Mark S. Senak's Blog, page 40
February 28, 2014
Weekly Roundup 2.28.14
Last weekend was so warm I got to take my first bike ride and went around the Mall. It was delightful with temps in the 60′s. Early in the week, I was excited to find a blooming crocus where just a few days before, there had been a huge drift of snow. There it was on my dog walk, blooming purple against dark earth.
Later in the week I took the same picture. Here it is.
This weekend is slated to be in the teens. Definitely no bike ride.
But on the chance that you are more interested in FDA-related stuff, here is some of what happened this week that was notable:
Proposal for New Food Labels – FDA announced that it was proposing changes to food labeling that would reflect current nutritional science to help consumers make more informed choices. The proposed changes to the label include formatting changes that emphasize some aspects of the same information that was included on the earlier version such as number of calories and the serving size. The proposed new version also seeks to add new information not present before, such as including the amount of “added sugars” . The release has links to a wealth of information about the new proposed label.
New Hispanic Health Data – Ok, it is not FDA, but it is significant – NIH released comprehensive data on Hispanic/Latino health and habits derived from a study that enrolled over 16,000 adults in geographically diverse cities around the U.S. and who had origins that included Central America, Cuba, Dominican Republic, Mexico, Puerto Rico and South America. The study contains a good deal of information and some of it that was highlighted in the press release included the percentage of people who reported having asthma ranged from 7.4 among those of Mexican ancestry to 35.8 among those of Puerto Rican ancestry. You can see the full data book here.
Another Approval for a Rare Condition - FDA seems to be on a roll when it comes to approving drugs intended to treat conditions that are rare. This week it was a drug called Myalept marketed by Amylin Pharmaceuticals intended to treat the complications of leptin deficiency, in addition to diet, in patients with congenital generalized, or acquired generalized lipodystrophy – a condition associated with a deficit of fat tissue. According to the press release from FDA, the product has a REMS program that will require prescribers to enroll and certified after completing training. By my count, that appears to be the fifth approval for a rare condition this year.
First Stop Sale Order for Tobacco Products – While tobacco products used to come on and off the market without oversight, under the authority of the Tobacco Control Act, FDA now as the authority to review products and determine which may be sold on the market by determining if market entries are substantially equivalent to a valid predicate product. According to the press release, FDA identified four products for removal from the market.
That’s it for me this week. A little more than one more week until Daylight Savings!
Share this:





February 27, 2014
FDA Press Releases – What They Say
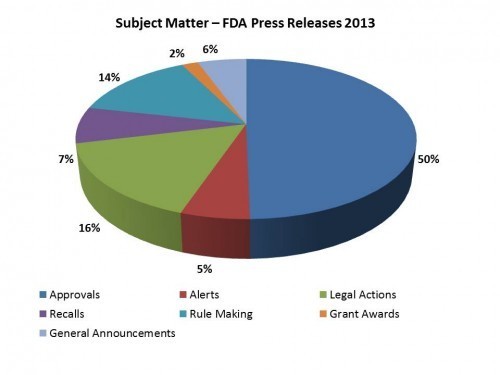
Keeping my eye on FDA, and being most interested in communications, AND having a penchant for looking at patterns through databases, I started categorizing FDA press releases to see what they talked about and in what way. I only started as of January 1, 2013 and tracked them by category. What was the subject matter – an approval? A legal action? Rule making? Then I looked at what sector was affected – Drug, Device, Food/Beverage? Some of the releases are in Spanish, so we tracked that too. Lastly we tracked the product area/use/indication affected and the product name, if there was one involved. In this way, I can look solely at approvals, or approvals in a particular treatment area.
A little of what i found. I counted 165 press releases for 2013 with 49 of them being co-produced in Spanish. What you find in many respects is what you might expect – the largest category was related to approvals. But there are some unexpected things as well – the second largest category was about FDA enforcement actions. About half of all press releases were about product approvals, which one might expect. However, the number of bilingual releases varies considerably by category. In the end, while each press release is important, it is also good to get perspective about the body of communications issued by the agency. A piece of pie is great, but one should appreciate the whole as well.
And here is a breakout as the releases affected different sectors:
Approvals (81)
61 Drug Approvals
19 Device Approvals
1 Tobacco-related Approval
26 of these were also produced in Spanish
Alerts (9)
6 Regarding Drugs
2 Regarding Food and Beverage
1 Device
3 of these were also produced in Spanish
Legal Actions (26)
10 related to Drugs
10 related to Food and Beverage
5 related to a Supplement
1 realted to Devices
3 of these were also produced in Spanish
Recalls (12)
10 Drug recalls
1 Device recall
1 Pet food recall
1 of these was also produced in Spanish
Rule Making (23)
7 related to Drugs
7 related to Devices
7 related to Food/Beverage
1 related to Soap
1 related to Tobacco
12 of these were also produced in Spanish
Grant Making (3)
1 related to Drugs
2 related to Devices
None of these were also produced in Spanish
The balance fell into a category I called General Announcements which was a collecting place for miscellaneous releases.
Share this:





February 21, 2014
Weekly Roundup – 2.21.14
The snow has melted and the ground is soggy. Looking at the ground there are daffodil tops poking out of the dirt and this morning on my dog walk, I spotted my first two crocus blooming purple. The volume of birdsong has definitely increased. And I find myself in moments where there is a quick mental break, with a wandering mind considering the scheduling and destinations of spring vacations. Daylight savings is just 16 days away…
In the meantime, it looks like it was a fairly eventful week and here is a bit of what happened:
Supply Chain Pilot Program for Drugs Launched - In August 2013, FDA published a notice in the federal register seeking pharmaceutical company participation in a pilot program aimed at bolstering the security of the supply chain for drugs imported into the U.S. The participating companies would agree to have in place a supply chain protocol validated by U.S. Customs-Trade Partnership Against Terrorism; a plan in place to quickly address potential problems; effective recall plans in place and maintenance of control over the drugs from time of manufacture through entry into the U.S. Participants are permitted to have up to five drugs receive expedited priority import entry review. You can see the thirteen companies that have been “pre-qualified” to participate in the FDA press release and there is a Web page on the FDA site devoted to the topic.
Another Orphan Product Approval – This week FDA approved Chelsea Therapeutics’ Northera for the treatment of neurogenic orthostatic hypertension which is a rare, chronic and debilitating condition often affecting people with Parkinson’s Disease. People with the condition face a sudden drop in blood pressure upon standing. The product was approved with a boxed warning and under FDA’s accelerated approval process.
FDA Looking to Overhaul OTC Monograph System – FDA announced this week that it would be holding a public hearing on March 25-26 at the agency’s White Oak campus with an eye toward modernizing the process and regulatory framework for OTC Drug Review under the Monograph Process to make it more responsive. The purpose of the hearing is to solicit opinions about how to better position the system in today’s environment. The OTC Monograph system currently provides for the ability to categorically set standards for OTC products so that they do not have to get approval under the new drug approval process individually. However this process put into place four decades ago is somewhat cumbersome. FDA cites particular challenges including the fact that there are a large number of products marketed for which there is not a final monograph available. FDA has set up a meeting information site on the Web site. This will likely be the most important meeting regarding OTC drugs in many years.
That’s it for me this week. The weekend feels like it was a long time in coming. I plan to greet it with open arms. Enjoy.
Share this:





February 18, 2014
Building a Case, FDA AdComm Prep – Part 3 (final)
In Part 1, we covered the need for good communications planning, in Part 2, a little bit about how to go about it and in Part 3, some thoughts about the day of your meeting.
You have developed your narrative and from it derived the individual components of the presentation on need, safety, efficacy and any other considerations. You have looked at the warts – and asked the hard questions about the data and issues that relate to the risk/balance ratio – like the number needed to treat and the possible safety issues, the potential for a REMS program, how special populations might be affected, how the trials were conducted and recruited, the makeup of the clinical trials, etc.
Now you have chosen your presentation team. It may be a combination of internal and external team members. External consultants can be very useful and credible, particularly if they have a background associated with the drug approval process or connection with a major medical society related to the treatment area. You establish “leads” for responses in key areas, with back up experts to be called upon for highly specific information.
 The presentation team needs training not only in presentation technique, but in question response. Speakers particularly need to be taught flagging and headlining as it related to providing the answer in a meaningful way; and need to be practiced and rehearsed on a range of questions. In fact, the presentation team should be prepared not only with mock panels made up of outside experts to simulate the day of experience, but drilled frequently on the range of potential questions they will face. And conduct as many mock panels as are necessary.
The presentation team needs training not only in presentation technique, but in question response. Speakers particularly need to be taught flagging and headlining as it related to providing the answer in a meaningful way; and need to be practiced and rehearsed on a range of questions. In fact, the presentation team should be prepared not only with mock panels made up of outside experts to simulate the day of experience, but drilled frequently on the range of potential questions they will face. And conduct as many mock panels as are necessary.
In addition, a sponsor must identify those people who will be responsible for speaking with the media and they must be media trained – not the same as presentation training. Which brings us to an important point.
The AdComm meeting is not just a scientific meeting about the approval of a compound – it is also where the baton gets handed off from the regulatory side of an organization to the marketing side. The AdComm meeting is where the branding of the product draws its first breath – it is the public unveiling of the drug – warts and all, and therefore it is important that there be a coordination of communication between regulatory and marketing at this time.
A good deal of sponsor thought goes into the Open Public Comment (OPC) period of a meeting when third parties and patients can state a point of view. Should a sponsor encourage third parties to participate? Support and connections of sponsors for third parties need to be disclosed at the time. Such connections have an impact. Purely organic commentary is perhaps the most desirable. However, in any case, OPC should be considered carefully. No panel is interested in being condescended to or patronized during this part of the meeting. The most useful kind of OPC participation occurs when those offering commentary are shedding real light on an issue – particularly the human face to the condition. If the condition is one commonly understood by everyone, that is probably not necessary. If, on the other hand, it is a condition that needs a human face, it can be quite useful. I have seen good examples of this and bad. Each must be assessed on a case by case basis.
Finally, from a media point of view, it is a good idea to understand the media environment for your day. Are there other FDA AdComms meeting that day – unusual, but not unheard of – that could overshadow or blunt your coverage? Are there hearings on the Hill that could do the same? Look at the social media commentary from the last meeting in this space and develop your own social media listening approach to the meeting.
In the end, it is about data, data and data. But good skills, good planning and even good luck still are factors not to be ignored.
Share this:





February 16, 2014
Weekly Roundup 2.14.14
Well, I am really tardy with this posting – the Weekly Roundup is not only late, it is real late. Sorry, it was not Valentine’s Day festivities, nor was it a result of the snow storms – some of which left my herd grazing on snow. It was just really busy and I was a bit stretched. And I didn’t think in the end, anyone would truly mind.
But there were a few things of note, particularly this first item, so here it is, better late than never:
FDA Seeking Public Comment on DTC – The agency announced this week in the Federal Register that it would be seeking public comment on its research related to “Disclosure Regarding Additional Risks in DTC Prescription Drug Television Ads”. The comment is on the collection of information, but in this notice FDA lays out its hypothesis about DTC ads as currently implemented with the “major statement” are too long thereby undermining patient comprehension. In other words by being thorough and complete one is being boring and uncommunicative. The ad warnings may be so long and involved that it does not penetrate with the audience. On the other hand, how to get the information that is important into the ears and minds of patients? How to solve the issue? FDA is going to embark on a study with participants who have been diagnosed with one of three possible medical conditions who will view different versions of ads to assess risk comprehension. Should be interesting. You can see more about the study design in the Federal Register notice.
First Drug to Receive Rare Pediatric Disease Priority Review Voucher Approved - The agency announced approval of Vimizim to treat a rare congenital enzyme disorder called Morquio A syndrome. The condition is caused by a deficiency in a particular enzyme which can lead to problems with bone development, growth and mobility and is so rare that the press release states that there are only 800 patients diagnosed in the United States. The drug got priority review designation and prior to the approval there were no approved drug treatment options.
New Approval to Treat CLL - A new treatment for chronic lymphocytic leukemia called Imbruvica was approved under accelerated approval to treat patients who have received at least one previous therapy. In addition to being approved under accelerated approval, the new treatment was orphan-product designation. According to the press release, CLL is a rare disease of the blood and bone marrow that gets increasingly worse over time and causes a gradual increase in white blood cells.
There (finally) is the Weekly Roundup for this week. For those of you having a long weekend due to the holiday, I hope you have a good and restful one.
Share this:





February 13, 2014
Building a Case, AdComm Preparation, Part 2
 Last week, after a talk I gave on the subject, I put up a posting Building a Case, AdComm Preparation, Part 1, where I talked about some of the reasons why communications is almost as important of a factor in AdComm preparation as the data and science. There is a sweet spot between good data and good communications that is the place where any sponsor ought to be aiming. Today, let’s look a little more at some of the mechanics of good prep.
Last week, after a talk I gave on the subject, I put up a posting Building a Case, AdComm Preparation, Part 1, where I talked about some of the reasons why communications is almost as important of a factor in AdComm preparation as the data and science. There is a sweet spot between good data and good communications that is the place where any sponsor ought to be aiming. Today, let’s look a little more at some of the mechanics of good prep.
First off with regard to the presentation itself – as with any communications challenge – it is best not to approach it on a piecemeal basis. Before getting to the individual sections of a presentation – the need for the compound, the safety, the efficacy, etc., it is a good idea to sit down and develop the overall narrative first. If you were going to write it as a story, what would you have to say? This master document then is the master messaging document and works to ensure that the individual components hang together to be able to tell a convincing story. A piecemeal approach can work, but there is also a chance that it can result in a presentation that is halting and at times, even inconsistent. The overarching narrative provides a master messaging document from which the individual presentations can flow and relate to one another.
But before you even get to the presentation, there is the issue of messaging and organization around the issues one faces. So prior to developing the narrative around those issues, one must begin to catalog and prioritize the issues. That work primarily is done in two areas: research into the history of the committee and the drug class and a good, hard look at the candidate compound itself.
The committee research is comprised of several components. First look at the history of the committee going back for a few years. Note that in part 1, I mentioned that I recently databased each committee – the number of meetings, the number of recommendations for approval, etc. That is a start. Many people like to track the voting patterns of individual members, and in addition, it is important to go through the transcripts provided on the committee site and assess current sitting members for their hot button issues or recurrent themes or concerns. Particular attention is paid to compounds that have the same or similar indications.
The other part in looking at the history of the committee is to look at each committee member up close. What is their expertise and research history that could shed light on the types of questions they may ask? What professional societies figure in importantly, or third party organizations? Looking at the committee as a whole, is there expertise that might be missing with regard to the compound and therefore signals a possibility that FDA would bring in a consultant for the meeting? Who might that consultant be? In addition, a media analysis of the committee is important. How have media covered this committee in the past – are there “go-to” spokespeople among the committee members who important members of the media seek out for comment after a meeting? Who are the likely third parties who will be sought after for comment?
With this outward assessment going on, it is also important to look inward. Every candidate compound is different and everyone has warts. A fresh set of eyes – those of someone unburdened by an investment of time and effort in the compound or its success – can be very helpful in cataloging the issues that the particular compound has which may be of concern. Data, efficacy, design of clinical trials, recruitment of clinical trials, makeup of clinical trials, special populations, outliers… all issues to be assessed. Here the messaging must be built around each of those issues, taking into account a number of angles for questioning.
A strong master narrative, an environmental research effort and an inward assessment area all key components to get you into the sweet spot. In Part 3, we will look at a few more issues regarding the meeting itself.
Share this:





February 10, 2014
Reflections on 8 years of Blogging – Eye on FDA Starts a 9th Year
Hello. Eight years ago yesterday – February 9, 2006 – I began this blog with my first posting. Since then, I have published 1250 more postings.
Though it seems like only yesterday, 2006 was a very different time:
George W. Bush was President of the United States.
There was no FDA Commissioner at the time – Lester C. Crawford had left FDA in September 2005 and Andrew C. Von Eschenbach would not come in until December 2006.
FDA approved 22 new molecular entities that year.
People were getting nervous about Avian flu.
Google bought YouTube.
Facebook was two years old and in September 2006, it was opened up to anyone over 13 years of age who had an email address.
MySpace was a top web site.
Twitter would not exist for another month.
Certainly Pharma wasn’t engaged in social media – nor were many other companies. Journalists, with the exception of some bloggers, weren’t there either.
Back then, professional blogs were kind of scarce. Blogging was considered the realm of teenagers keeping diaries online, or sensationalists spreading gossip, to a large degree. A colleague of mine began writing a blog that had intelligent musings on what was going on – with a regular Friday posting called This Week in Jewish Baseball. I thought to myself, “Hey, I could do that – only write about the area in which I work professionally…” I was – and am – fortunate enough to work for a company that saw the wisdom in that. And so I began writing about the regulation of the marketplace for medicines and the actions of the agency that regulates one-fourth of our economy.
 Since then, I have tried to cover the stuff that is important related to the developments that affect not only those who communicate about the pharmaceutical market place, but patients who are consumers in that marketplace. There have been a few basic driving tenets along the way – I have always wanted to give readers useful information – things that would make them look smart and resources that would help them make important decisions. I have always wanted to call attention to things that, while important, might have their strategic implications overlooked because we are so busy in our daily lives. Sometimes, the blog has given me a little room to provide personal insight, particularly about my time working in the HIV/AIDS epidemic. And from time to time even, there has even been news – such as when FDA provided its first insight into the regulation of social media in a podcast in March, 2009.
Since then, I have tried to cover the stuff that is important related to the developments that affect not only those who communicate about the pharmaceutical market place, but patients who are consumers in that marketplace. There have been a few basic driving tenets along the way – I have always wanted to give readers useful information – things that would make them look smart and resources that would help them make important decisions. I have always wanted to call attention to things that, while important, might have their strategic implications overlooked because we are so busy in our daily lives. Sometimes, the blog has given me a little room to provide personal insight, particularly about my time working in the HIV/AIDS epidemic. And from time to time even, there has even been news – such as when FDA provided its first insight into the regulation of social media in a podcast in March, 2009.
Certainly times have changed. Advisory committees activities used to be reported on by reporters who attended meetings and wrote about them in their publications. Today tweets from those watching the proceedings tell the story. Patients have not only become e-patients, but one-fourth of the people using the FDA website are doing so from a mobile device. The pharma industry has scores of Twitter feeds, Facebook pages, YouTube channels, Pinterest accounts and presence on Google+. Even FDA has 13 Twitter feeds that it manages. All of this has had profound ramifications and implications for the communications around medicines and how we use them. It has been nothing short of fascinating to see communications so changed in such a short span of time.
Eight years is a long time and a lot has happened and yet amazingly, I have not aged at all.
There are about 3300 subscribers to the blog – divided about half and half between people who subscribe by RSS feed and people who subscribe by email. You are mostly FDA beat writers, people who work in pharma and folks in other communications firms who work with industry, as well as patient organizations. The Eye on FDA Twitter feed has over 10,500 followers. I want to thank everyone for reading and watching and sticking around. And I look forward to talking about more in the years to come.
Thanks everyone.
Share this:





February 7, 2014
Weekly Roundup 2.7.14
Well, it has been one long winter. I have the February blues and I am blaming that fact on my being a bit tardy with the Weekly Roundup here. While I love a good snow fall, the fact is that here in Washington, we aren’t even getting much snow. We get forecasts like “ice pellets” and things like that. No lovely vistas of snowscapes. There has also been a handy amount of dark ice. Good times. But just to cheer us all up, here is a nice close up of Marky the Cow. She is growing up so fast…
And also to cheer you up, here is a bit of what happened this week:
FDA Launches Campaign to Reduce Youth Tobacco Use – The agency acted this week to reduce the number of people in the next generation of smokers by launching a campaign called “The Real Cost” directed to young people ages 12-17. The agency said that it was the first of several campaigns that would be launched over time. FDA says that it is using a comprehensive, multimedia approach with compelling facts and vivid imagery designed to change beliefs and behaviors over time, using several social media platforms. The campaign will be evaluated to measure its effectiveness over time and is designed to target subcategories of the audience including multicultural youth, rural youth and LGBT kids. You can view the ads on the FDA’s YouTube channel and visit the campaign web site here.
FDA Update Requirements on Infant Formula – FDA issued an interim final rule to further safeguard the health of infants being fed infant formula. One of the interesting facts from the FDA’s press release on the issuance of this rule was that the agency cited the fact that despite the recommendation that mothers breastfeed their babies “only 75 percent of infants in the United States start out being breast fed” – and actually I was surprised to know it was that high. In any case, this interim rule amends the agency’s quality control procedures, notification and record and reporting requirements for manufacturers. The agency will be accepting comments on the interim final rule for a 45 day period.
That’s it for me – like February, today’s posting is short. Have a good weekend everyone and know that we only have a month from Monday for Daylight Savings!
Photo courtesty of Anne Becker.
Share this:





February 6, 2014
Building a Case – AdComm Preparation, Part 1
 Today I am giving a talk here in Washington about preparation for advisory committees. Over the years, I have had occasion to attend more meetings of FDA Advisory Committees than I care to think about, often with the purpose of helping a company on their own approach to an FDA Advisory Committee meeting – sometimes on product approval, sometimes regarding a policy issue. I thought it might be worthwhile to cover a little bit of what I will be talking about today.
Today I am giving a talk here in Washington about preparation for advisory committees. Over the years, I have had occasion to attend more meetings of FDA Advisory Committees than I care to think about, often with the purpose of helping a company on their own approach to an FDA Advisory Committee meeting – sometimes on product approval, sometimes regarding a policy issue. I thought it might be worthwhile to cover a little bit of what I will be talking about today.
First of all, of course my remarks are aimed at the non-clinical aspects of AdComm preparation. To prepare for this task, I began assembling a data base – no surprise to those who know me. I went back to 2009 and profiled every Advisory Committee meeting regarding a drug, tracking the committee, the date, whether it was held jointly with another committee, whether it was a meeting to consider an approval or whether it was a meeting regarding an issue of policy, the company, the compound, the indication and whether or not there was a definitive positive vote. As of now, the data is pretty raw, meaning I haven’t had the chance to go through and ensure that all of the entries are correct. Nor did I look at every angle the way I would like to – for instance, what is the track record of individual companies – so the numbers I will have today will not be final. But I did find that of the over 200 meetings held since 2009, roughly just over half of those that considered approvals had positive votes (again without the benefit of the yet to come review of the entries).
When I broke it down by committee, one can see that some committees, like Oncology and Endocrinology, have been very busy – but the positive vote rate varied considerably between the two. Without going into the specifics of the numbers (until I have had a chance to check the entries), it does indicate that it is important to know your committee and to glean the best practices from the positive outcomes that were related to that committee – they can provide a blue print for a new sponsor in that treatment category.
Let’s turn away from those numbers to content and delivery.
There is a sweet spot in AdComm preparation to be realized at the intersection of having really good data with having really good communications and delivery. Can you get a positive outcome with good data and bad communications? Yes, you probably can. Can you get a positive outcome with bad data and really good communications. No, you probably cannot. Still, the place where you are best off is within the sweet spot of good data and good comms.
Why? The content of this meeting echoes on long after everyone leaves the room. It is important to remember that the AdComm is where branding for the product takes its first breath. It is here that the public is really being introduced to the compound for the first time – all the more reason why communications place an important role. That is the focus on the first part of the talk.
When it comes to fashioning the actual presentation, the presenters have more at hand than the task of conveying a simple presentation – they need to build a convincing case on the need for the compound, and that there is a favorable risk/benefit ratio. On this front, it is a great pitfall to get lost in the weeds, without thinking about the entirety of the case being made.
In the end, while most people treat this as a scientific meeting – and it is – it is also important to look at it like an adversarial proceeding. There is a judge (the Chair), there is a jury (the AdComm), media cover it (FDA beat reporters and trades), there is a prosecutor (FDA reviewers) and the sponsor is the defense. Witnesses may show up as special experts or speakers at the Open Public Comment period. That said, it is important that you be able to present your evidence-based best case. In part 2, more on that topic.
Share this:





February 3, 2014
Upcoming AdComms by Subject Matter
The Advisory Committee calendar put up by FDA is very useful, but lists the upcoming meetings chronologically by date and by their named committee. I thought it might be helpful every so often to provide an overview of upcoming AdComm meetings by topic instead so that one can readily discern where there is likely to be news. So here is my first effort, notable perhaps for the fact that there is little action related to new drug approval.
ASSISTED REPRODUCTION
Oocyte Modification – The Cellular, Tissue and Gene Therapies Advisory Committee will meet to discuss oocyte modification in assisted reproduction for the prevention of transmission of mitochondrial disease on February 25-26.
ASTHMA
Inhaler Replacement – There will be a joint meeting of the Pulmonary Drugs Advisory Committee with the Non-Prescription Drugs Advisory Committee to discuss use of an epinephrine inhaler for OTC use that would replace a discontinued version – on February 25.
OTC Bronchodilators – There will be a meeting of the Non Prescription Drugs Advisory Committee to consider whether OTC bronchodilators administered by hand-held rubber bulb nebulizers for the temporary relief of mild symptoms of intermittent asthma should be removed from the monograph on February 26.
CARDIOVASCULAR
NSAIDS and CV Risk – Meeting of the Arthritis Drugs AdComm with the Risk Management Advisory Committee, February 10-11;
NDA for cangrelor Injection - Submitted by The Medicines Company – Meeting of the Cardiovascular and Renal Drugs Advisory Committee for the proposed indication of reduction of thrombotic cardiovascular events, including stent thrombosis, in patients with coronary artery disease undergoing PCI, February 12;
BLA for seralaxin Injection – Submitted by Novartis – Meeting of the Cardiovascular and Renal Drugs Advisory Committee for the proposed indication to improve symptoms of acute heart failure, February 13.
HPV
New Indication for Test – The Microbiology Devices Panel will meet to discuss the premarket approval application for a new indication as a first line primary cervical screening test for the cobas Human Papillomavirus (HPV) Test, sponsored by Roche Molecular Systems, on March 12.
INFLUENZA
Flu Strains – The Vaccines and Related Biological Products Committee will meet to hear an overview of a research program in the Laborary of Respiratory Viral Diseases, CBER and then will discuss and make recommendations on the selection of strains to be included in the flu virus vaccine for the 2014-2015 season on February 28.
OPHTHALMOLOGY
PreMarket Application for new lens - Meeting of Ophthalmic Devises Panel to discuss application from Starr Surgical Company for Visian Toric Implantable Collamer Lens (TICL) for new lens for the treatment of addressing myopic astigmatism in adults 21-45, February 14.
REGULATORY/POLICY
Regulatory Considerations - The Orthopaedic and Rehabilitation Devices Panel will meet to discuss the regulatory classification of iontophoresis devices on February 21.
SLEEP APNEA
PreMarket Application for new device – Meeting of the Anesthesiology and Respiratory Therapy Devices Panel to consider application for Inspire Medical Systems’ Inspire II Upper Airway Stimulator to be a permanently implanted device to treat moderate to severe sleep apnea in patients not effectively being managed with continuous positive airway pressure devices, February 20.
In addition to this, I have begun a data base on Advisory Committees held since 2009 that will profile a number of characteristics, including outcomes. More on that later.
Share this: