The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two
The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two
Jeffrey Dach MD
Brenda is a young woman who came to my office for evaluation and upon testing, was found to have essentially no urinary iodine excretion, indicating iodine deficiency.
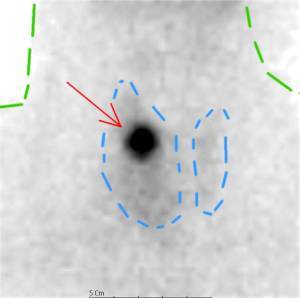
Above header image: Autonomous Nodule in right thyroid lobe (red arrow) showing “hot” , avid update of radio-tracer, with suppression of remaining thyroid gland (blue dotted line) Outline of neck (blue dotted line). Courtesy of wikimedia commons.
Although Brenda had been in the US for twenty years, she was born and grew up in a foreign country noted for low iodine levels. Because of her iodine deficiency, Brenda began a program of iodine supplementation with iodized salt. About a week later, Brenda experienced an episode of tachycardia, severe rapid heart rate, with a sustained pulse rate of 220 beats per minute. Shortly after arriving at the local hospital Emergency Room, the tachycardia resolved spontaneously. The doctors had no explanation for the tachycardia, and Branda was sent home with a Beta-Blocker Drug, called Atenolol to slow the heart rate should the tachycardia return.
What Caused Brenda’s Tachycardia? The Autonomous Nodule.
Brenda’s tachycardia was a manifestation of hyperthyroidism from an autonomous thyroid nodule. When Brenda started taking iodized salt, the increased iodine was converted rapidly into thyroid hormone by the autonomous nodule, a well described phenomenon of hyperthyroidism associated with dietary Iodine intake. The autonomous thyroid nodule manufactures thyroid hormone uncontrollably depending on iodine availability, outside of the normal control mechanism of TSH (thyroid stimulating hormone). When these patients ingest dietary iodine, they become thyrotoxic with very low TSH, high Free T3 and FreeT4, yet the autonomous nodule continues to take up iodine and produce large amounts of thyroid hormone despite the very low TSH. Yet, the low TSH will suppress the iodine uptake in the surrounding normal thyroid tissue, rendering the nodule “hot” on a suppressed background of normal thyroid on the radionuclide scan.
The Sonogram and Radio-Nuclide Thyroid Scan
Brenda was advised to stop the dietary Iodine and sent for diagnostic evaluation for an autonomous thyroid nodule, also called a “hot nodule”. Sure enough, Brenda’s thyroid sonogram showed a thyroid nodule in the right upper lobe, and her Technetium 99M radionuclide scan showed the nodule avidly took up radio-tracer, a “Hot Nodule”, indicating an autonomous nodule. (25-29)
Thyroxine Suppression Test
In some cases, the nodule may not be visible on the radionuclide scan. The radioactive tracer uptake may not be increased enough or “Hot” enough to be visible. To make the nodule more visible, standing out from the surrounding normal thyroid gland, and an additional step may be required. This is called a Thyroxine suppression test. The scan is repeated after giving the patient Levothyoxine which suppresses uptake in the remaining normal thyroid tissue, yet does not affect the autonomous nodule, making the hot nodule stand out from the suppressed background thyroid tissue. (25-29)
In 1998, Dr John Stanbury concluded the most common cause of iodine induced hyperthyroidism is the autonomous nodule, writing:
The biological basis for IIH (Iodine induced hyperthyroidism) appears most often to be mutational events in thyroid cells that lead to autonomy of function. When the mass of cells with such an event becomes sufficient and iodine supply is increased, the subject may become thyrotoxic. These changes may occur in localized foci within the gland or in the process of nodule formation. IIH may also occur with an increase in iodine intake in those whose hyperthyroidism (Graves’ disease) is not expressed because of iodine deficiency. The risks of IIH are principally to the elderly who may have heart disease, and to those who live in regions without medical care.(21)
What is an Autonomous Nodule?
Since 1998, more recent medical research reveals that autonomous thyroid nodules are clones of cells that have a mutation in the TSH Receptor (Thyroid Stimulating Hormone Receptor). These mutations usually arise as a result of iodine deficiency in patients who spend their childhood in iodine deficient countries or geographic regions. Mutations in the gene for the TSH receptor make these nodules independent of TSH control usually found in normal thyroid tissue. Thus, based on the availability of Iodine, the autonomous nodule makes thyroid hormone uncontrollably. (9-13)(35)
Etiology of Autonomous Nodules
What causes the autonomous nodule? Remember, iodine is needed for the production of thyroid hormone, and Iodine deficiency impairs this process causing hypothyroidism. The pituitary responds to low thyroid hormone levels by secreting more TSH, causing elevation of the TSH, which then travels to the thyroid gland and instructs increased generation of hydrogen peroxide, as well as all other steps in thyroid hormone production. If the patient is also selenium deficient, the the selenium-based antioxidants system will be deficient, with lack of degradation of hydrogen peroxide. The excess hydrogen peroxide is mutagenic, leading to mutations in the TSH receptor. A clone of thyroid cells with such a mutation is an autonomous nodule. In 2007, Dr. Knut Krohn writes:
We reconstruct a line of events that could explain the predominant neoplastic character (i.e. originating from a single mutated cell) of thyroid nodular lesions. This process might be triggered by the oxidative nature of thyroid hormone synthesis or additional oxidative stress caused by iodine deficiency or smoking. If the antioxidant defense is not effective, this oxidative stress can cause DNA damage followed by an increase in the spontaneous mutation rate, which is a platform for tumor genesis. The hallmark of thyroid physiology—H2O2 production during hormone synthesis—is therefore very likely to be the ultimate cause of frequent mutagenesis in the thyroid gland. DNA damage and mutagenesis could provide the basis for the frequent nodular transformation of endemic goiters. (51-53) Emphasis Mine.
The Autonomous Nodule Originates in an Iodine Deficient Region
Chronic iodine deficiency, usually from living in an iodine deficient region, is considered the main cause of autonomous nodules. Iodine deficiency is a risk factor for mutations in the TSH receptors and development of the autonomous thyroid nodule. Here in the US, we started a program of iodine fortification of table salt back in 1924, and since iodine deficiency has been considerably reduced, autonomous nodules are now quite rare in the US. Most of the cases we see here in the US are patients who migrate here from iodine deficient regions outside the US. These people may harbor autonomous nodules, and may present with features of transient hyperthyroidism when supplemented with iodine in the diet. (1-13)
Hyperthyroidism After Iodized Salt Fortification
Even the small amounts of dietary iodine found in Iodized Salt can cause transient hyperthyroidism in patients with autonomous nodules. Public records show a transient increase in mortality from thyrotoxicosis in 1926-1928 after the introduction of Iodized Salt. This was thought to be due to presence of pre-existing autonomous nodules in the population. Likewise, in various other countries, Iodized salt and Iodized Bread programs were introduced, and again a transient increase in thyrotoxicosis was reported shortly afterwards. Again, these cases were thought to be autonomous nodules responding to dietary iodine supplements. Note: some of these cases were toxic nodular goiter, with one or more autonomous nodules. In addition to iodized salt programs, thyrotoxicosis in the autonomous nodule patient may be caused by various iodine containing drugs such as SSKI, amiodorone, and iodinated radiographic contrast. In case reports of iodine causing hyperthyroidism in normal thyroid glands, one might suggest these normal glands harbor autonomous nodules that are simply missed. (1-13)(20-23) (33-34)
Marine-Lenhart Syndrome
In Japan, potassium iodine is commonly used as a thyroid blocking drug in Grave’s disease patients. However about 10 per cent of cases do not respond or are made worse by iodine. Could some of these cases be explained by autonomous functioning thyroid tissue? This is the Marine-Lenhart Syndrome defined as Graves’ disease with thyroid nodular lesions and clinical characteristics of both Graves’ disease and Plummer disease (toxic nodular goiter). In 2021, Dr. Hirosuke Danno found 0.26 per cent prevalence of MLS among Graves Disease patients in Japan. This is not high enough prevalence to explain the 9-10 per cent rate of iodine escape found in 2015 by Dr. Yoshihara when switching pregnant Graves’ disease patients from Methimazole to Iodine. However, the co-existence of Plummers’ with Graves’ is something to keep in mind when clinical features are atypical. (54-58)(64)
Over Expression of NIS in autonomous nodules
In 1999, Dr. Meller demonstrated over expression of the Na+/I- symporter (NIS) in autonomous nodules, providing an explanation for enhanced uptake and clearance of iodine on radionuclide scan. (27)
Treatment of Toxic Adenoma and Toxic Multinodular Goiter
Treatment of thyro-toxicosis caused by autonomous thyroid nodule or toxic nodular goiter consists of the following: (38-48)(59-63)
1) Thyroid Blocking Drugs, also called medical treatment. The most used drug is Methimazole, 15 -30 mg per day. Lithium Carbonate 300 mg TID has also been found useful as a thyroid blocking drug, and may be given prior to radioactive iodine (I-131) therapy to enhance retention of Iodine in the thyroid gland. To slow the heart rate, Beta Blockers (atenolol 25-40 mg/day) are commonly prescribed for relief of tachycardia. (44-48)
2) Thyroid Ablation with Radiation. Radioactive Iodine (I-131) therapy for autonomous nodule is a form of radiation therapy which ablates the nodule, and is quite successful. Autonomous nodules are highly active and soak up most of the radioactive iodine, sparing the remaining normal gland. (45-47)
3) Surgical removal of the nodule with thyroid lobectomy procedure. (63)
4) Percutaneous ethanol injection into the autonomous thyroid nodule under ultrasound control. (38-40)
Another way to check Iodine Sufficiency- Serum Thyroglobulin
Serum thyroglobulin is also useful in evaluating iodine status. (17-19)
Conclusion: Although quite rare in the US, the autonomous nodule is an important cause of iodine induced hyperthyroidism. Unlike Graves’ Disease in which iodine serves as a useful thyroid blocking agent, iodine is contraindicated for the autonomous nodule or toxic nodular goiter patient, as in these cases, iodine causes thyrotoxicosis, which may represent a life-threatening medical emergency. The autonomous nodule is frequently missed by mainstream medicine, representing another error.
References
1) Azizi, F. “Iodized oil: its role in the management of iodine deficiency disorders.” International Journal of Endocrinology and Metabolism 5.2 (2007): 91-98.
2) Kohn, LAWRENCE A. “The Midwestern American” epidemic” of iodine-induced hyperthyroidism in the 1920s.” Bulletin of the New York Academy of Medicine 52.7 (1976): 770.
3) Connolly, R. J., G. I. Vidor, and J. C. Stewart. “Increase in thyrotoxicosis in endemic goitre area after iodation of bread.” The Lancet 295.7645 (1970): 500-502.
4) Vidor, G. I., et al. “Pathogenesis of iodine-induced thyrotoxicosis: studies in northern Tasmania.” The Journal of Clinical Endocrinology & Metabolism 37.6 (1973): 901-909.
5) Todd, C. H., et al. “Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe.” The Lancet 346.8989 (1995): 1563-1564.
6) Bourdoux, Pierre, et al. “Iodine-induced thyrotoxicosis in Kivu, Zaire.” Lancet 347.9000 (1996): 552-553.
7) Delange, F., B. De Benoist, and D. Alnwick. “Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt.” Thyroid 9.6 (1999): 545-556.
8) Livadas, D. P., et al. “The toxic effects of small iodine supplements in patients with autonomous thyroid nodules.” Clinical endocrinology 7.2 (1977): 121-127.
9) Tonacchera, Massimo, et al. “Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter.” The Journal of Clinical Endocrinology & Metabolism 85.6 (2000): 2270-2274.
10) Bülow Pedersen, Inge, et al. “Large differences in incidences of overt hyper-and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey.” The Journal of Clinical Endocrinology & Metabolism 87.10 (2002): 4462-4469.
11) Bülow Pedersen, Inge, et al. “Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark.” The Journal of Clinical Endocrinology & Metabolism 91.10 (2006): 3830-3834.
12) Davies, Terry F., et al. “Thyrotropin receptor–associated diseases: from adenomata to Graves disease.” The Journal of clinical investigation 115.8 (2005): 1972-1983.
13) Gozu, Hulya Ilıksu, et al. “Similar prevalence of somatic TSH receptor and Gsα mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey.” European journal of endocrinology 155.4 (2006): 535-545.
14) Beck-Peccoz, Paolo. “Antithyroid drugs are 65 years old: time for retirement?.” Endocrinology 149.12 (2008): 5943-5944.
15-16) deleted
17) Brodowski, Jacek, et al. “Thyroglobulin concentration after introduction of population iodine prophylaxis in random selected group of children and adolescents in Szczecin region.” Polski Merkuriusz Lekarski: 20.116 (2006): 164-167.
18) van den Briel, Tina, et al. “Serum thyroglobulin and urinary iodine concentration are the most appropriate indicators of iodine status and thyroid function under conditions of increasing iodine supply in schoolchildren in Benin.” The Journal of nutrition 131.10 (2001): 2701-2706.
19) Vejbjerg, Pernille, et al. “Thyroglobulin as a marker of iodine nutrition status in the general population.” European Journal of Endocrinology 161.3 (2009): 475-481.
20) Gołkowski, Filip, et al. “Increased prevalence of hyperthyroidism as an early and transient side-effect of implementing iodine prophylaxis.” Public health nutrition 10.8 (2007): 799-802.
21) Stanbury, John Burton, et al. “Iodine-induced hyperthyroidism: occurrence and epidemiology.” Thyroid 8.1 (1998): 83-100.
22) Corvilain, Bernard, et al. “Autonomy in endemic goiter.” Thyroid 8.1 (1998): 107-113.
23) Schwarzfischer, P., et al. “Iodine-induced hyperthyroidism in the aged. 2. Pathomechanism, differential diagnosis and therapy problems.” Fortschritte der Medizin 100.5 (1982): 153-158.
24) Joseph, K., J. Mahlstedt, and U. Welcke. “Early recognition of autonomous thyroid tissue by a combination of quantitative thyroid pertechnetate scintigraphy with the free T4 equivalent (author’s transl).” Nuklearmedizin. Nuclear Medicine 19.2 (1980): 54-63.
25) Fricke, Eva, et al. “Scintigraphy for risk stratification of iodine-induced thyrotoxicosis in patients receiving contrast agent for coronary angiography: a prospective study of patients with low thyrotropin.” The Journal of Clinical Endocrinology & Metabolism 89.12 (2004): 6092-6096.
26) Ratnos, Celso D., et al. “Thyroid suppression test with L‐thyroxine and [99mTc] pertechnetate.” Clinical endocrinology 52.4 (2000): 471-477.
27) Meller, J., and W. Becker. “Scintigraphy with (99m) Tc-pertechnetate in the evaluation of functional thyroidal autonomy.” The Quarterly Journal of Nuclear Medicine and Molecular Imaging 43.3 (1999): 179.
28) Meller, J., and W. Becker. “The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound.” European journal of nuclear medicine and molecular imaging 29.2 (2002): S425-S438.
29) Meller, J., and W. Becker. “Scintigraphic evaluation of functional thyroidal autonomy.” Experimental and clinical endocrinology & diabetes 106.S 04 (1998): S45-S51.
30) Nolte, Wilhelm, et al. “Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study.” European journal of endocrinology 134.3 (1996): 337-341.
31) Hehrmann, R., et al. “Risk of hyperthyroidism in examinations with contrast media.” Aktuelle Radiologie 6.5 (1996): 243-248.
32) Rieu, Max, et al. “Prevalence of subclinical hyperthyroidism and relationship between thyroid hormonal status and thyroid ultrasonographic parameters in patients with non‐toxic nodular goitre.” Clinical endocrinology 39.1 (1993): 67-71.
33) Savoie, J. C., et al. “Iodine-induced thyrotoxicosis in apparently normal thyroid glands.” The Journal of Clinical Endocrinology & Metabolism 41.4 (1975): 685-691.
34) Shilo, Shmuel, and Harry J. Hirsch. “Iodine-induced hyperthyroidism in a patient with a normal thyroid gland.” Postgraduate Medical Journal 62.729 (1986): 661-662.
35) Krohn, Knut, and Ralf Paschke. “Progress in understanding the etiology of thyroid autonomy.” The Journal of Clinical Endocrinology & Metabolism 86.7 (2001): 3336-3345.
36) Lima, N., and G. Medeiros‐Neto. “Transient thyrotoxicosis in endemic goitre patients following exposure to a normal iodine intake.” Clinical endocrinology 21.6 (1984): 631-637.
37) Laurberg, Peter, et al. “The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives.” European journal of endocrinology 155.2 (2006): 219-228.
38) Monzani, Fabio, et al. “Five‐year follow‐up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients.” Clinical endocrinology 46.1 (1997): 9-15.
39) Tarantino, Luciano, G. Froncica, and Ignazio Sordelli. “Percutaneous ethanol injection of hyperfunctioning thyroid nodules: long-term follow-up in 125 patients.” American Journal of Roentgenology 190.3 (2008): 800.
40) Lippi, Francesco, et al. “Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group.” The Journal of Clinical Endocrinology & Metabolism 81.9 (1996): 3261-3264.
41) Iagaru, Andrei, and I. Ross McDougall. “Treatment of thyrotoxicosis.” Journal of nuclear medicine 48.3 (2007): 379-389.
42) Rose, Noel R., et al. “Linking iodine with autoimmune thyroiditis.” Environmental Health Perspectives 107.suppl 5 (1999): 749-752.
43) Xue, Haibo, et al. “Selenium upregulates CD4+ CD25+ regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD. H-2h4 mice.” Endocrine Journal 57.7 (2010): 595-601.
44) Azizi, Fereidoun. “Long-term treatment of hyperthyroidism with antithyroid drugs: 35 years of personal clinical experience.” Thyroid 30.10 (2020): 1451-1457.
45) Płazińska, Maria Teresa, Leszek Królicki, and Marianna Bąk. “Lithium carbonate pre-treatment in 131-I therapy of hyperthyroidism.” Nuclear Medicine Review 14.1 (2011): 3-8.
46) Ross, Douglas S., et al.. “Successful treatment of solitary toxic thyroid nodules with relatively low-dose iodine-131, with low prevalence of hypothyroidism.” Annals of internal medicine 101.4 (1984): 488-490.
47) Huysmans, Dyde A., Frans H. Corstens, and Peter W. Kloppenborg. “Long-term follow-up in toxic solitary autonomous thyroid nodules treated with radioactive iodine.” Journal of Nuclear Medicine 32.1 (1991): 27-30.
48) Ross, Douglas S. “Treatment of toxic adenoma and toxic multinodular goiter.” UpToDate. 12th ed. Waltham, MA: Wolters Kluwer (2019).UpToDate_
49) Ratcliffe, Guy E., et al. “Radioiodine treatment of solitary functioning thyroid nodules.” The British Journal of Radiology 59.700 (1986): 385-387.
50) Nygaard, Birte, et al. “Long‐term effect of radioactive iodine on thyroid function and size in patients with solitary autonomously functioning toxic thyroid nodules.” Clinical endocrinology 50.2 (1999): 197-202.
51) Song, Yue, et al. “Roles of hydrogen peroxide in thyroid physiology and disease.” The Journal of Clinical Endocrinology & Metabolism 92.10 (2007): 3764-3773.
52) Krohn, Knut, Jacqueline Maier, and Ralf Paschke. “Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors.” Nature clinical practice Endocrinology & metabolism 3.10 (2007): 713-720.
53) Maier, J., et al. “Iodine deficiency activates antioxidant genes and causes DNA damage in the thyroid gland of rats and mice.” Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1773.6 (2007): 990-999.
54) Nishikawa, Mitsushige, et al. “Coexistence of an Autonomously Functioning Thyroid Nodule in a Patient with Graves’ Disease An Unusual Presentation of Marine-Lenhart Syndrome.” Endocrine journal 44.4 (1997): 571-574.
55) Charkes, N. David. “Graves’ disease with functioning nodules (Marine-Lenhart syndrome).” Journal of Nuclear Medicine 13.12 (1972): 885-892.
56) Danno, Hirosuke, et al. “Prevalence and Treatment Outcomes of Marine-Lenhart Syndrome in Japan.” European Thyroid Journal 10.6 (2021): 461-467. 0.26% in Japan
57) The Marine-Lenhart syndrome (MLS), first described by Charkes in 1972 [1], is now commonly defined as “a combination of Graves’ disease and autonomous functioning thyroid nodule(s) (AFTN)”
58) Miyazaki, Megumi, et al. “A case of Marine-Lenhart syndrome with predominance of Plummer disease.” Journal of UOEH 41.2 (2019): 165-170.
59) Azizi, Fereidoun, et al. “Treatment of toxic multinodular goiter: comparison of radioiodine and long-term methimazole treatment.” Thyroid 29.5 (2019): 625-630.
60) Bawand, Rashed, et al. “Comparison of clinical efficacy of antithyroid drugs, radioactive iodine, and thyroidectomy for treatment of patients with graves’ disease, toxic thyroid adenoma, and toxic multinodular goiter.” Biomedical and Biotechnology Research Journal (BBRJ) 6.4 (2022): 569.
61) Roque, Catarina, et al. “Long-term effects of radioiodine in toxic multinodular goiter: thyroid volume, function, and autoimmunity.” The Journal of Clinical Endocrinology & Metabolism 105.7 (2020): e2464-e2470.
62) Racaru, L. Vija, et al. “Management of adenomas and toxic multinodular goiters with Iodine 131.” Médecine Nucléaire 44.4 (2020): 272-276.
63) Kırdak, Türkay. “Surgery in Hyperthyroidism: Toxic Adenoma and/or Multinodular Goiter.” Thyroid and Parathyroid Diseases. Springer, Cham, 2019. 45-49.
64) Yoshihara, Ai, et al. “Substituting potassium iodide for methimazole as the treatment for Graves’ disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan.” Thyroid 25.10 (2015): 1155-1161.
Published on February 7th, 2023 by Jeffrey Dach MD
The post The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two appeared first on Jeffrey Dach MD.
Jeffrey Dach's Blog
- Jeffrey Dach's profile
- 3 followers



