, saccharin, was linked to cancer. Thus Hayes' approval of NutraSweet profoundly changed the eating habits of millions of Americans, handing Searle a financial bonanza.
It also climaxed a topsy-turvy, eight-year FDA review process in which the agency approved the sweetener, then banned it and demanded a grand jury investigation of its manufacturer, only to reverse course again after reexamining the issue at least five times.
Now six years after Hayes' ruling, its uses expanded, the sweetener is widely consumed in diet sodas, puddings, cereal, drink mixes and even chewing gum and vitamins. yet NutraSweet and its FDA approval remain at the center of controversy, the sweetener's safety questioned by a small corps of independent scientists; defended by its manufacturer and the diet food and drink industry.
In a recently released report, the General Accounting Office concluded that the FDA "adequately followed" its food additive approval process on NutraSweet. Congress's investigative arm did not evaluate the sweetener's safety. A federal appeals court also has rejected court suits by consumer groups challenging the NutraSweet approval.
United Press International has learned that more than 1O federal officials involved in the NutraSweet review have taken private sector jobs linked to the industry _ among them Hayes, an acting FDA commissioner and former chiefs and acting chiefs of the agency's Bureau of Foods.
In addition, many of the scientists who have produced favorable studies or served as outspoken advocates of NutraSweet's safety have received grants or consulting fees from Searle and the industry.
Consumer lawyer James Turner, who has unsuccessfully pressed petitions for a NutraSweet ban as part of an 11-year campaign against the sweetener, asserted, "NutraSweet is an opportunity for the entire country to look in great detail at how we make food safety decisions. it is a rickety, 19th Century process."
G.D. Searle began to study the artificial sweetener aspartame soom after a company laboratory chemist, James Schlatter, stumbled on the compound when he licked it off his finger while conducting ulcer research in 1965.
197O, a Searle official laid out a plan for winning FDA approval for the sweetener. "We must create an affirmative atmosphere in our dealing with them," Herbert Helling wrote senior company executives.
Helling suggested that Searle representatives carefully order proposals to the FDA to put Bureau of Foods officials "into a yes-saying habit." If FDA officials could be swayed to do Searle some favor, he asserted, it would "help bring them into a subconscious spirit of participation."
On July 26, 1974, just 15 months after Searle petitioned for approval, FDA commissioner Alexander Schmidt approved aspartame use in dry foods, allowing a 3O-day period for public hearings and comment. he acted on a strong endorsement from the Bureau of Foods, now called the Center for Food Safety and Applied Nutrition.
At that point, consumer attorney Turner, author of a 197O book about food additives, objected to the short comment period. Turner was joined in his protest by a now-defunct public interest group and by Dr. John Olney, a Washington University neuropathologist who had linked aspartame to brain lesions in mice.
Schmidt promptly froze the approval. In an action that was the first of its kind, he ordered that a Public Board of Inquiry be named to look into aspartame.
Schmidt also had been alerted to conflicts between Searle's research reports and conclusions from independent animal studies that the firm's anti-infective drug Flagyl and its cardiovascular drug Aldactone may cause cancer. he named a Bureau of Drugs task force to investigate.
Philip Brodsky, the unit's since-retired lead investigator, said aspartame was included in a broad inquiry into Searle animal studies on five drugs and the Copper-7 intrauterine device to surprise the company "We didn't think they'd expect us to cover it."
The task force assailed Searle's conduct of research on most of the products, including aspartame, in a searing, 84-page report.
"At the heart of the FDA's regulatory process," the report said, "is its ability to rely upon the integrity of the basic safety data submitted by sponsors of regulated products. Our investigation clearly demonstrates that, in the G.D. Searle Co., we have no basis for such reliance now."
The task force charged, for example, that the company removed tumors from live animals and stored animal tissues in formaldahyde for so long that they deteriorated. Instead of performing autopsies on rhesus monkeys that suffered seizures after being fed aspartame, the company had financed a new monkey seizure study with a different methadology that showed no problems.
For the next seven years, Searle's petition was tied up in reviews by the task force and other sharply critical FDA panels.
At the task force's request, Richard Merrill, the FDA's general counsel, demanded in a letter that Samuel Skinner, the U.S. attorney in Chicago, open a grand jury investigation of Searle and three of its employees.
One Searle official named by merrill was Robert McConnell, who had been director of Searle's Department of Pathology and Toxicology and oversaw most of the company's aspartame research.
McConnell's Detroit lawyer, Gerald Wahl, said that as the inquiries heated up, his client suddenly was awarded a $15,OOO bonus and asked to take a three-year sabbatical by director Wesley Dixon. Wahl said Dixon told McConnell he had become a "political liability," a remark Dixon later denied making.
McConnell received his annual salary of more than $6O,OOO during the sabbatical at the Massachusetts Institute of Technology, but he never got his job back and ended up suing the company, Wahl said.
"I've represented hundreds of executives, but I've never seen anybody get the deal that McConnell got," he said. "When you boil it all down, they were looking for continued support from McConnell" during the inquiries.
Wahl said McConnell had felt pressure to hurry his research because of the "profit motive," but that the company never ordered him to alter test results.
Chief investigator Brodsky said that "politicized" handling of the task force disclosures, at hearings chaired by Sen. Edward Kennedy, D-Mass., was one reason he retired in 1977. He said the main witnesses, Searle executives and top FDA officials uninvolved in the investigation, gave "the wrong answers to the wrong questions ... They didn't even let the experts answer the questions."
The FDA, rocked by the controversy, established a set of "good laboratory practices" _ minimum standards for future corporate research work.
Richard Ronk, deputy Bureau of Foods chief, stressed that Searle's practices were typical of the industry at the time, not "the worst on the block."
Searle's fortunes did not begin to change until 1977, when Donald Rumsfeld, White House chief of staff under Gerald Ford, was named its new president.
Turner alleged that Searle chose, with Rumsfeld's hiring, not to redo the questioned studies on the belief he could handle aspartame as "a legal problem rather than a scientific problem."
The company also hired another Ford White House official, William Timmons, as a Washington lobbyist.
Before deciding on Merrill's grand jury request, U.S. Attorney Skinner and an aide agreed in February 1977 to meet with lawyers for Searle, including Newton Minow, a partner in the law firm of Sidley & Austin. A month later, Skinner, a Republican appointee who was looking a job as a result of Jimmy Carter's election, informed aides in a memo that he had begun preliminary employment discussions with the law firm. Withdrawing from the Searle matter, Skinner suggested his designated successor, Thomas Sullivan, be left to decide whether to open a grand jury inquiry _ a move that delayed action for at least four months. Sullivan took office just 12 weeks before expiration of the statute of limitations for prosecuting alleged false statements on aspartame. While a grand jury inquiry ultimately was convened, those allegations were not
explored. Skinner has denied any conflict of interest.
Assistant U.S. Attorney William Conlon worked with the grand jury until Oct. 12, 1977, two days after the statute of limitations expired on the aspartame allegations. No indictments were brought on the few matters investigated. Conlon, who declined comment, joined Sidley & Austin 15 months later.
Following issuance of the task force report in March 1976 and facing a dilemma as to how to proceed, the FDA sought new reviews of several "pivotal" studies _ long-term animal tests to see whether aspartame causes cancer. (See Part #2)
February 21, 2012
Cancer Risks from Aspartame in Studies
ATTENTION: ASSIGNMENTS EDITORS
Interview Opp
For Immediate Release September 1, 2005
Keywords: FDA/ FAA / Health and Safety / Medical / Science/ Transportation
Contact: Mary Stoddard
Latest Scientific Studies Show Aspartame Causes Lymphomas and Leukemia Reports Leading Consumer Advocate
Dallas - New studies, published in scientific journals, link the artificial sweetener aspartame to lymphoma and leukemia in rats and spontaneous changes in thinking and behavior in people, Aspartame Consumer Safety Network founder reports. Stoddard is the keynote speaker at a health and nutrition conference in Detroit, Michigan, Thursday evening, September 8th.
"The release of important scientific studies - in Italy and Africa, warns that the artificial sweetener, aspartame, commonly found in diet drinks and 7,000 products worldwide, may cause more harm than once thought possible," said network founder Mary Nash Stoddard of Frisco, Texas.
“The first study was released, in the European Journal of Oncology by Morando Soffritti and coworkers,” Stoddard said.
"This study clearly demonstrates a significant increase in several types of lymphomas and leukemia in rats. . . . These malignancies have increased dramatically, since the widespread use of aspartame," said neurosurgeon and author of Excitotoxins The Taste That Kills, Russell Blaylock, M.D., in Jackson, Mississippi.
The second is The dose-dependent effects of Aspartame on Serotoninergic Parameters in Albino Rats, by B.A. Iwalokun, Department of Biochemistry, Lagos State University, Lagos, Nigeria. Study results provided "strong indications that aspartame may alter serotoninergic parameters and associated behaviors," Stoddard said. The abstract was published in the abstract book from the Third International Conference on Mechanisms of Action of Neutraceuticals, at Maggie Valley Resort, North Carolina.
Dr. Richard Wurtman, a neurologist at the Massachusetts Institute of Technology [MIT] in Cambridge, says aspartame may have effects that are not detectable by standard neurotoxicological texts. Specifically, Wurtman and his collaborators are concerned that excess phenylalanine in the blood can reduce the production of brain neurotransmitters - catecholamines and serotonin - by competing with their precursor amino acids for transport across the blood-brain barrier. "There is no question that you can demonstrate changes in neurotransmitter release in rats given large doses of aspartame," says Wurtman. [from an interview with writer, Tim Stephens in The Journal of NIH Research, April, 1999; VOL 3 pg. 35; title: Some Still Bitter Over NutraSweet.]
A 1993 study, published in Biological Psychiatry by Professor of Clinical Psychiatry at Northeastern Ohio Universities College of Medicine, Ralph G. Walton, M.D. shows: "Administration of this substance [aspartame], has also been associated with aggression and bingeing."
An evolving view in modern psychiatry is that although depression, obsessive compulsive disorder, panic disorder, impulse control disorders and eating disorders have been viewed as separate entities, they should be viewed as a continuum of disorders - all involving some degree of dysregulation of serotonin. "I believe there is overwhelming evidence that aspartame contributes to this dysregulation," said Dr. Walton.
In a 1997 lecture at University of Texas Southwestern Medical School in Dallas, published, 1998 in toxicology sourcebook, Deadly Deception Story of Aspartame, Stoddard told students: "Individuals are reporting, to ACSN, cases of brain tumors and non-Hodgkins lymphoma."
In 1986, Stoddard was diagnosed with a life threatening blood disease, eosinophilia myalgia, after adding aspartame to her daily diet. When she ceased using the sweetener, the illness went away. [www.aspartamesafety.com]
###
Mary Nash Stoddard, Founder Aspartame Consumer Safety Network and Pilot Hotline [1987-present]
email: marystod@airmail.net
http://www.aspartamesafety.com
http://www.marystod.blogspot.com/
HEART SYMPTOMS LINKED TO ASPARTAME USE
By Sheryl Ubelacker
TORONTO (CP) - For those who drink diet pops in the belief that sugar-free beverages are healthier than regular soft drinks, new research suggests they should think again.
A huge U.S. study of middle-aged adults has found that drinking more than one soft drink a day - even a sugar-free diet brand - may be associated with an elevated risk for metabolic syndrome, a cluster of factors that boosts the chance of having a heart attack or stroke and developing diabetes.
Officials of a leading aspartame awareness group, Aspartame Consumer Safety Network, reported today to confirm the findings of the Framingham Heart Study
"We found that one or more sodas per day increases your risk of new-onset metabolic syndrome by about 45 per cent, and it did not seem to matter if it was regular or diet," Dr. Ramachandran Vasan, senior investigator for the Framingham Heart Study, said Monday from Boston.
"That for me is striking."
Metabolic syndrome is associated with five specific health indicators: excess abdominal fat; high blood sugar; high triglycerides; low levels of the good cholesterol HDL; and high blood pressure.
"And other than high blood pressure, the other four . . . all were associated with drinking one or more sodas per day," said Vasan, a professor of medicine at Boston University.
Having metabolic syndrome is known to double the risk of heart attack and stroke, as well as boosting the risk of diabetes.
The study included nearly 9,000 observations of middle-aged men and women over four years at three different times. The study looked at how many 355-millilitre cans of cola or other soft drinks a participant consumed each day.
The researchers found that compared to those who drank less than one can per day, subjects who downed one or more soft drinks daily had a:
-31 per cent greater risk of becoming obese (with a body mass index of 30 or more).
-30 per cent increased risk of adding on belly fat.
-25 per cent higher risk of developing high blood triglycerides or high blood sugar.
-32 per cent higher risk of having low HDL levels.
But Vasan and his colleagues, whose study was published Monday in Circulation: Journal of the American Heart Association, are unsure what it is about soft drinks that ratchets up the risk of metabolic syndrome.
"We really don't know," he said. "This soda consumption may be a marker for a particular dietary pattern or lifestyle. Individuals who drink one or more sodas per day tend to be people who have greater caloric intake. They tend to have more of saturated fats and trans fats in their diet, they tend to be more sedentary, they seem to have lower consumption of fibre."
"And we tried to adjust for all of these in our analysis . . . but it's very difficult to completely adjust away lifestyle."
Dr. David Jenkins, director of the Risk Factor Modification Centre at St. Michael's Hospital in Toronto, said previous studies have suggested that diet pops did not have the same effects on weight and health as do naturally sweetened soft drinks.
"The unusual thing that needs comment is they (the study authors) say that the diet colas are the same as the calorically sweetened colas," said Jenkins. "So I think that is the piece that they've put into this puzzle . . . I think we need a lot more scrutiny of that."
Jenkins said he believes that high consumption of soft drinks likely goes along with eating a high-calorie diet.
"I think the disappointing thing is if you thought you were doing (yourself) a major service - which you always used to think - by taking diet drinks, this is not helping you," he said. "Before we were saying take the diet (drink) and you're OK. Now were saying: 'Watch it."'
The study also begs the question whether there is some ingredient in soft drinks - regular or diet - that may encourage metabolic syndrome.
But Dr. Arya Sharma, chair of cardiovascular obesity research at McMaster University, said there is nothing suggested by the authors of the study that would lead to that conclusion.
"One thing that they say and other people have said before is if you drink a lot of sweet things, then you are sort of conditioning yourself for that sweet taste," Sharma said Monday from Hamilton. "So people who drink diet pop may be eating other sweets, whether that comes in the form of dessert or other things, I don't know."
"It may be that people who are drinking diet pop - and we have this effect often with people who go on diets or when people go running or whatever - that you do a little bit of something that you think is good, and then you overcompensate by doing more of something that is bad."
"The idea could be because I'm drinking diet pap, I can afford to splurge on dessert."
Vasan said he cannot out-and-out recommend that people stop drinking soft drinks based on this study, because the findings are based on association, not clear cause and effect.
"The simple message is eat healthy, exercise regularly and everything should be done in moderation," he said. "If you're a regular soda drinker you should be aware that this study adds to the evidence that regular soda may be associated with metabolic consequences."
"If you're a diet soda drinker, stay tuned for additional research to confirm or refute these findings."
From: marystod@airmail.net Subject: Fwd: Sudden Cardiac Death Date: April 5, 2007 7:13:38 PM CDT Heart Problems Associated With Aspartame/Methanol Ingestion:
Sudden Cardiac Death is not a "heart attack" or myocardial infarction caused by clogged arteries. It's an electrical problem in which the cardiac conduction system that generates the impulses regulating the heart suddenly puts out rapid or chaotic electrical impulses, or both. The heart ceases its rhythmic contractions, the brain is starved of oxygen and the victim loses consciousness in seconds.
Aspartame's biochemical route to SCD
Dr. J. Bowen believes the evidence is pointing the finger at aspartame as the toxin responsible for sudden death in many of these instances. "The combination of aspartame consumption with the stresses of strenuous athletic competition lead to activation of the shock mechanism including the elaboration of arginine vasopressin in the hypothalamus. This results in cerebral edema, cardiac congestion and pulmonary edema in combination with severe potassium wastage which is a sure ticket to sudden death, especially in the face of the many damages inflicted by aspartame.
"Aspartame is already well known for causing neuroendocrine abnormalities such as serotonin elevations and suppression in various areas of the brain. Due to its phenylalanine isolate poisoning, which depletes dopamine, and hypothalamic damage from its extreme excitotoxic effect and resultant formaldehyde/formic acid poisoning especially focused in the hypothalamus, any biochemist could verify the direct effect of aspartame poisoning in producing the fatal aberrant shock mechanism in those exposed to it. The mere occurrence of severe athletic stress does not cause this to happen all by itself," Dr. Bowen reasoned.
Indeed, only in recent history is it mentioned that people have a habit of simply "dropping dead" from routine exertion.
Aspartame triggers an irregular heart rhythm and interacts with cardiac medication. It damages the cardiac conduction system and is a direct cause of sudden death. What Dr. Bowen is saying, of course, is that it's not just hitting their hearts but their hypothalamus and neuroendocrine systems as well.
Of interest is the report from The Telegraph in the UK regarding an investigation of why children, having mild seizures that normally don't cause death, die before they can get to a hospital. Dr. Bowen responded: "Sudden death during seizures is almost always from cardiac standstill due to arrhythmias. There are several ways that aspartame can cause this damage. Aspartame and methyl alcohol poisoning are noted for damaging myocardium as well as the cardiac conduction system itself.
"This kind of damage leads to susceptibility to irregular heartbeats, or arrhythmias. The aspartame and methyl alcohol poisoning cause immense damage to the mitochondria and to MtDNA which perpetuates the mitochondria damage. The myocardium and cardiac conduction system never get to rest. They are constantly at work pumping blood, therefore they are very highly concentrated in mitochondria to accommodate the metabolic needs of this tremendous work load.
"Therefore, mitochondrial damage is more highly reflected in the heart. Damaged mitochondria produce increased amounts of free radicals and other abnormal metabolite-producing arrhythmias.
"The person using NutraSweet may have a markedly decreased intake of mineral and vitamin co-enzyme factors which also sensitizes the heart to arrhythmias. Seizures always put unusual demands on the cardiorespiratory system and seizures due to NutraSweet occur more frequently and in spite of otherwise adequate anti-seizure medication. Aspartame creates unusual medical toxicity from the anti-seizure medication.
"It should be no surprise then that people are dropping dead from this aspect of aspartame toxicity."
_________________________________________________________________________________
CDC reviewIn Nov. 1984 the CDC compiled a report reviewing 213 of 592 cases of aspartame complaints. Some of these included cardiac arrest, seizures, disorientation, hyperactivity, extreme numbness, excitability, memory loss, loss of depth perception, liver impairment, severe mood swings and even DEATH. Frederick L. Trowbridge added an executive summary that conflicted with the information in the report by stating that the complaints were "generally of a mild nature."
Original:
Pituitary - Markedly enlarged; slightly hyperemic. Heart - Left Ventricle has undergone dilitation walls thin. Lung - Right, anterior, medial and post-caval lobe have undergone consolidation. Testes - Marked atrophy, bilaterally.
Seminal Vesicle - Marked atrophy, bilaterally.
Thyroid - Moderately enlarged, bilaterally. A 2 mm in dia., dis- crete, sl raised, moderately firm yellowish-grey lesion is located in the posterior tip, bilaterally. (Thyroid submitted in toto wrapped in a lens paper).
Heart - Wall of left ventricle thin Brain - Marked autolysis Pituitary - Marked autolysis Pituitary - Marked enlargement. Pit - appears markedly hyperemic Heart - Left ventricle has undergone a moderate amount of dili- tation. Wall, left ventricle is thin.
Female Rat No. F16CF (Path No. 95619) Heart - Focal Fibrosis. Kidney - Mild chronic nephritis.
Female Rat No. K29CF (Path No. 95631) Heart - Focal fibrosis Kidney - Focal calcification
Female Rat No. M4CF (Path No. 95632) Liver - Focal hyperplasia
Female Rat No. M10CF (Path No. 95634) Kidney - Focal calcification. Pituitary - Adenoma Ovary - Fibrosis and Pigmentation.
(65)
Female Rat No. M15CF (Path No. 95635) Pituitary - Adenoma. Ovary - Cyst.
Female Rat No. B30CF (Path No. 95801) Kidney - Focal calcification.
Female Rat D29CF (Path No. 95803) Urinary Bladder (1) Chronic diffuse inflammation. (2) Diffuse mild hyperplasia.
The second phase of the review consisted of the microscopic examination of all tissues from the high dose females - a total of 36 animals. The inconsistencies are listed below:
Female Rat No. B14HF (Path. No. 95657) Eye was reported as not examined but eye was present and normal.
Female Rat No. F25HF (Path. No. 95823) Urinary Bladder - Mild diffuse hyperplasia.
Female Rat No. H7HF (Path No. 95623) Ovary - Neoplasm - probably granulosa cell tumor.
Female Rat No. H9HF (Path No. 95665) Heart - Focal fibrosis. Urinary Bladder - Mild focal hyperplasia.
Female Rat No. H15HF (Path No. 95665) Lymph Node - The diagnosis of lymphoma, benign, was present on the Searle microscopic report. According to Dr. Frith, lymphoma is generally not considered to be benign and he would diagnose lympphosarcoma.
Female Rat NO. H18HF (Path No. 95667) Pituitary - Adenoma. Brain - Mild bilateral hydrocephalus.
Female Rat No. K18HF (Path No. 95824) Pituitary - Adenoma
Female Rat No. K24HF (Path. No. 95671) Mass noted grossly - nothing consistent with mass reported microscopically.
(66)
Female Rat No. - M2HF (Path. No. 95672) Uterus - Chronic mild endometritis.
Female Rat No. M30HF (Path. No. 95343) Kidney - Focal calcification. Uterus - Chronic mild endometritis.
Female Rat No. M30HF (Path. No. 95675) Pancreas - Focal hyperplasia.
The third phase of this review consisted of microscopic verification of all masses reported grossly at necropsy from all female animals not examined in phases 1 and 2 and included a total of 73 animals. The inconsistencies are listed below:
Female Rat No. D10Lf (Path No. 92521) Subcutaneous mass was diagnosed as an angiofibroma on Searle report. The lesion is more consistent with an angiosarcoma.
Female Rat No. K9MF (Path. No. 95707) Uterus - Polyp.
Female Rat No. M1LF (Path. No. 95844) Tissue mass seen grossly was reported as missing and not available for microscopic examination. The tissue was present and was a mammary fibroadenoma.
In summary, Dr. Frith reviewed:
1) All 36 high dose females (all slides) including 3 that had been excluded from the study due to autolysis.
2) 36 (one-half) of the control females (all slides) including 1 animal that had been excluded from the study due to auto- lysis.
3) Remaining 73 female animals with grossly observed masses. (sufficient slides were reviewed to substantiate the masses)
4) 5 additional animals selected by the investigators (A1HM, A9HM, A29HM, C2CM, C24HM).
(67)
The slides reviewed in the first two categories above constituted 20% of the total animals on the study. Dr. Frith reviewed these slides blindly and then compared his findings with the Searle microscopic reports. According to Dr. Frith, his findings were in agreement with those of SEarle, for the most part. In his opinion, some of the lesions that he reported as inconsistencies were small, and might be considered insignificant by some pathologists. Dr. Frith did feel, however, that the ovarian neoplasms (animals H10CF, H19CF, and H7HP, and chronic cystitis and diffuse hyperplasia (animal D29CF) should have been reported.
Dr. Frith also considered two other discrepancies to be significant. They were:
1) The reporting of a mass (by Searle) as missing which was actually present (MlLF).
2) The finding of a polyp of the uterus which was not diagnosed by Searle (K9MF)
The second of the above two discrepancies assumes even more significance in view of the following:
The Histopathologic Summary table (table 11) in Volume I of the submission to FDA lists the following incidence of Uterine Polyps on page 87:
Incidence of Uterine Polyps
Controls Low Medium High 1 of 69 1 of 34 4 of 34 6 of 33 (1%) (3%) (12%) (18%)
The finding of one additional uterine polyp by Dr. Frith (in animal K9MF) increases the incidence in the mid dose to 5 of 34 (15%).
On page 82 of Volume I of the submission to FDA, is the statement: "other sporadic findings is included endometrial hyperplasia, polyp, cyst, congestion and squamous metaplasia." The term "sporadic findings" was used to characterize the incidence of uterine polyps, in spite of the fact that Searle had done a statistical analysis of these findings.
(68)
The errors are tabulated below:
Wt. Shown In Wt. Recorded on Animal No. Organ Submission Original Pathology Sheet
A12CM Kidneys 3.75 G 3.45 G L28LM Ven. Prostrate 747 mg. 474.7 mg. C0lMM Kidneys 9.40 G 9.219 G. C02HM Kidneys 1.46 G 4.259 G E14HM Kidneys 11.74 G 4.746 G J12HM Pituitary 3.0 mg. 3.3 mg. J30HM Ven. Prostrate 444 mg. 444.8 mg. F17CF Ovaries 36.7 mg. 233.5 & 36.7 mg. H30CF Liver 9.4 G 9.493 G B20HF Uterus 1115 mg. 1155 mg. K11HF Adrenals 799.1 mg. 797.1 mg.
(42)
[PRES. CLINTON DRINKS DIET COKE]Heart Attack
[DICK CHENEY DRINKS DIET SPRITE]Heart patientHere is the short piece from Globe tabloid
CHENEY THE DIVA
VEEP'S SUITE DEMANDS
The 'suite' life of Vice President Dick Cheney included full celelebrity-style treatment in his hotel rooms while on the road. Here are some of Dick Cheney's rules; All the room lights must be turned on when he walks in and all the TV's must be tuned to the FOX news Channel. And Cheney's suite has to be kept at a chilly 68 degrees with a piping hot pot of decaffeinated coffee waiting for his arrival, according to a list of "downtime requirements" supplied by the veep's advance team to one hotel.
America's second in command also requires four--not two or six--cans of caffeine-free Diet Sprite, says the document obtained by The Smoking Gun, after a Cheney staffer gave it to a hotel employee.
When Cheney, 65, travels with his wife Lynne, 64, she needs two bottles of sparkling water, either Calistoga or the Perrier brand, according to the document.
Metabolic Disorders:Graves Disease
Following from WOR Radio Dr. Robert Atkins Interview w/Mary Nash Stoddard:
All right, let's start taking calls you're on WOR, let's talk to Herb on Long Island.
Herb: Hello, Dr. Bob, I'd just like to relay a story about my daughter who just happened to be the first female jet pilot in the USAF. About five years ago, she came home on Thanksgiving weekend leave and she drank a lot of diet sodas. She had a bad spell here. A weak spell. But, when she got back to base, she went through a medical, and they determined that she had heart palpitations and arrhythmia.
Dr. Atkins : Were they alert to the possibility at this point in history, five years ago of diet sodas being the cause? Did they themselves think of diet sodas as the possible problem?
Herb: Yes. And, they determined that it might be the diet sodas and the artificial sweeteners.
Dr. Atkins: Mary, you should, take credit for that, I think. For giving that index of suspicion to everybody connected with caring for pilots that that is a possibility.
Mary : That's wonderful. We have done a lot of work with the FAA. Off the record, they are with us. But, on the record they can't say anything.
Herb: Also, in her group, in the Air Force, there were other pilots who were grounded because of heart problems. They discontinued using all their artificial sweeteners for one month and their flying status was restored to them. My daughter's retired now.
Dr. Atkins: Let's talk to Joy in New Jersey, Joy, you're on WOR.
Joy: Yes, good evening, Dr. Atkins and Ms. Stoddard. I experienced a severe problem with aspartame about ten years ago. I put it in my coffee. Within three minutes, I had such a headache I couldn't stand on my feet. I had palpitations and dizziness. I was deathly sick. I thought I was going to end up in the hospital. _____________________________________________________________________________________Statement of H. J. Roberts, M.D. Concerning Cardiac and CHEST ComplaintsAttributed to Aspartame (NUTRASWEET (R))
Many patients and correspondents have asked whether products containingaspartame can cause or aggravate symptoms relating to the heart, bloodpressure and chest. Based on my experience, as detailed in multiplepublications and books (see below), the answer is YES.
Hundreds of such instances have been documented in my database of over 950 aspartame reactors. There was dramatic improvement after avoidingaspartame, and a prompt and predictable recurrence of these problems whenthe patient resumed aspartame products ... knowingly or inadvertently.
ABNORMAL HEART RHYTHM
More than 120 individuals experienced a detectable change in theirheart rate and rhythm after consuming aspartame - including gum andproducts that did not contain caffeine. This included "fluttering",(palpitations) and rapid heart action (tachycardia). A number had evenundergone heart monitoring (Holter testing) and other studies, especiallyfor the associated weakness and faint.
One patient developed a slow pulse and complete heart block within hoursafter consuming an aspartame drink for the first time. His attackspontaneously subsided within a day without a pacemaker; and there hasbeen no recurrence.
This subject has obvious relevance to reports of unexplained sudden deathin persons who had been consuming considerable aspartame. ATYPICAL CHEST PAIN
More than 50 aspartame reactors experienced unexplained pain in the chest. (Many others have atypical pain elsewhere in the body). A numberunderwent stress tests and coronary angioplasty for suspected coronaryheart disease; they proved normal in the majority.
SHORTNESS OF BREATH
Over 70 aspartame reactors with "shortness of breath" promptly improvedafter abstaining from these prducts, and predictably suffered a recurrenceon rechallenge. Clinical sleep apnea also dramatically stopped in these patients when they avoided aspartame.
February 13, 2012
Aspartame: Possibly The World's Deadliest Neurotoxin
Is Aspartame dangerous? Judging by the ingredient lists of over 5,000 food products that list Aspartame or NutraSweet/Equal™, one of its name brand versions, the average American believes this product must be completely safe and harmless. But according to Zoh Show guest, Mary Nash Stoddard, founder of The Aspartame Consumer Safety Network and author of the source book, The Deadly Deception, Aspartame "has the potential of being the deadliest neurotoxin known to man." Mary has become the point woman for the citizens' "right to know" about the dangers of Aspartame and joined Zoh on July 2, 1997 to describe the work of the Aspartame Consumer Safety Network (ACSN) and what we can do to avoid this dangerous substance.
"Aspartame was discovered in the 60s as a drug for peptic ulcers," Mary explained. "What the Aspartame Consumer Safety Network is attempting to do is to call the FDA into responsibility and act upon the fact that it was discovered as a drug, still is a drug and [they should] recall and retest it as a drug. It will never pass those tests, and that is the reason it wasn't tested as a drug initially.... Aspartame is 50% Phenylalanine which lowers the seizure threshold in individuals. It's 40% Aspartic Acid which caused lesions in the brains of mice and lab animals, and it can cause silent lesions in the brain according to the top scientists in the amino acid studies. That further breaks down into methanol, 10% wood alcohol by weight, formaldehyde, formic acid and DKP [diketopiperazine] which is the brain tumor agent. So it's a toxic mix we have here and it breaks down in anything above 85º Fahrenheit. Remember [our] body temperature is 98.6º, so it breaks down within ten minutes of ingestion."
MYSTERIOUS REACTIONS
Mary was also having simultaneous mysterious reactions to the Weight Watchers products she was using with Aspartame in them. Weight Watchers also recommended using Equal™ and diet soft drinks, and she was becoming ill. "Mine started with a knee joint problem which was arthritic in nature, I thought, and progressed into severe muscle spasms, cramping, twitching, low grade fever [and] fibromyalgia symptoms." Mary was eventually diagnosed with eosinophilia myalgia, a potentially fatal condition. She began her exhaustive search for answers for her child and herself, and one of the results was a compiled 200 page fact report "which is simply reprints of articles from the medical and science journals. [They] were very difficult to find. It was almost hidden in the literature, but it was there, and that's how I got started."
Mary was asked, in 1987, to testify at the third Senate hearing on Aspartame safety in Washington, D.C. While she was there she met a lot of other people who were claiming the same symptoms. She remembered it being "kind of like that movie, 'Close Encounters Of The Third Kind' where everybody got together and said 'Wow, we thought we were the only ones.' " The long list of symptoms on Mary's website were compiled from her twelve years of research and interviews with other sufferers. Aspartame can be the cause of headaches, nausea, vertigo, hearing loss, tinitus, insomnia, numbness of extremities, eye problems, blurred vision, blindness, memory loss, slurred speech, mild to suicidal depression, personality changes, violent behavior, anxiety attacks, hyperactivity, gastro intestinal disorders, seizures, skin lesions, muscle cramps, joint pain, fatigue, PMS, menstrual irregularities, chest pain, arrhythmia, edema and INCREASED appetite. Death is also listed as a symptom. Mary's website continues, "Aspartame may trigger or mimic the following illnesses: Chronic Fatigue Syndrome, Epstein-Barr, Post-Polio Syndrome, Lyme Disease, Alzheimer's Disease, ALS Disease, Epilepsy, Multiple Sclerosis, EMS, Hypothyroidism, mercury sensitivity from amalgam fillings, Fibromyalgia, Graves, Lupus, Non-Hodgkins Lymphoma and ADD."
With the variety of symptoms and conditions Aspartame can trigger, Mary says it is essential for doctors to remove if from patients' diets before conducting neurological tests. She says they should take "all of their patients off of this substance and then do their MRIs, their CAT scans, their PET scans and diagnostic testing full speed ahead, but don't do that until the patient is taken off of Aspartame."
BRAIN TUMOR REGISTRY
After looking at lab animal studies, The Aspartame Consumer Safety Network created a brain tumor registry. Lab animals fed Aspartame had "brain tumors, mammary tumors, which translates into breast cancer in humans, they had uterine tumors and they had pancreatic cancer tumors. So the brain tumor registry is very interesting," she says. "We have discovered a 10% rise in the advent of the very kinds of brain tumors that occurred in the lab animals and those are Astrocytomas and Glioblastomas [glio cell cancers]. These have become almost epidemic in the population. If you go on the Internet and get into any brain tumor news group, you'll find that almost everybody there started with Astrocytomas and these people have been using the diet sodas and the powdered sweetener, Equal™.... Maybe that got them [started] into what they have developed."
READ THE LABELS
Aspartame is included in 5,000 products across 60 countries, so avoiding it is increasingly difficult. Become a careful label reader, advises Mary. "Even labels of over the counter medications and prescription meds, which it is in.... The Deadly Deception contains a list of some of the childrens' prescription medications it's in, like their pain medications that they're given for fevers, and their antibiotics that they're given for infections, ear infections and such, it's in those. You have got to either ask your pharmacist or see it on the label. Please, reject anything that has the word Aspartame [or] Phenylalanine on it. Sometimes it is listed as Phenylalanine."
DIABETICS DECEIVED
"Diabetics are some of the most insidiously affected people," Mary warned, "because they are not being told that Aspartame will harm them. They're being told that it's their lifeline [when] just the opposite is true." She suggests diabetics test themselves by drinking "two or three, preferably warm [room temperature], diet drinks and then begin testing about an hour later. You'll find [that] the blood sugar levels are not in control with Aspartame."
There are many alternatives for diabetics, however. Raw unrefined honey, tubinado sugar, which is an unrefined sugar, Stevia, a sweet, dietary supplement, and rice syrup, a grain sweetener that does not metabolize like a raw sugar. These and many others can be found in the health food stores in most areas.
WHERE IS THE HEALTH
CARE INDUSTRY?
Zoh reports the trouble had by medical researcher, Dr. Roberts, M.D., in publishing his brief study called "Aspartame Associated Confusion and Memory Loss: A Possible Human Model For Early Alzheimer's Disease". None of the standard medical peer reviewed journals would publish it. This was peculiar, he thought, "considering the increasing magnitude of Alzheimer's Disease and the relevance of my observations to newer biochemical findings and avenues of research." He can "personally vouch for the enormous difficulty in getting published [any] articles concerning reaction to Aspartame products.... The options are generally limited to burying the findings in a small circulation journal such as the bulletin of a county medical society, reporting the results as a letter to the editor or, unfortunately, discarding the project."
Unfortunately, the peer reviewed journals are not always about the latest findings in research. More often than not, the articles and research are slanted to benefit the companies who underwrite the studies. The articles that appear in the journals are censored by those who sit on the peer review panels, often the same people who sit on the boards of major chemical and drug manufacturing companies--the real control of health care in this country. This monopoly over research and publishing may in part explain why foundations such as the Epilepsy Institute and the diabetic organizations endorse a product that is really injurious to the consumer. "It's funding," says Mary Nash Stoddard succinctly. "The Diabetic Association is funded by all the diet soft drink and sweetener companies." She has a photo showing an oversize check for $75,000 presented to the American Dietetic Association from the NutraSweet™ Co. The American Dietetic Association is the board that oversees all registered dietitians and the food served in institutions, hospitals and care facilities. "These are licensed professionals and they have prostituted themselves to the artificial sweetener companies.... They have allowed, because of this funding, the NutraSweet™ PR people to write their facts in their fact sheet on artificial sweeteners -- on their own product! They are definitely in bed together. You can't get around that. The Epilepsy Association, the Diabetic Association, the Dietetic Association and many, many other [health organizations] are beholden to the sweetener industry.
"Virtually all of the research that has been done since the 80s has been tainted by Monsanto and NutraSweet™ funding of the studies," continued Mary.
POLITICAL INTRIGUE
"If you go into the political scenario behind it, which we do in our report called Deadly Deception Story of Aspartame, you will see all of the intrigue up to the Supreme Court with the former Monsanto Chemical [attorney] now seated on the Supreme Court, Judge Clarence Thomas.... It's definitely a horrible web of political and scientific intrigue."
PILOTS AT RISK
Mary says she met an F16 pilot in Washington, DC who asked her to continue her public fight against Aspartame because it poses a serious risk for pilots. She says he reported "We're having seizures. We're losing our medical certification to fly. This is a danger that the flying public needs to know about... and they're not being told. You know, the FAA will talk about just about anything except the danger to the flying public associated with the pilot using Aspartame or the controller in the tower using it and getting blurred vision at the wrong time. This has caused many accidents, I believe, in aviation. In our Deadly Deception Story of Aspartame, we have a whole section, reprints from flying magazines, including The U.S. Air Force Flying Safety Magazine which warned its pilots, two different times in 1992, to stay away from diet drinks and Aspartame if you want to keep your medical certification to fly. If you don't get seizures from it, you may have heart attack problems and you'll lose your medical, you'll lose your job and all that training and all that money for your license is down the drain. Believe me, pilots do not want to lose that."
WHAT YOU CAN DO
"One of the top priority poisons in our food supply today, and that of our children," Mary maintains, "is Aspartame. Beyond a doubt, it has the potential of being the deadliest neurotoxin known to man." She advises the first thing we do is to eliminate this poison from your diet. Don't buy any product that has the word Aspartame, NutraSweet™ or Equal™ (and now: Neotame) listed in the ingredients. Also keep an eye open for a new product coming out called Joe Sweet which is a combination of sugar and Aspartame.
Mary also urges us to return the products with these ingredients that we already have in our homes to the store, even if they are already opened. That way the manufacturer, not the grocer, will absorb the cost. The manufacturer needs to know that a growing number of concerned consumers will not buy anything with this poison in it.
You can join the all-volunteer Aspartame Consumer Safety Network and order Deadly Deception Story of Aspartame on amazon.com. This 200 page fact report by Mary Nash Stoddard is an excellent resource for information. . Visit them on the web at http://www.aspartamesafety.com/
This article was prepared by Sally O'Hara.
February 12, 2012
ASPARTAME & LIVER CANCERS (peer-reviewed medical Journal article)
(My Letter to a Liver Cancer patient: "A cursory search of our literature produced the following compelling examples of liver damage from the methanol and formaldehyde breakdown components of aspartame. If you were using aspartame prior to your liver cancer diagnosis then there is a very strong possibility your aspartame use played a part in the development of your particular cancer. See the evidence below. If you need more, we have it. Best - Mary Nash Stoddard/author Deadly Deception Story of Aspartame)"
J Pharm Pharmacol. 2000 May;52(5):547-52.Protective effect of N-acetylcysteine on rat liver cell membrane during methanol intoxication.Dobrzyñska I, Skrzydlewska E, Kasacka I, Figaszewski Z.Institute of Chemistry, University in Bialystok, Poland.AbstractMethanol is oxidized in-vivo to formaldehyde and then to formate, and these processes are accompanied by the generation of free radicals. We have studied the effect of N-acetylcysteine on liver cell membrane from rats intoxicated with methanol (3.0 g kg(-1)). Evaluation of the effect was achieved by several methods. Lipid peroxidation and surface charge density were measured. An ultrastructural study of the liver cells was undertaken. The concentration of marker enzymes of liver damage (alanine aminotransferase and aspartate aminotransferase) in blood serum was measured. Methanol administration caused an increase in lipid peroxidation products (approximately 30%) as well as in surface charge density (approximately 60%). This might have resulted in the membrane liver cell damage visible under electron microscopy and a leak of alanine aminotransferase and aspartate aminotransferase into the blood (increase of approximately 70 and 50%, respectively). Ingestion of N-acetylcysteine with methanol partially prevented these methanol-induced changes. Compared with the control group, lipid peroxidation was increased by approximately 3% and surface charge density by approximately 30%. Alanine aminotransferase and aspartate aminotransferase activity increased by 9 and 8%, respectively, compared with the control group. The results suggested that N-acetylcysteine was an effective antioxidant in methanol intoxication. It may have efficacy in protecting free radical damage to liver cells following methanol intoxication.PMID: 10864143 [PubMed - indexed for MEDLINE]METHANOL (10% OF ASPARTAME MOLECULE)Methanol is a type of alcohol not intended for human or animal consumption. It's common to find methanol in products like anti-freeze, canned heating sources like Sterno, varnish, windshield wiper fluid, paint thinner, and fuel additives. Methanol poisoning is extremely serious and can result in death within a few hours if not immediately treated.
As little as a couple of tablespoons (about 14-28 ml) of methanol can causemethanol poisoning in children. In adults, two ounces (56 ml) may causemethanol poisoning. Ingestion of methanol is cause for very grave concern and immediate hospital treatment.
Methanol poisoning has a number of symptoms. These include bizarre behavior, falling into a coma, extreme dizziness, severe headaches and seizures. Methanol poisoning can render a person temporarily blind, dilate the pupils and cause blurred vision. The digestive system starts to immediately reject methanol and symptoms may include severe stomach pain, nausea, and diarrhea. Methanol also disrupts liver and pancreatic function. Even with treatment, methanol poisoning can cause permanent liver damage.
Other symptoms in methanol poisoning are difficulty breathing, signs of lowoxygen levels through blue fingernails and lips, complete fatigue and cramps in the legs. These symptoms when taken together represent a medical emergency, and you should contact 911 or emergency services in your country immediately if you suspect methanol poisoning. You should not attempt to induce vomiting with syrup of ipecac unless you are instructed to do so by emergency services.
Treatment for methanol poisoning is fairly intensive. Many patients have a nasal-gastric tube inserted with activated charcoal, which helps "pump the stomach." Patients are observed for sharp declines in blood pressure, seizures, or stopping breathing. It is quite common for people to need dialysis, and most people are also given an antidote to methanol in the form of ethanol. As symptoms develop, other medications, like anticonvulsants may be needed. Many people require breathing support like a respirator.
If you do suspect methanol poisoning, it's important to take any suspected containers with methanol with you to the hospital. Lab analysis can roughly determine how much methanol a product contains which can help predict the degree of symptoms. You should not, however, waste time searching for methanol containers if you don't know what a person has consumed.
Most cases of methanol poisoning are accidental. Unfortunately, some alcoholics may actually consume methanol on purpose. This frequently causes irreversible blindness and can quite easily result in death. To prevent this, some methanol products have emetic properties added, which make a person immediately throw up after drinking them. This may not completely rid the body of methanol and hospital treatment is still necessary, even if a person immediately throws up after drinking methanol.
http://avogadro.chem.iastate.edu/MSDS/methanol.htm
Material Safety Data Sheet
Methyl Alcohol, Reagent ACS, 99.8% (GC)
FORMALDEHYDE IS A BREAKDOWN PRODUCT OF METHANOL IN ASPARTAME:
Life Sci. 1998;63(5):337-49.Formaldehyde derived from dietary aspartame binds to tissue components in vivo.Trocho C, Pardo R, Rafecas I, Virgili J, Remesar X, Fernández-López JA, Alemany M.Departament de Bioquímica i Biologia Molecular, Facultat de Biologia, Universitat de Barcelona, Spain.AbstractAdult male rats were given an oral dose of 10 mg/kg aspartame 14C-labelled in the methanol carbon. At timed intervals of up to 6 hours, the radioactivity in plasma and several organs was investigated. Most of the radioactivity found (>98% in plasma, >75% in liver) was bound to protein. Label present in liver, plasma and kidney was in the range of 1-2% of total radioactivity administered per g or mL, changing little with time. Other organs (brown and white adipose tissues, muscle, brain, cornea and retina) contained levels of label in the range of 1/12 to 1/10th of that of liver. In all, the rat retained, 6 hours after administration about 5% of the label, half of it in the liver. The specific radioactivity of tissue protein, RNA and DNA was quite uniform. The protein label was concentrated in amino acids, different from methionine, and largely coincident with the result of protein exposure to labelled formaldehyde. DNA radioactivity was essentially in a single different adduct base, different from the normal bases present in DNA. The nature of the tissue label accumulated was, thus, a direct consequence of formaldehyde binding to tissue structures. The administration of labelled aspartame to a group of cirrhotic rats resulted in comparable label retention by tissue components, which suggests that liver function (or its defect) has little effect on formaldehyde formation from aspartame and binding to biological components. The chronic treatment of a series of rats with 200 mg/kg of non-labelled aspartame during 10 days resulted in the accumulation of even more label when given the radioactive bolus, suggesting that the amount of formaldehyde adducts coming from aspartame in tissue proteins and nucleic acids may be cumulative. It is concluded that aspartame consumption may constitute a hazard because of its contribution to the formation of formaldehyde adducts.PMID: 9714421 [PubMed - indexed for MEDLINE]_____________________________________________________________________________________________________________________________________________________
February 5, 2012
KOMEN FOUNDATION / BREAST CANCERS: SINCE ASPARTAME IN FOOD SUPPLY (1982-PRESENT)
Komen for the Cure continues to lie to women about the nature of breast cancer and studies showing a clear link between Aspartame consumption and breast cancers.
Komen for the Cure is notorious for denying a link between any chemical exposure and breast cancer, fueling instead the myth that most cases of breast cancer are inherited. In reality, less than ten percent of breast cancer cases have a hereditary link, while the more than 90 percent of other cases have a definitive environmental link.
The sad reality is that the Komen Foundation, which is supported by millions of highly-driven individuals who believe the group is working in the best interests of women, continues to expose itself as nothing but a pseudo-scientific, fraudulent research group.
"The organization's continuous denial of sound science in favor of its own baseless opinion on the matter shows that another agenda is at work -- and it is one that, by all appearances, has no basis in actually curing or preventing breast cancer,"claims NaturalNews.com.
Sources for this article include:
http://thetruthaboutstuff.com/published/Bittersweet%20Breast%20Cancer.pdf http://marystod.blogspot.com/b/post-preview?token=G0-FUTUBAAA.CK9aEHdPnp1QDtB8AbLOpA.mMCOITRk46L234SOHWwAWA&postId=968020619959722604&type=POST
http://motherjones.com/environment/2011/09/breast-cancer-komen-bpahttp://www.aspartamesafety.com
http://naturalnews.com/
December 8, 2011
Expert on Methanol Alerts Public to Imminent Health Crisis
Date: Wed, Nov 2, 2011 at 8:17 PM
Subject: Aspartame Submission from Professor Woodrow Monte
To: maud.paques@efsa.europa.eu
Cc: Hugues.KENIGSWALD@efsa.europa.eu
Hugues Kenigswald, Chief
3 November 2011
Food Additive and Nutrient Division
European Food Safety Authority
Mr. Kenigswald:
Please consider this a response to your letter of October
14th to Rich Murray requesting my work by November 5th. This is also
a continuation of my official submission to the EFSA call for data on
aspartame on the 7th of July, 2011.
I explicitly give EFSA permission to exchange the book
chapter I attach to this email with the Joint FAO/WHO Expert Committee
on Food Additives.
This is my work, I am the author and I own the copyright
to the work.
My concern about the safety of aspartame centers on its
11% methanol content. My reservation about its safety has only
deepened over the twenty five years since my first scientific
publication questioning its safety in the Journal of Applied Nutrition
(previously sent to Sandra Adedapo).
Every molecule of aspartame liberates a molecule of
methanol on consumption and each molecule of methanol metabolizes into
a molecule of formaldehyde within the brain and other vulnerable
tissue of the unsuspecting consumer.
Formaldehyde is now universally classified as a known
human carcinogen with no safe level of consumption. The health threat
is magnified when this highly reactive substance is produced within
the body from methanol.
My recent scientific article explains in detail the
premise by which methanol may act as a etiologic agent of
disease.(586)* The reference numbering of this version of my article
can be used on the reference section of my website,
http://www.whilesciencesleeps.com/, to easily retrieve original
references where needed for elucidation.
I am in press of a review of the entire body of methanol
literature as pertinent to aspartame poisoning in a book entitled
While Science Sleeps. The book will be available on Amazon.com very
soon. I recommend it to your committee as a thorough review of the
methanol literature by a food scientist who has never been a
consultant to the industry that profits from the sweetener.
The recent controversial admission by the U. S.
Environmental Protection Agency that "methanol is a possible cause of
developmental birth defects"(627) and the recent release (this year)
of an internal U.S. FDA memo(677) pointing to aspartame as having
caused birth defects in laboratory animals are significant evidence to
suspect aspartame as the cause of the 25-year-old epidemic of autism,
whose point of origin appears to coincide to within one gestational
period of the date of aspartame's addition to carbonated beverages.
I have attached a prepublication copy of Chapter 12 of my
book, While Science Sleeps, that deals with autism and aspartame in
order to expedite your committee's reevaluation of the safety of this
substance. I ask that any use of the material herein be sourced to my
in press book, While Science Sleeps: A Sweetener Kills, by Woodrow C.
Monte, Ph.D.
If I can do anything further to help in your review,
please let me know. In the recent past, I have registered with Sandra
Adedapo of your staff and sent her other information that I hope will
be helpful.
Kindest regards,
Woodrow C. Monte, Ph.D - woodymonte@gmail.com
Emeritus Professor of Food Science - Arizona State University
* All references from submitted Chapter 12 of While Science Sleeps.
Wh i l e S c i e n c e S l e e p s © W. M o n t e ( P r e p u b l i c
a t i o n ) P a g e | 1
Reproduction with permission only - http://www.whilesciencesleeps.com/contact/
P a g e | 2
Table of Contents
Introduction 3
I A Time When All the Easy Questions Have Been Answered 7
II Methanol: Where Is It Found? How Can It Be Avoided? 20
III Man And Methanol: A Tragic History of Mutation and Deceit 27
IV Formaldehyde Is The Real Problem 44
V The Silent Battle That Turns Methanol Into Disease 64
VI How Methanol Kills 73
VII Atherosclerotic Cardiovascular Disease (Heart Disease) 94
VIII Alzheimer's Disease and its Perivascular Nature 122
IX Multiple Sclerosis 136
X Classic Autoimmune Diseases Lupus and Rheumatoid Arthritis 184
XI Cancers of Aspartame 194
XII Autism and other Birth Defects 216 [ 27 p ]
Bibliography 242
http://www.whilesciencesleeps.com/references/
740 full text pdfs
http://www.whilesciencesleeps.com/pdf/1.pdf
(1) Monte WC. Aspartame; Methanol and the Public Health. Journal of
Applied Nutrition 1984;36(1):42-58.
http://www.whilesciencesleeps.com/pdf/627.pdf
(627) Shelby M. NTP-CERHR Expert Panel report on the reproductive and
developmental toxicity of methanol. Reproductive Toxicology
2004;18:303-90.
http://www.whilesciencesleeps.com/pdf/677.pdf
(677) Collins TFX. Memorandum: Aspartame shown to cause nural tube
birth defects in the New Zealand rabit, an animal very resistant to
methanol poisoning. Freedom of information: Department of Health
Education and Welfare, Food and Drug Administration; 1978.
A very important message to the European Food Safety Authority
Link below.
http://www.whilesciencesleeps.com/open-letter-to-hugues-kenigswald/
Read the open letter
http://www.whilesciencesleeps.com/While%20Science%20Sleeps%20-%20Chapter%2012%20(ref).pdf
Download Chapter 12 of the book "While Science Sleeps"
A compelling must read full of shocking facts
Chapter 12 Slideshow for Download [ 5 MB ]
http://www.whilesciencesleeps.com/files/Chapter%2012%20Birth%20Defects%20caused%20by%20Aspartame.ppt
Birth Defects caused by Aspartame
Open Letter to European F. S. A.
http://www.whilesciencesleeps.com/multiple-sclerosis/
Chapter 9 Slideshow 1
http://www.whilesciencesleeps.com/the-teachers-paradigm/
Chapter 9 Slideshow 2
http://www.whilesciencesleeps.com/birth-defects-caused-by-aspartame/
Chapter 12 Slideshow
Birth Defects caused by Aspartame
http://www.amazon.com/Solving-Mystery-Multiple-Sclerosis-Poisoning/dp/B003L783I0/ref=rsl_mainw_dpl?ie=UTF8&m=ATVPDKIKX0DER
Dr Monte's DVD on Amazon.com $ 15
Solving the Mystery of Multiple Sclerosis: Is Your Diet Secretly Poisoning You?
November 19, 2011
92 Symptoms FDA
The following are symptoms attributed to aspartame in complaints submitted to the FDA by the Department of Health and Human Services April 20, 1995.
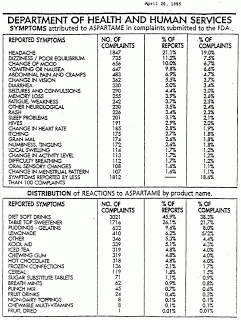
How Sweet It Isn't: Cancer Expert Keith Block, MD, Advises Avoiding Artificial Sweetener Aspartame
By Leni Kass
Health concerns are mounting about aspartame, the artificial sweetener consumed by more than 350 million people worldwide. Data from a long term, large scale animal study released by scientists from the Ramazzini Foundation for Cancer Research in Bologna, Italy, strongly link the chemical additive to cancer. This research has prompted the European Food Safety Authority to announce the group will review the research "as a matter of high priority, in the context of previous extensive safety data available on aspartame."
In the U.S., the New Mexico Environmental Improvement Board is considering whether it has statutory authority to ban aspartame in the state due to worries the sweetener could be contributing to citizens' health problems. In addition, Christine Lydon, MD, a consultant for several of the world's largest sports nutrition companies who has recommended aspartame-containing products to clients for years, has written an article published in the October edition of Oxygen magazine in which she says a review of the scientific research on the substance has convinced her aspartame is a health hazard. "I sat down with a pile of literature two inches thick. After making it through the first 10 pages, I stormed into my kitchen and fed every item of food containing aspartame to the garbage," she writes in the magazine article. "Since that time, I have not had so much as a stick of aspartame sweetened gum."
First declared safe and approved by the U.S. Food and Drug Administration (FDA) in 1981 for use in powdered mixes and tabletop sweeteners, by 1996 aspartame was approved for use in all foods and beverages. However, Ralph Walton, MD, a psychiatry professor at Northeastern Ohio Universities College of Medicine, analyzed the research. He documented that all of the research showing no health risks had aspartame industry-linked sponsorship. On the other hand, 92% of the independent, non-aspartame industry sponsored studies identified one or more problems with aspartame. "That's a glaring disparity," notes Keith I. Block, MD, one of the nation's leading cancer specialists, who is editor-in-chief of the peer-reviewed journal Integrative Cancer Therapies. Dr. Block says he has been troubled for years by the possibility the sugar substitute may, in fact, be anything but a healthy dietary choice. He is particularly concerned about the possibility aspartame might be a carcinogen. The Ramazzini Foundation's study concluded aspartame caused cancer of the kidney and peripheral nerves, mainly in the head. Earlier data from the same study published in July showed aspartame in doses closely equivalent to the acceptable daily intake for women caused an increased risk for leukemia and lymphomas in female lab rats.
In addition to concerns about a possible aspartame/cancer link, Dr. Block, Medical/Scientific Director of the Block Center for Integrative Cancer Care and Optimal Health in Evanston, Illinois, and a Clinical Professor, Department of Medical Education, at the University of Illinois College of Medicine at Chicago (UIC), and at the Department of Pharmacology, and his colleagues believe the sweetener may produce a host of distressing symptoms in some people. "Women in particular have reported neurotoxic reactions to aspartame and we have observed that skin reactions and gastrointestinal symptoms often disappear in patients who stop consuming aspartame-containing products."
Dr. Block also explains that phenylalanine and aspartic acid, the amino acid components of aspartame, are known to stimulate insulin release – which could actually sabotage a dieter's effort to lose weight. " In addition, high insulin levels tend to amplify the adverse effects of some other growth factors that could ultimately fuel the growth and spread of cancers such as colorectal cancer, prostate cancer and breast cancer," he notes. "I think such theoretical links should at least warrant considering cutting back or eliminating aspartame containing foods in the diet."
What sweetener does Dr. Block recommend? "There are a variety of natural grain and plant-derived sweeteners that do not cause a big spike in blood sugar. nd fruit can often satisfy a 'sweet tooth' while providing many health benefits," he answers. "I also recommend eating smaller meals throughout the day, and eating foods that have a low-glycemic index, such as corn, peas, and black beans. The body requires more time to break down and absorb these foods, and this leads to a more gradual and moderate rise in blood glucose levels. Much research suggests that this kind of dietary strategy will result in better health and greater longevity – and it is a far healthier way to eat than relying on artificial sweeteners."
Leni Kass has been in marketing and public relations for over 15 years. Previously, she worked with teens, and facilitated a therapy group for adolescents with eating disorders. She is cofounder and CEO of Hey U.G.L.Y., Inc. NFP, a 501c3 nonprofit organization that empowers teens with self-esteem building tools, to help them counter challenges such as eating disorders, bullying, violence, substance abuse and suicide. U.G.L.Y. is an acronym that stands for meaning Unique Gifted Lovable You.
Article Source: http://EzineArticles.com/?expert=Leni_Kass
_____________________________________________________________________________________________________
Symptoms which May be Due to Aspartame
What kinds of symptoms may occur as a result of ingesting aspartame? They may involve almost any system of the body. Probably the most common are headaches, including migraines. As I mentioned, one can also experience seizures. Some pilots have lost their licenses after having experienced seizures from aspartame. Several articles have appeared in flying magazines.
Other neurologic or psychiatric symptoms include dizziness, unsteadiness, confusion, severe drowziness and sleepiness, numbness, hyperactivity--especially in children, severe depression, irritability, anxiety, aggression, personality changes, insomnia and phobias.
Visual changes may include blurred vision, blindness, pain and reduced tears. Ringing or buzzing in the ears, hearing impairment or noise intolerance occur in some people. Palpitations, shortness of breath or recent high blood pressure may mimic a heart condition.
Other systems that can be affected are the gastrointestinal system, including diarrhea, nausea and abdominal pain; the skin, including itching and hives; and the endocrine system, including loss of control of diabetes, menstrual changes, marked weight loss or gain and aggravated low blood sugar.
To see if you are being affected by aspartame, eliminate all aspartame products for about two weeks. If some of your symptoms improve, you may then reintroduce aspartame and see if your symptoms return. If they do, you should probably eliminate aspartame entirely. - Michael Schachter M.D., F.A.C.A.M.
_____________________________________________________________________________________________________

Mary Nash Stoddard, Founder
Aspartame Consumer Safety Network and Pilot Hotline
[Promoting FDA Recall of Aspartame - since 1987]
phone: 1-214-387-4001
email: marystod@airmail.net
http://www.aspartamesafety.com



