Margaret McCartney's Blog, page 11
April 2, 2012
Myrios and their adverts on the tube
Myrios are running adverts on the London tube.
This company offers blood testing for various conditions. I am troubled by the evidence and practice of their testing.
Here's what they test for
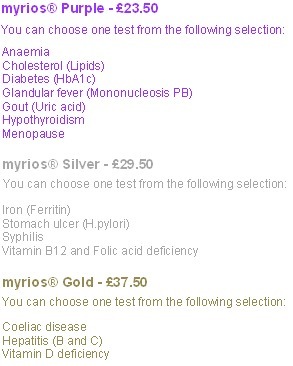
"We always advise you visit your local pharmacist who will be able to recommend the right test based on your health concerns."
They also say, in their FAQs,
"When should I get a test?
With all conditions, preventative medicine is key, so it is important to check your health and wellbeing periodically, especially if you feel or notice any changes."
This is not only wrong, but misleading.
First of all, if you have symptoms 'any changes' – you don't need a single blood test (and how would you choose which one?) but to discuss your symptoms with a doctor, who should work with you to decide what to do, which may not just involve blood tests – and it may not – but clinical examination or other interventions.
Second, the idea that we need a check of our 'wellbeing periodically' via one of their blood tests simply isn't true. These tests are being offered as screening tests, and screening, as I keep on saying, is complicated, with pros and cons. The UK National Screening committee looks at each screening intervention and makes recommendations based on the evidence.
Then there is the problem of false reassurance. For example, testing for syphilis isn't usally done in isolation – if there is a concern about a sexual infection, for example, multiple tests for many other pathogens are usually run.
And of course, there is the maddening problem of the NHS being left out to sort out results, as Myrios say "You should always speak to your doctor (GP) about your results." This is very unfair. Normally, before any tests are done, the doctor explains the purpose of the tests and ensures they are done with the cost/benefit ratio is reasonable. Myrios are running tests as screening tests, without NHS approval for them being used as such, and then expect the NHS to deal with them.
It's a bad deal for the NHS and for the people taking up the tests.
that weren't
March 29, 2012
BMJ – private screening clinics are not regulated well enough
Free link to BMJ article here.
March 21, 2012
The Lancet and aspirin and all cause mortality
There are three papers today in the Lancet about aspirin. I'm going to ignore the two papers about the effect on cancer metastasis, just now, and concentrate on the third, which is titled
Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials
It's been covered at the BBC, here. They say that
"Taking a low (75-300mg) daily dose of the drug appeared to cut the total number of cancer cases by about a quarter after only three years – there were nine cancer cases per 1,000 each year in the aspirin-taking group, compared with 12 per 1,000 for those taking dummy pills.
It also reduced the risk of a cancer death by 15% within five years (and sooner if the dose was higher than 300mg)
And if patients stayed on aspirin for longer, their cancer death risk went down even further – by 37% after five years."
This is the relevant bit from the paper
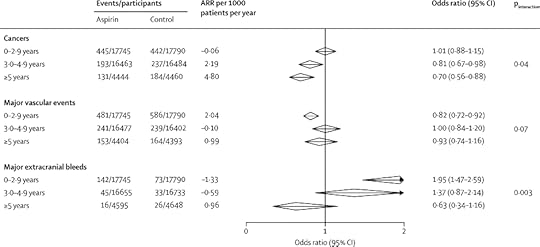
"Allocation to aspirin reduced cancer deaths (562 vs 664 deaths; odds ratio [OR] 0·85, 95% CI 0·76–0·96, p=0·008; 34 trials, 69 224 participants), particularly from 5 years onwards (92 vs 145; OR 0·63, 95% CI 0·49–0·82, p=0·0005), resulting in fewer non-vascular deaths overall (1021 vs 1173; OR 0·88, 95% CI 0·78–0·96, p=0·003; 51 trials, 77 549 participants). In trials in primary prevention, the reduction in non-vascular deaths accounted for 87 (91%) of 96 deaths prevented. In six trials of daily low-dose aspirin in primary prevention (35 535 participants), aspirin reduced cancer incidence from 3 years onwards (324 vs 421 cases; OR 0·76, 95% CI 0·66–0·88, p=0·0003) in women (132 vs 176; OR 0·75, 95% CI 0·59–0·94, p=0·01) and in men (192 vs245; OR 0·77, 95% CI 0·63–0·93, p=0·008). The reduced risk of major vascular events on aspirin was initially offset by an increased risk of major bleeding, but effects on both outcomes diminished with increasing follow-up, leaving only the reduced risk of cancer (absolute reduction 3·13 [95% CI 1·44–4·82] per 1000 patients per year) from 3 years onwards. Case-fatality from major extracranial bleeds was also lower on aspirin than on control (8/203 vs 15/132; OR 0·32, 95% CI 0·12–0·83, p=0·009)."
I have cut and pasted the numbers below.
It seems that, at 5 years, 1.42% of the the control group had cancers, and 2.94% if the control group. This is a difference, small to an individual, potentially large to a population. But we really need to know about all cause mortality – if all we are doing is reducing cancer incidence by increasing bleeding risk (eg brain haemorrhage) we might not be doing a great deal of good.
This is a very important sentence:
"Allocation to aspirin reduced the risk of non-vascular death in the 51 trials (1021 vs 1173 deaths; OR 0·88, 95% CI 0·78–0·96, p=0·003; 152 deaths avoided in 40 269 participants allocated aspirin; table 1). The proportion of deaths classed as non-vascular varied (heterogeneity pfigure 1), [15], [16], [17], [18],[19], [20], [21], [22], [23], [24], [25] and [26] aspirin reduced non-vascular death (OR 0·88, 95% CI 0·78–0·98, p=0·02; 87 deaths avoided), but not vascular death (OR 0·99, 95% CI 0·87–1·12, nine deaths avoided), such that the effect on all-cause mortality was non-significant (1165 vs 1261 deaths; OR 0·92, 95% CI 0·85–1·00, p=0·06)."
Repeat: the effect on all cause mortality was not significant.
I think we need to discuss this better – rather a lot of people need to be treated with aspirin to prevent cancer, and we don't know that this will reduce overall death rates.
I need to read the other two papers, but busy day today.
March 20, 2012
Why I don’t have smears
article today in the Independent.
In answer to criticism received; no, I don’t think that discussing why I don’t have a smear test is irresponsible.
I do think it’s bad practice to cajole and occasionally frighten women into smear tests. These are the Scottish information booklets presented to women having a first smear, and thereafter. There is no information about the NNT – it does say that ’1 in 10′ will be recalled, but it doesn’t say what proportion need treatment and what proportion of these will benefit. This information is also missing from the English leaflet.
Currently, women are encouraged to have a smear. GPs are incentivised to do so. This means that when a woman comes in with a symptom that needs sorted, there is a detraction from the woman’s agenda. This presents a conflict. I can’t quote research because it, shamefully, hasn’t been done – but there are women who do not wish to have a smear and who are asked, time and again, why they are not ‘complying’ with the medical screening targets. This can cause distress and can even prevent women from coming to see the doctor about symptoms. Many doctors have seen this pattern.
I don’t expect people in general to realise this, but it cannot be ignored. There are many discussion forums on the internet where women discuss the issues they have faced when they have wished to decline a smear. Women have felt humiliated, bullied, harried. It’s not fair and it’s not right.
To me the problem is that we advertise smears as the usual. We don’t individually consent and explain screening in the same way as we would other interventions – like a prescription or an operation. We do not recognise that it is a completely valid choice not to have screening. Doctors cannot and should not force their own value judgements onto anyone else – either to have or not to have a cervical screening.
I’m happy to explain my rationale because I am concerned that a great many women are being directed into cervical screening without knowing that it’s a choice and without giving fully informed consent. I want to see better discussions of the pros and cons. This isn’t recognised well – here’s a response to some research on the uptake of cervical screening written by myself and Professor Susan Bewley. It didn’t occur to these researchers that some women may have wanted not to be screened.
Here’s Angela Raffle’s, and colleagues, bottom line
In the NHS cervical screening programme around 1000 women need to be screened for 35 years to prevent one death
Over 80% of women with high grade cervical intraepithelial neoplasia will not develop invasive cancer, but all need to be treated
For each death prevented, over 150 women have an abnormal result, over 80 are referred for investigation, and over 50 have treatment
Before the 1988 relaunch of screening with strict quality standards, for each death prevented there were 57000 tests and 1955 women had abnormal results
That’s useful, clear information, to women considering a cervical screening test. It explains the high risk of a false positive relatively speaking, so that an abnormal smear can be contextualised. It also makes clear the small but present chance of a smear stopping a death from cervical cancer.
It’s not for me to tell women what to do, or what not to do. It is my job, as a GP, to explain the pros and the cons – and that’s what we need to be doing better. Respecting women’s autonomy means respecting a decision to have, or not to have, the test.
It’s worth noting what Archie Cochrane said of cervical screening: “never has there been less appeal to evidence and more to opinion”
Why I don't have smears
article today in the Independent.
In answer to criticism received; no, I don't think that discussing why I don't have a smear test is irresponsible.
I do think it's bad practice to cajole and occasionally frighten women into smear tests. These are the Scottish information booklets presented to women having a first smear, and thereafter. There is no information about the NNT – it does say that '1 in 10′ will be recalled, but it doesn't say what proportion need treatment and what proportion of these will benefit. This information is also missing from the English leaflet.
Currently, women are encouraged to have a smear. GPs are incentivised to do so. This means that when a woman comes in with a symptom that needs sorted, there is a detraction from the woman's agenda. This presents a conflict. I can't quote research because it, shamefully, hasn't been done – but there are women who do not wish to have a smear and who are asked, time and again, why they are not 'complying' with the medical screening targets. This can cause distress and can even prevent women from coming to see the doctor about symptoms. Many doctors have seen this pattern.
I don't expect people in general to realise this, but it cannot be ignored. There are many discussion forums on the internet where women discuss the issues they have faced when they have wished to decline a smear. Women have felt humiliated, bullied, harried. It's not fair and it's not right.
To me the problem is that we advertise smears as the usual. We don't individually consent and explain screening in the same way as we would other interventions – like a prescription or an operation. We do not recognise that it is a completely valid choice not to have screening. Doctors cannot and should not force their own value judgements onto anyone else – either to have or not to have a cervical screening.
I'm happy to explain my rationale because I am concerned that a great many women are being directed into cervical screening without knowing that it's a choice and without giving fully informed consent. I want to see better discussions of the pros and cons. This isn't recognised well – here's a response to some research on the uptake of cervical screening written by myself and Professor Susan Bewley. It didn't occur to these researchers that some women may have wanted not to be screened.
Here's Angela Raffle's, and colleagues, bottom line
In the NHS cervical screening programme around 1000 women need to be screened for 35 years to prevent one death
Over 80% of women with high grade cervical intraepithelial neoplasia will not develop invasive cancer, but all need to be treated
For each death prevented, over 150 women have an abnormal result, over 80 are referred for investigation, and over 50 have treatment
Before the 1988 relaunch of screening with strict quality standards, for each death prevented there were 57000 tests and 1955 women had abnormal results
That's useful, clear information, to women considering a cervical screening test. It explains the high risk of a false positive relatively speaking, so that an abnormal smear can be contextualised. It also makes clear the small but present chance of a smear stopping a death from cervical cancer.
It's not for me to tell women what to do, or what not to do. It is my job, as a GP, to explain the pros and the cons – and that's what we need to be doing better. Respecting women's autonomy means respecting a decision to have, or not to have, the test.
It's worth noting what Archie Cochrane said of cervical screening: "never has there been less appeal to evidence and more to opinion"
March 13, 2012
New things – Inside Health and screening
My favourite subject – screening.
There's a column on Inside Health about it, as well as a feature on private companies who offer screening for aortic aneurysms.
The references I used are here
http://www.annals.org/content/152/8/505.full?aimhp
http://www.scielo.org.ar/pdf/rac/v76n2/en_v76n2a20.pdf
http://www.ncbi.nlm.nih.gov/books/NBK33513/
http://www.ncbi.nlm.nih.gov/books/NBK33507/
http://summaries.cochrane.org/CD001923/carotid-endarterectomy-for-asymptomatic-carotid-stenosis
http://www.mrc.ac.uk/Newspublications/News/MRC006173
http://www.nejm.org/doi/full/10.1056/NEJMoa070972
There is also a feature in the Times today, but it's behind a paywall, about the misinformation some healthcare charities give out, and the influence of the pharmaceutical industry – who have moved on from 'educating' doctors.
It's cheaper from the publishers
compared with Amazon – see here
(plus, Pinter and Martin get to reinvest more in their next titles.)
March 9, 2012
NEJM Alzheimers study: all it seems?
The study published yesterday has made the headlines across the media;
" The study they funded, led by Professor Robert Howard from the Institute of Psychiatry at Kings College London, and published in the New England Journal of Medicine, has concluded that the drugs carry on working in people whose illness has become severe.
"For the first time, we have robust and compelling evidence that treatment with these drugs can continue to help patients at the later, more severe stages of the disease," he said. "We observed that patients who continued taking donepezil were better able to remember, understand, communicate and perform daily tasks for at least a year longer than those who stopped taking the drugs. These improvements were noticeable to patients, their caregivers and doctors."
I'm not quite so sure. The trial took 295 patients living in the community who had moderate to severe Alzheimers disease and who were already on donepezil. They were divided into groups;
treated with donepezil,
discontinue donepezil,
discontinue donepezil and start memantine,
continue donepezil and start memantine.
They were followed for a year. At the start, in the 'methods' section, the researchers say
" The minimum clinically important differences were 1.4 points on the SMMSE and 3.5 points on the BADLS."
These are the'standardised mini mental state examination', and 'Bristol Activities of Daily Living'. The latter is a functional assessment and a better guide for useful improvements.
The important bits in the results are in a box that I can't manage to cut and paste, but the relevant bits are
BALDS – Difference between scores for continued vs discontinued donepezil, which were -2.9 at 52 weeks, with an 'overall difference' of -3.0.
Active versus placebo memantine scores were different by -1.5.
The BALDs score is a 60 point scale. The authors told us in the discussion that a 3.5 point difference would be clinically significant, but the difference in the results is a points score on this scale of 3. What does this mean? I am not sure, because small improvements in function may be very clinically meaningful (eg the ability to keep up with personal hygiene).
The other thing that bothers me is this.
"The study was overseen by King's College London and was funded by the U.K. Medical Research Council (MRC) and the Alzheimer's Society."
and "
Recruitment was slower than anticipated, and it was not possible to extend the recruitment period, since the public funder of the study (MRC) believed that the disadvantages of a delay in reporting results outweighed the benefits of increasing the power of the study."
This is quite unusual, because a better powered study usually means more definitive results. Around 50 patients in each of the 4 groups who completed the study. I'm not sure this is definitive enough evidence to make a large change in practice. Additionally, patients were excluded who were felt unable to "adhere to the study regimens". This may have produced a bias – the cognitively best or most socially supported may have been over-represented and may not reflect normal practice.
The other note is the amount of potential conflicts of interest.
"Dr. McShane reports receiving payment for work as the local principal investigator for commercial trials from Abbott, Novartis, i3 Innovus, and Medivation; Dr. Lindesay, receiving consulting fees from Novartis NEURONET and lecture fees from Janssen, Novartis, Eisai, and Pfizer; Dr. Ritchie, receiving consulting and lecture fees from Pfizer and Eisai and grant support and reimbursement for travel expenses from Eisai; Dr. Barber, receiving royalties from Arnold Press; Dr. Burns, receiving royalties from John Wiley; Dr. Findlay, receiving lecture fees and reimbursement for meeting expenses from Eisai, Pfizer, and Lundbeck; Dr. Jones, receiving consulting fees from Merz Pharmaceuticals and Janssen, grant support from Eisai, Lundbeck, and Merz Pharmaceuticals, and lecture fees from Lundbeck, Merz Pharmaceuticals, Eisai, Pfizer, and Novartis and being a board member of Merz Pharmaceuticals, Lundbeck, Eisai, Pfizer, and Lilly; Dr. McKeith, receiving lecture fees from Novartis; Dr. O'Brien, receiving consulting fees from GE Healthcare, Bayer Healthcare, and Servier and lecture fees from Pfizer, Eisai, Novartis, Lundbeck, Eli Lilly, Shire, and GE Healthcare; Dr. Passmore, receiving consulting fees from Pfizer, Lundbeck, Novartis, Shire, and Johnson & Johnson and lecture fees and reimbursement for travel expenses from Lundbeck and Pfizer; Dr. Katona, receiving consulting fees from Lundbeck and Eli Lilly, grant support from Lundbeck, lecture fees from Lundbeck, Lilly, Shire, and Pfizer, payment for development of educational presentations from Lundbeck, and reimbursement for travel expenses from Pfizer and being a board member of Lundbeck; Dr. Ballard, receiving consulting and lecture fees from Lundbeck, Eisai, Bristol-Myers Squibb, Janssen, Acadia, and Novartis and grant support from Lundbeck and Acadia; Dr. Brown, receiving lecture fees from UCB Pharma, GlaxoSmithKline, Solvay, and Lundbeck; Dr. Banerjee, receiving grant support from Pfizer, lecture fees from Lundbeck, and reimbursement for travel expenses from Pfizer and Eisai; and Dr. Bentham, receiving consulting fees from TauRx Therapeutics."
This work is interesting, but it didn't achieve the level of functional benefit the authors had said would be clinically significant. I'm wary of false hope being generated.
March 3, 2012
Prostate awareness month
[image error]is here.
Here's an advert from their sponsors, Marks and Spencers.
You'll note that it says "My husband had prostate cancer. He had no signs or symptoms. How were we going to cope?"
This sounds suspiciously like screening (although it may mean that he had no prostate signs or symptoms, but e.g. metastatic symptoms. I don't know.) We know that screening for prostate cancer using PSA blood tests doesn't work. Richard Albin, discoverer of PSA, has written that it's no more effective than a coin toss. (There's lots about this in my book.) The last time I criticised the use of women as a means to PSA awareness, an editorial in 'Prostate Cancer and Prostate Diseases' came back, saying "it is hard to see the harm in providing the public with more information " – well, if it leads to more men being screened, it's hard to see that harm wouldn't be created.
The problem with this advert is that, whether they intend to or not, they create fear that a symptom free person may have prostate cancer and that screening will help. The chief executive of the Prostate Cancer Charity told the Guardian in 2010 that "every man over 50 who doesn't have symptoms of prostate cancer is entitled to ask his GP for a PSA test. Yet, 70% of men aged 50-70 don't even know that the test exists, let alone their right to request it". Back to Albin, who writes from the US: " Testing should absolutely not be deployed to screen the entire population of men over the age of 50, the outcome pushed by those who stand to profit. I never dreamed that my discovery four decades ago would lead to such a profit-driven public health disaster." Of course, we have plenty of private clinics here in the UK pushing the screening agenda too.
I can only conclude that awareness campaigns are very bad for our health.
February 28, 2012
Inside Health – doctors are, of course, human
about whether it matters if your doctor smokes or is overweight. …
Listen again is here. I note that Mark Porter says this is a soapbox – no way!
The references I used are here, if anyone's interested.
http://www.bmj.com/content/344/bmj.d8041
http://www.ncbi.nlm.nih.gov/sites/entrez/8861843?dopt=Abstract&holding=f1000,f1000m,isrctn
http://ntr.oxfordjournals.org/content/6/2/369.abstract
http://www.ncbi.nlm.nih.gov/sites/entrez/8861843?dopt=Abstract&holding=f1000,f1000m,isrctn
http://www.ncbi.nlm.nih.gov/pubmed/18425860
http://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2010.00821.x/abstract;jsessionid=CAC4
Margaret McCartney's Blog
- Margaret McCartney's profile
- 13 followers



