Jeffrey Dach's Blog, page 7
July 9, 2023
Natural Thyroid Toolkit by Jeffrey Dach MD
 Natural Thyroid Toolkit by Jeffrey Dach MD
Natural Thyroid Toolkit by Jeffrey Dach MD
Natural Thyroid Toolkit: Hashimoto’s, Graves,’ Iodine and Natural Desiccated Thyroid is the breakthrough book on using natural desiccated thyroid.
Foreword by David Brownstein, MD
“This book needs to be read by all physicians, and by all who are suffering from thyroid problems. I am a voracious reader. I have read hundreds of health books. Natural Thyroid Toolkit by Jeffrey Dach, MD is one of the best. It is a must-read.” end quote, David Brownstein, MD in the Foreword to the book.
About the Author
Jeffrey Dach, M.D. is co-author of Stop the Thyroid Madness Volume II published in 2014. Dr. Dach is sole author of Cracking Cancer Toolkit published in 2020, Heart Book, published in 2018, Bio-Identical Hormones 101 published in 2011, and Natural Medicine 101 published in 2008.
Jeffrey Dach MD was originally board certified in diagnostic and interventional radiology, and worked 25 years as a hospital based physician. Dr. Dach retired from hospital based medicine 20 years ago and opened an outpatient clinic specializing in natural thyroid and bioidentical hormones. This book, Natural Thyroid Toolkit, represents 20 years of experience using natural thyroid in the out-patient setting. Author’s web site: https://jeffreydachmd.com/
The Silent Epidemic and Unsuspected illness
Depending on which source you read, between 10-30 percent of the population has some kind of thyroid disorder, and many of these suffer needlessly. This state of affairs has been expressed as a silent epidemic or an unsuspected illness. In 1891, thyroid extract was first used to treat thyroid patients, representing one of the first medications in the history of western medicine. Since these early days, the basic science of thyroid has made a quantum leap. Although publicly available in the medical literature, current breakthroughs in thyroid science are largely ignored by mainstream medicine. The practice of thyroid endocrinology remains outdated, hampered by dogmatic reliance on older tests and treatments.
The goal of this this book, Natural Thyroid Toolkit, is to bring state of the art thyroid diagnosis and treatment into the public realm. The reader is provided with a unique blend of information from both clinical experience and from the thyroid medical literature. This book includes copious references to in vitro and in vivo basic science studies as well as the more formal clinical trials in human populations. This book represents 20 years of prescribing natural desiccated thyroid in the outpatient clinical setting.
Errors in Modern Thyroid Endocrinology
This book discusses many of the errors in modern thyroid endocrinology. One of the errors of modern endocrinology is the dogmatic reliance on the TSH laboratory test, a classic example of the misapplication of a laboratory test. The second error is sole reliance on levothyroxine (T4-monotherapy) in the treatment of hypothyroidism. Combination therapy with natural desiccated thyroid containing both T3 and T4 is a superior and more robust treatment. There are more errors:Treating thyroid hormone levels while ignoring the autoimmune component of thyroid disease. Failing to treat euthyroid Hashimoto’s patients promptly with thyroid medication. Failing to treat pregnant women with thyroid medication when anti-thyroid antibodies are elevated. Failing to test for and treat with iodine because of “Medical Iodophobia,” the irrational fear of using iodine. Another error is ignoring the beneficial effects of selenium, magnesium, vitamin D3, and a gluten-free diet for autoimmune thyroid disease. My sincere thanks and gratitude goes to Dr. David Brownstein for writing the foreword for this book. Join our online thyroid community by signing up for my free monthly newsletter.
Click Here for Paperback Version on Amazon:
Click Here for E-Book Version on Amazon
Table of Contents
Foreword by David Brownstein, MD………………………………………….6
Introduction by Jeffrey Dach MD……………………………………………….8
Chapter 1: Natural Thyroid as Anti-Aging…………………………………..13
Chapter 2: Why Natural Thyroid is Better than Synthetic Part One..18
Chapter 3: Why Natural Thyroid is Better than Synthetic Part Two…23
Chapter 4: Why Natural Thyroid is Better than Synthetic Part Three..29
Chapter 5: Errors in Modern Thyroid Endocrinology……………………….33
Chapter 6: TSH is Inadequate for Levothyroxine Dosing…………………48
Chapter 7: TSH Suppression Benefits and Adverse Effects……………..53
Chapter 8: Paradigm Shift from Levothyroxine to Combination T3/T4..63
Chapter 9: Which Thyroid is Best, Natural, Synthetic, or Combination?.68
Chapter 10: The Unreliable TSH Lab Test………………………………………76
Chapter 11: Hypothyroidism and Reversible Cardiomyopathy……………82
Chapter 12: Hypothyroidism and the Immune System………………………89
Chapter 13: Thyroid Hormone Prevents Heart Attacks……………………..93
Chapter 14: The Production of Thyroid Hormone……………………………101
Chapter 15: Graves’ Hyperthyroidism Remission with Iodine Part One
Chapter 16: Iodine Treatment of Graves’ Disease Part Two…………….151
Chapter 17: Combined Lithium and Potassium Iodide for Graves’ …..164
Chapter 18: Addressing the Auto Immune Component ………………….173
Chapter 19: Autonomous Thyroid Nodule…………………………………….192
Chapter 20: Hashimoto’s, Iodine and Selenium Part One………………..210
Chapter 21: Hashimoto’s Iodine and Selenium Part Two…………………214
Chapter 22: Selenium and Thyroid, More Good News Part Three…….218
Chapter 23: Does Iodine Cause Hashimoto’s Thyroiditis?……………….225
Chapter 24: Origin and Features of Hashimoto’s …………………………..231
Chapter 25: Low Thyroid, Hashimoto’s, and Pregnancy…………………235
Chapter 26: Hashimoto’s with Normal TSH, When to Treat?……………238
Chapter 27: Hashimoto’s Thyroiditis, Manic Depressive Psychosis…..244
Chapter 28: Myo-inositol for Hashimoto’s Thyroiditis………………………253
Chapter 29: Bromine Detoxification with Unrefined Sea Salt…………….263
Chapter 30: Maternal Iodine Supplements and Smarter Children………267
Chapter 31: Breast Cancer Prevention with Iodine Supplementation….271
Chapter 32: Iodine Treats Breast Cancer, Overwhelming Evidence…..277
Chapter 33: The Thyroid Nodule Epidemic…………………………………….290
Chapter 34: Adrenal Insufficiency, HPA Dysfunction, and Fatigue…….299
Index………………………………………………………………………………………308
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
http://sci.med.narkive.com/covV2Qo2/jeffrey-dach-book-announcment-natural-medicine-101
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2023 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given. See Repost Guidelines.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
Published on July 9th, 2023 by Jeffrey Dach MD
The post Natural Thyroid Toolkit by Jeffrey Dach MD appeared first on Jeffrey Dach MD.
June 21, 2023
Reverse T3 Helpful or Waste of Time?
 Reverse T3, Helpful, or Waste of Time?
Reverse T3, Helpful, or Waste of Time?
by Jeffrey Dach MD
Linda is a stay at home mom sitting in my office with Hashimoto’s autoimmune thyroid disease with typical symptoms of fatigue, hair loss and weight gain despite taking levothyroxine 125 mcg/d prescribed by her endocrinologist. Linda asks me the question:
Why isn’t the thyroid pill working for me?
Linda’s initial thyroid labs last year before starting Levothyroxine, showed the following:
TSH of 8.4 (0.40-4.50 mIU/L)
Free T4 of 0.6 (0.8-1.8 ng/dL)
After seeing Linda in the office for the first visit, I sent Linda back to the Lab while still on the levothyroxine 125 mcg. These follow up labs showed:
TSH has decreased to 1.46 mIU/L (0.40-4.50 mIU/L)
Free T4 has increased to 1.4 ng/dL (0.8-1.8 ng/dL)
Free T3 was low end of range 240 ng/dL (range 230-420)
Reverse T3 was upper end of range 22 (range 8-25 ng/dL)
This laboratory panel is typical for patients not doing well with Levothyroxine. The TSH has gone down, and the Free T4 has gone up. The Free T3 is at the lower end of normal range, and the reverse T3 is at the upper end of the normal range. What does this mean? This means trouble.
The Deiodinase System
This lab pattern means the Linda’s D1 deiodinase system in the periphery is preferentially converting the levothyroxine (T4 monotherapy) to reverse T3, the inactive form of the thyroid hormone. At the same time, centrally in the hypothalamus and pituitary of the brain, the deiodinase system is converting the T4 to T3 normally. The pituitary responds to this abundant T3 by lowering the TSH to reduce production of thyroid hormone. The resulting lower TSH looks good to the endocrinologist thinking the thyroid function is normal, even though the patient is still suffering from peripheral cellular hypothyroidism. Typically, the endocrinologist will tell the patient the labs are perfect, and ignore the patient’s complaints of continued hypothyroid symptoms. The endocrinologist may give the patient a pat on the back and a referral to a psychiatrist for an SSRI antidepressant, obviously the wrong treatment.
Switching from Levo to NDT
We then switched Linda’s thyroid medication from the levothyroxine (Levo) to natural desiccated thyroid, NDT (NP thyroid from Acella). Linda was started on 60 mg/ day (one grain) and gradually increased to 120 mg/day (two grains), and then returned to the lab 6 weeks later for a follow up thyroid lab panel which showed:
TSH has decreased further to 0.26 mIU/L (0.40-4.50 mIU/L)
Free T4 has decreased to 1.0 ng/dL (0.8-1.8 ng/dL)
Free T3 has increased to 340 ng/dL (range 230-420)
Reverse T3 has decreased to 14 (range 8-25 ng/dL)
This shows the D1 deiodinase is working nicely, and the circulating Free T3 has gone up from the original 240 on Levo to 340 ng/dL on the NDT. The reverse T3 which had been higher, is now back down to the middle of the range. Linda now reports all her low thyroid symptoms have resolved and she is feeling so much better. This is a typical recurring scenario when seeing patients not doing well on Levothyroxine.
The typical pattern on levothyoxine shows skewed values: Although still within the lab range, the labs show a high reverse T3, high free T4 and low Free T3. This pattern indicates these patients will feel better switched to NDT. The reason for this is levothyroxine is T4 only mono-therapy, while NDT contains both T4 and T3, a form of combination therapy which can be replicated with the use of levothyroxine (T4) combined with generic cytomel (T3). Some endocrinologists are starting to use combination therapy. Most are not.
What is Happening at the Cellullar Level?
At the cellular level, Linda’s D1 and D2 deiodinase are being inhibited by the T4 in levothyroxine. The cells recognize the T4 load as hyperthyroidism, and in order to protect the cell, the D1 and D2 deiodinases are downregulated, while the D3 deiodinase is upregulated. The final result is lower free T3 causing tissue level hypothyroidism, and higher reverse T3, representing conversion of T4 to its inactive form. At the same time the TSH is looks good, at the lower end of the range because the D2 deiodinase in the hypothalamus and pituitary is a different type, relatively insensitive to the inhibitory effects of T4. Note: D1 deiodinase and D2 deiodinase convert T4 to T3, while D3 deiodinase converts T4 to reverse T3.
Free T3 to reverse T3 Ratio
Notice the Free T3 to reverse T3 ratio is very useful here. It alerts the astute physician to the problem with T4 monotherapy, showing the ineffectiveness of levothyroxine. The more levothyroxine the endocrinologist gives, the greater the inhibition of D1 deiodinase in the periphery, and the more profound is the cellular hypothyroidism.
Combination Therapy is the Solution to D1 Deiodinase Inhibition by Levothyroxine.
Animal studies show the solution to this problem is combination therapy with both T4 and T3. The only manufactured combination thyroid pill at the moment is NDT, natural desiccated thyroid, such as NP Thyroid from Acella or Armour from Abbvie. Another combination therapy is to add a small dose of generic Cytomel to the levothyroxine.
Animal Studies Show Only Combination T3 and T4 Restores T3 Metabolic Markers
D2 in the Pituitary (Centrally) Acts Differently from D2 in Periphery
According to a 2015 animal study by Dr. De Castro, the D2 deiodinase enzyme system in the pituitary acts differently from the D2 in the peripheral tissues. In the peripheral tissues, D2 is inactivated by T4. High T4 levels inactivate D2 deiodinase as a safety mechanism to
protect the cells from local hyperthyroidism. Elevated T4 levels inactivate the D2 enzyme in the peripheral tissues, thereby preventing the conversion of T4 to its active form, T3. However, the D2 in the hypothalamus and pituitary is a different type that is not inactivated by T4. In the hypothalamus and pituitary, the abundant T4 in circulation is promptly converted to intracellular T3, which then suppresses the TSH to low levels. This results in the pattern we see with Linda’s labs, relatively higher serum T4 and relatively lower serum T3. In the periphery, cells are starved of T3 because of the inactivation of the D2 enzyme by T4, thus inhibiting the conversion of T4 to T3 in the peripheral tissues.
Animal Studies of Combination Therapy
The benefit of combination therapy with both T3 and T4 was demonstrated in animal studies by Dr. de Castro. In 2015, Dr. De Castro studied the deiodinase system in mice, finding only constant infusion of both T4 and T3 normalized thyroid levels. Dr. De Castro writes:
These studies reveal that tissue-specific differences in D2 ubiquitination are an inherent property of the TRH/TSH feedback mechanism and indicate that only constant delivery of L-T4 and L-T3 fully normalizes T3-dependent metabolic markers and gene expression profiles in Tx rats.(6)

Above Image: Schematic of chemical structures of Thyroxine (T4), and conversion of T4 to either T3 (lower left) or reverse T3 (lower right) courtesy of Dr. Cristiane Gomes-Lima (1)
Header Image: Young woman sleeping, oil on canvas by
Domenico Fetti, circa 1615, Budapest Museum of Fine Arts. Courtesy of wikimedia.
The Reverse T3 Debate
Linda’s endocrinologist does not use the reverse T3 test, believing reverse T3 to be of no clinical value. Why is this? The medical literature says so. In 2019, Dr. Cristiane Gomes-Lima states there is no evidence to support the use of reverse T3 to monitor T4 monotherapy with levothyroxine. Dr Gomes-Lima writes:
Reverse T3 is physiologically relevant to thyroid economy. However, its clinical use as a biochemical parameter of thyroid function is very limited. Currently, no evidence supports the use of rT3 to monitor levothyroxine therapy, either given alone or in combination with liothyronine. (1)
In my opinion, future studies will demonstrate the above conclusion to be in error, and the pattern of higher reverse T3, higher Free T4, and lower Free T3 (within the lab range) will be adopted as a valid strategy for predicting good outcomes when switching from T4 monotherapy to NDT or combination T4/T3 therapy.
Free T4 and Reverse T3/Free T3 Ratio, A Useful Window into Status of Deiodinase System
In 1984, 25 years before Dr. Gomes-Lima wrote her article in 2019, Dr. Shimada studied T3, T4, and reverse T3 in 61 hyperthyroid, 31 hypothyroid patients, 8 subacute thyroiditis, and 40 normal
subjects. Dr. Shimada concluded “the relationship between serum T4 level and rT3/T3 ratio should be examined for adequate information concerning the peripheral conversion of thyroid hormones under various thyroid diseases.” Examining the Free T4 and ratio of free T3 to reverse T3 is a useful window into the status of the deiodinase system. If the T4 in levothyroxine is being preferentially converted to reverse T3, this is useful information the levothyroxine is not working, and best to try a combination drug containing both T4 and T3 such as NDT. In 1984 Dr. Shimada writes:
In order to clarify the conversion of thyroxine (T4) to triiodothyronine (T3) or to reverse T3 (rT3), serum concentrations of T4, T3, rT3, thyrotropin (TSH), thyroxine-binding globulin (TBG) and values of T3 uptake (T3U) were measured in 61 hyperthyroid and 31 hypothyroid patients, 8 patients with subacute thyroiditis, and 40 normal subjects. Then, free T4 index (FT4I), T3/T4, rT3/ T4, and rT3/T3 ratio were calculated…The rT3/T3 ratio was high in the hyperthyroid patients and low in the hypothyroid patients compared with that in the normal subjects…Our results indicated that thyroid hormones themselves could regulate the conversion of T4 to T3 or rT3 by activating 5-monodeiodinase [D3 deiodinase] in hyperthyroidism and by activating 5’-monodeiodinase [D2 deiodinase] and suppressing 5-monodeiodinase [D3 deiodinase] in hypothyroidism. Serum rT3 level was a more sensitive parameter than serum T4 or T3 for evaluating thyroid dysfunction….we concluded that the relationship between serum T4 level and rT3/T3 ratio should be examined for adequate information concerning the peripheral conversion of thyroid hormones under various thyroid diseases. (2)
Dr. Alan B. McDaniel in Townsend Letter
In agreement with Dr. Shamadzu is Dr. Alan B. McDaniel who says in 2021, “the ratio of tT3/ RT3 is the most accurate measure of the actual thyroid hormone function in the body,” writing in the Townsend Letter:
Unfortunately, TSH is often suppressed by the NDT [natural desiccated thyroid] dose that gives the best symptom-relief. My best explanation is that the patient’s thyroid gland continues making too much T4 (converted to RT3) until it is suppressed by NDT’s richer mix of T3. Simply put, more than 80% T4 is often too much…As long as blood levels of the thyroid hormones are normal, low TSH is no physiological problem. Low TSH does not damage bones – high T4 does! …However, some practitioners incorrectly assume low TSH means that you’ve made the patient hyperthyroid. So, your TSH-suppressed patient must understand this to defend her treatment from “good intentions.” …Over the years during which I logged hundreds of patients for whom T4-only treatment gave poor results, I also recorded many scores of patients who had suboptimal results from NDT thyroid. Here again, the “post-analytical analysis” of lab reports is so important! As with T4, incorrect dosing occurs—either too much or too little, as the patients above demonstrate—but by far the most frequent problem was dysfunctional deiodination of T4, indicated by low tT3/RT3…In closing, I’d like to remind you of the four most important points I have tried to prove in this review:
1. Thyroid hormone doses should be divided at least every 12 hours.
2. Therapeutic blood levels must be tested according to peak/trough fluctuations; preferably at mid-dose.
3. The ratio of tT3/ RT3 is the most accurate measure of the actual thyroid hormone function in the body.
4. Some people need to take T3 along with T4 for their best clinical results. (3) Note: RT3= reverse T3. Note: tT3=total T3.
Reverse T3, Helpful or Waste of Time?
In 2020, Dr. Theodore Friedman measured reverse T3 (rT3) in 98 consecutive hypothyroid patients seen in a tertiary Endocrinology clinic, all with severe fatigue, and many of them already treated with different thyroid preparations. Dr. Theodore Friedman writes:
Measuring rT3 may be helpful in patients who are already on T4-containing thyroid treatments who still have hypothyroid symptoms.
In 2021, Dr. Theodore Friedman again writes:
Measuring rT3 may be helpful in patients who are already on thyroid treatments, and is of greater importance in patients taking synthetic preparations [levothyroxine T4 monotherapy].
Conclusion: When someone asks about the Reverse T3, Helpful or a Waste of Time? You can say with confidence, yes it is helpful in the levothyroxine treated patient to determine if the levothyroxine is working, and if not, then the patient should be switched from levothyroxine to combination therapy with NDT. And no, it is not a waste of time to measure reverse T3.
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida, 33314
954-792-4662
Articles with Related Interest
All Thyroid Articles by Jeffrey Dach MD
Links and References:
1) Gomes-Lima, Cristiane, Leonard Wartofsky, and Kenneth Burman. “Can Reverse T3 Assay Be Employed to Guide T4 vs. T4/T3 Therapy in Hypothyroidism?.” Frontiers in Endocrinology 10 (2019): 856.
2) Shimada, T. “The Conversion of Thyroxine to Triiodothyronine (T3) or to Reverse T3 In Patients with Thyroid Dysfunction.” Nihon Naibunpi Gakkai Zasshi 60.3 (1984): 195-206
3) Diagnose and Treat Hypothyroidism in 2021, Part 3: New Endocrinology By Alan B. McDaniel, MD Townsend Letter
4) Friedman, Theodore C., and Julian B. Wilson. “SUN-410 Reverse T3 in Patients with Hypothyroidism, Helpful or a Waste of Time?.” Journal of the Endocrine Society 4.Supplement_1 (2020): SUN-410.
5) Wilson, Julian Bryant, and Theodore C. Friedman. “Reverse T3 in Patients With Hypothyroidism, Helpful or a Waste of Time?.” Journal of the Endocrine Society 5.Supplement_1 (2021): A952-A952.
6) De Castro, Joao Pedro Werneck, et al. “Differences in Hypothalamic Type 2 Deiodinase Ubiquitination Explain Localized Sensitivity to Thyroxine.” The Journal of Clinical Investigation 125.2 (2015): 769.
—————————————————————————–
Free T4 and Reverse T3/Free T3 Ratio, A Useful Window into Status of Deiodinase System
In 1984, Dr. Shimada studied T3, T4, and reverse T3 in 61 hyperthyroid, 31 hypothyroid patients, 8 subacute thyroiditis, and 40 normal
subjects. Dr. Shimada concluded “the relationship between serum T4 level and rT3/T3 ratio should be examined for adequate information
concerning the peripheral conversion of thyroid hormones under various thyroid diseases.” Examining the Free T4 and ratio of free T3 to reverse T3 is a useful window into the status of the deiodinase system.
If the T4 in levothyroxine is being preferentially converted to reverse T3, this is useful information the levothyroxine is not working, and best to try a combination drug containing both T4 and T3 such as NDT.
Dr. Shimada writes:
In order to clarify the conversion of thyroxine (T4) to triiodothyronine (T3) or to reverse T3 (rT3), serum concentrations of T4, T3, rT3, thyrotropin (TSH), thyroxine-binding globulin (TBG) and values of T3 uptake (T3
U) were measured in 61 hyperthyroid and 31 hypothyroid patients, 8 patients with subacute thyroiditis, and 40 normal subjects.
Then, free T4 index (FT4I), T3/T4, rT3/ T4, and rT3/T3 ratio were calculated…The rT3/T3 ratio was high in the hyperthyroid
patients and low in the hypothyroid patients compared with that in the normal subjects. …
Our results indicated that thyroid hormones themselves could regulate the conversion of T4 to T3 or rT3 by activating 5-monodeiodinase [D3 deiodinase] in hyperthyroidism and by activating 5’-monodeiodinase [D2 deiodinase] and suppressing 5-monodeiodinase [D3 deiodinase] in hypothyroidism. Serum rT3 level was a more sensitive parameter
than serum T4 or T3 for evaluating thyroid dysfunction….we concluded that the relationship between serum T4 level and rT3/T3 ratio should be examined for adequate information concerning the peripheral conversion of thyroid hormones under various thyroid diseases. (10)
Shimada, T. “The Conversion of Thyroxine to Triiodothyronine (T3) or to Reverse T3 In Patients with Thyroid Dysfunction.” Nihon Naibunpi Gakkai Zasshi 60.3 (1984): 195-206
—————————————–
In the hypothyroid patient on levothyroxine, the lab finding of shunting
to reverse T3 predicts the patient will do well switching from levothyroxine to NDT (natural desiccated thyroid), which contains a combination
of T3 and T4, the solution to T4 shunting to reverse T3. In these cases, the Free T4 may be higher than usually seen, and the Free T3
lower than usually seen. Unfortunately, the use of the reverse T3 test for this purpose has been ignored by conventional endocrinology which
dogmatically clings to the idea that reverse T3 has no clinical utility in monitoring levothyroxine therapy.
In 2019, Dr. Cristiane Gomes-Lima
writes:
Reverse T3 is physiologically relevant to thyroid economy. However, its clinical use as a biochemical parameter of thyroid function is very limited. Currently, no evidence supports the use of rT3 [reverse T3] to
monitor levothyroxine therapy, either given alone or in combination with liothyronine. (46-47)
In my opinion, future studies will demonstrate the above conclusion to be in error, and the pattern of higher reverse T3, higher Free T4, and lower Free T3 (within the lab range) will be adopted as a valid strategy for predicting good outcomes when switching from T4 monotherapy to NDT or combination T4/T3 therapy.
Gomes-Lima, Cristiane, Leonard Wartofsky, and Kenneth Burman. “Can Reverse T3 Assay Be Employed to Guide T4 vs. T4/T3 Therapy in Hypothyroidism?” Frontiers in Endocrinology 10 (2019): 856.
https://www.frontiersin.org/articles/...
Gomes-Lima, Cristiane, Leonard Wartofsky, and Kenneth Burman. “Can Reverse T3 Assay Be Employed to Guide T4 vs. T4/T3 Therapy in Hypothyroidism?.” Frontiers in endocrinology 10 (2019): 856.
Most physicians caring for hypothyroid patients on T4 monotherapy see a significant subset of subjects who still complain of symptoms suggestive of thyroid hormone insufficiency in spite of TSH levels within the reference range. The argument made is that these patients suffer from insufficient T3 generation from T4. To attempt to generate T3 levels equivalent to those seen with thyroidal secretion of T3, the potential role and efficacy of combination T4/T3 treatment has been assessed. Having a blood test like rT3 to successfully address appropriate dosing of a T4/T3 combination agent could allow clinicians to more effectively treat patients with primary hypothyroidism.
————————————————————
Chopra, Inder J., et al. “Opposite Effects of Dexamethasone on Serum Concentrations of 3, 3′, 5′-Triiodothyronine (Reverse T3) and 3, 3′,
5′-Triiodothyronine (T3).” The Journal of Clinical Endocrinology & Metabolism 41.5 (1975): 911-920.
Euthyroid Sick Syndrome
High reverse T3 may also be found in a condition referred to as “euthyroid sick syndrome” in acute or chronic illness, starvation, and eating disorders. Although elevated reverse T3 in chronic illness can be observed, it is poorly understood. Treatment with T3 (liothyronine)
is a matter of debate. Central hypothyroidism may resemble euthyroid sick syndrome, except that central hypothyroidism will have
a low reverse T3. In contrast, euthyroid sick syndrome will have a high reverse T3, as an adaptation to protect the severely ill patient
from excess thyroid hormone. (48-55)
After routinely measuring reverse T3 over the years, I have found this lab test usually confirms what we already know from the history,
physical exam, and other routine labs. For example, high reverse T3 is a protective mechanism in the thyrotoxic patient. A different scenario is
the reverse T3 in the normal range, yet higher than usually seen, a pattern found in patients who remain symptomatic while under treatment
with levothyroxine, as discussed above. Alternatively, a low reverse T3 usually confirms hypothyroidism in the untreated patient or confirms central hypothyroidism with HPA dysfunction.
Patients with central hypothyroidism typically show a low serum TSH even though they are clinically hypothyroid and have a low
Free T3 and T4. These patients benefit from treatment with thyroid hormone.
Reverse T3 Elevated in Metastatic Cancer
Occasionally, it may be challenging to interpret a high reverse T3. For example, I recall an asymptomatic 85-year-old female patient in no
acute distress. The thyroid labs were all normal except for an elevated reverse T3 of 28 ng/ dL (normal range= 10 – 24 ng/dL). Since the
patient seemed fine and the other thyroid labs were normal, I interpreted this as a lab error! A few weeks later, my office was informed the
patient developed shoulder pain; subsequent x-rays, and bone scans by the orthopedic surgeon showed lytic bone lesions indicating
extensive metastatic cancer. Sadly, the patient succumbed quickly to the extensive metastatic disease. In retrospect, the high reverse T3 was
a marker for metastatic cancer. (56-63)
In 2021 Dr. Annarita Nappi found D3 deiodinase which converts T4 to inactive reverse T3, is barely detectable in adult tissues yet is
upregulated in many cancer types and other chronic illnesses, thus explaining high reverse T3 levels in the cancer patient. Dr. Annarita
Nappi writes:
Although its expression is barely detectable in adult tissues, the D3 enzyme has been reactivated in several physiopathological
conditions in which cell proliferation is enhanced, such as chronic inflammation, myocardial infarction, tissue repair, and
critical illness… Interestingly, in adult life, D3 is also re-expressed in cancer. D3 was initially identified in various immortalized
cell lines derived from adenocarcinoma, breast cancer, endometrium carcinoma, neuroblastoma basal cell carcinoma, ovarian cancer, and colon. Accordingly, D3 is upregulated in many murine and human
tumors tissues, including the vascular tumors infantile hemangiomas and hepatic hemangioendothelioma as well as in various brain tumors, among which, gliosarcoma and glioblastoma multiforme. (60)
=============================
https://stopthethyroidmadness.com/rev...
Reverse T3 (also called Reverse Triiodothyronine)
======================
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
BEST
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Diagnose and Treat Hypothyroidism in 2021, Part 3: New Endocrinology
Diagnose and Treat Hypothyroidism in 2021, Part 3: New Endocrinology
By Alan B. McDaniel, MD
Unfortunately, TSH is often suppressed by the DTE dose that gives the best symptom-relief. My best explanation is that the patient’s thyroid gland continues making too much T4 (converted to RT3) until it is suppressed by DTE’s richer mix of T3. Simply put, more than 80% T4 is often too much.
As long as blood levels of the thyroid hormones are normal, low TSH is no physiological problem. Low TSH does not damage bones – high T4 does! 237
Low TSH doesn’t affect the heart; high T3 does.238
However, some practitioners incorrectly assume low TSH means that you’ve made the patient hyperthyroid. So, your TSH-suppressed patient must understand this to defend her treatment from “good intentions.”
Over the years during which I logged hundreds of patients for whom T4-only treatment gave poor results, I also recorded many scores of patients who had suboptimal results from DTE thyroid. Here again, the “post-analytical analysis” of lab reports is so important! As with T4, incorrect dosing occurs—either too much or too little, as the patients above demonstrate—but by far the most frequent problem was dysfunctional deiodination of T4, indicated by low tT3/RT3.
In closing, I’d like to remind you of the four most important points I have tried to prove in this review:
1. Thyroid hormone doses should be divided at least every 12 hours.
2. Therapeutic blood levels must be tested according to peak/trough fluctuations; preferably at mid-dose.
3. The ratio of tT3/ RT3 is the most accurate measure of the actual thyroid hormone function in the body.
4. Some people need to take T3 along with T4 for their best clinical results
https://www.optimaldx.com/blog/free-t...
Thyroid hormones are the spark plugs of metabolism.
Dicken Weatherby, N.D. and Beth Ellen DiLuglio, MS, RDN, LDN
Decreased FT3:FT4 ratio
A reduced FT3:FT4 ratio may indicate the use of T4 only therapy, hypothyroidism, selenium deficiency, disrupted deiodinase activity, and reduced production of T3 and free T3. A ratio of less than 2 suggests the presence of low T3 syndrome.[17] Critical illness, inflammation, and hypoxia may interfere with the conversion of T4 to T3 and increase degradation of T4, essentially leading to a rise in FT3:FT4 ratios.
================================================
https://pubmed.ncbi.nlm.nih.gov/19428...
Pimentel, Carlos Roberto Alves, et al. “Reverse T3 as a Parameter of Myocardial Function Impairment in Heart Failure.” International journal of cardiology 145.1 (2010): 52-53.
================================
free pdf
Click to access 9d19d173dbcd7334602b919ad5a4532824fd.pdf
Gomes-Lima, Cristiane. “Reverse T.” Cleveland Clinic Journal of Medicine 85.6 (2018): 451.
Unfortunately therefore, at the present time there are no data that support for or against the use of rT3 to monitor LT4 LT3 combination therapy.
Conclusion Reverse T3 is physiologically relevant to thyroid economy. However, its clinical use as a biochemical parameter of thyroid function is very limited. Currently, no evidence supports the use of rT3 to monitor levothyroxine therapy, either given alone or in combination with liothyronine.
————————-
2021
https://www.scirp.org/journal/paperin...
Exley, Sarah, Sonal Banzal, and Udaya Kabadi. “Low Reverse T3: A Reliable, Sensitive and Specific in Diagnosis of Central Hypothyroidism.” Open Journal of Endocrine and Metabolic Diseases 11.7 (2021): 137-143.
Reverse T3 is a reliable laboratory test differentiating between Central Hypothyroidism and “Euthyroid Sick Syndrome” in subjects with low free T4 and low/normal TSH levels.
Results: Reverse T3 established two distinct groups: 1) subnormal concentrations, 8.31 ± 0.52 [range, 11 – 14 ng/dl]; 2) supernormal levels; 32 ± 4 [normal Range 12 – 26]. Free T3 concentrations were subnormal or normal, 1.6 – 2.9 [normal range, 2.3 – 4.2 ng/ml] in individuals amongst both groups. On reassessment after 3 – 6 weeks, free T4, free T3, TSH and reverse T3 normalized in group with normal or elevated reverse T3 indicating recovery from “Euthyroid Sick Syndrome” whereas free T4 and reverse T3 remained subnormal in the other group suggesting presence of Central Hypothyroidism. Conclusion: Reverse T3 is a reliable laboratory test differentiating between Central Hypothyroidism and “Euthyroid Sick Syndrome” in subjects with low free T4 and low/normal TSH levels.
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
HELPFUL OR WASTE OF TIME ?
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
free pdf
Friedman, Theodore C., and Julian B. Wilson. “SUN-410 Reverse T3 in Patients with Hypothyroidism, Helpful or a Waste of Time?.” Journal of the Endocrine Society 4.Supplement_1 (2020): SUN-410.
Methods rT3 was measured in 98 consecutive patients seen
in a tertiary Endocrinology clinic with possible or confirmed
hypothyroidism (all with severe fatigue) with many of them
were already treated with different thyroid preparations.
Results: The figure shows the 25%-75% quartiles, ranges
and ratio of rT3 above the normal range/patients in that
category. The cutoff of 24 ng/dL (upper limit of normal for
rT3 at either Quest or LabCorp) is indicated by the line.
Overall, 18 of the 98 patients had a rT3 above the normal
range. Patients on L-T4 alone or desiccated thyroid plus
L-T4 had the highest levels of rT3 and the highest % above
the cut-off. Three of the patients with a high rT3 were not on
any thyroid medicine, and in 2 of them, the rT3 normalized
when repeated. The 8 patients with a high rT3 on L-T4 was
a relatively high percentage (29%).
Measuring rT3 may be helpful in patients who are already on T4-containing thyroid treatments who still have hypothyroid symptoms.
——————————————- —
Wilson, Julian Bryant, and Theodore C. Friedman. “Reverse T3 in Patients With Hypothyroidism, Helpful or a Waste of Time?.” Journal of the Endocrine Society 5.Supplement_1 (2021): A952-A952.
Background: Reverse T3 (rT3) is a biologically inactive form of T3 that is created by peripheral 5 deiodination of T4 by type 1 and type 3 deiodinases and may block T3 binding to the thyroid hormone receptor. As about 15% of patients on L-T4 replacement with a normalized TSH report continued fatigue and other hypothyroid symptoms, efforts are needed to understand why this occurs and how it can be corrected. Decades ago, endocrinologists realized that in severe illnesses, rT3 is often high and T3 is often low and termed this “sick euthyroid syndrome”. However, more recently, alternative doctors, including functional medicine doctors, have argued that high rT3 is detrimental and can block T3 from binding to the thyroid hormone receptor. Without peer-reviewed publications, these functional medicine doctors rely heavily on rT3 levels to treat patients that may have no other laboratory findings of hypothyroidism and often prescribe them L-T3-only preparations to try to lower the rT3. Also poorly characterized in the literature are the effects of hypercortisolism and hypopituitarism, both of which should modulate the expression of deiodinases to increase rT3. Hypotheses: 1) Patient rT3 levels will vary significantly with the type of thyroid medication taken. 2) Patient rT3 levels will be clinically significant in the management of patients on thyroid medications. 3) Hypercortisolism and hypopituitarism will increase rT3 levels. Methods: The most recent rT3 measurements were analyzed from 621 patients currently being managed by TCF. The upper limit of normal for rT3 at either Quest or LabCorp, which is usually 24.1 ng/dL was used as a cut-off for a high result and below 9.2 ng/dL as the low cut-off. Results: Elevate rT3 levels was seen in 3% of patients of patients not on thyroid replacement (5/143), seen in 8% of patients (17/203) taking desiccated thyroid. It was more prevalent in patients taking desiccated thyroid with synthetic T3 (27%, 7/26) or T4 (15%, 16/104) and was seen in 15% of patients (9/58) taking synthetic T4 alone. Changes were made to the amount or type of medications in 199 patients. Levels of rT3 levels were outside the normal range in 27% of these patients (54/199), being above normal range in 16% of these patients (32/199). Hypercortisolism was seen in 37 patients, 36 from Cushing’s disease, however above normal rT3 was seen in only 2 patients. Hypopituitarism was diagnosed in 22 patients, only one had above normal rT3 levels because they couldn’t afford their growth hormone replacement.
Conclusion: Measuring rT3 may be helpful in patients who are already on thyroid treatments, and is of greater importance in patients taking synthetic preparations. It is not recommended in patients who are not taking thyroid medicine (even if experiencing hypercortisolism) or in patients with hypopituitarism that are taking adequate hormone replacement.
https://pubmed.ncbi.nlm.nih.gov/31581...
McKeever, Liam, et al. “Higher caloric exposure in critically ill patients transiently accelerates thyroid hormone activation.” The Journal of Clinical Endocrinology & Metabolism 105.2 (2020): 523-533.
Higher caloric exposure in NTIS patients transiently attenuates the drop of the plasma T3/rT3 ratio, an effect that is minimized and finally lost over the following 3 days of continued higher caloric exposure.
—————————————————
https://pubmed.ncbi.nlm.nih.gov/30943...
Lin, Hung-Yun, et al. “Action of reverse T3 on cancer cells.” Endocrine Research 44.4 (2019): 148-152.
Background: Reverse T3 (rT3; 3,3′,5′-triiodo-L-thyronine) is widely regarded as an inactive naturally occurring analog of thyroid hormone. rT3 is known to bind to the thyroid hormone analog receptor on plasma membrane integrin αvβ3. This integrin is generously expressed by tumor cells and is the initiation site for the stimulation by L-thyroxine (T4) at physiological free concentrations on cancer cell proliferation. Results: In the present studies, we show that rT3 caused increases of proliferation in vitro of 50% to 80% (P < 0.05-0.001) of human breast cancer and glioblastoma cells. Conclusion: rT3 may be a host factor supporting cancer growth.
—————————–
1995
https://pubmed.ncbi.nlm.nih.gov/8808092/
Burmeister, Lynn A. “Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome.” Thyroid 5.6 (1995): 435-441.
To assess the efficacy of reverse T3 in differentiating between the hypothyroid and euthyroid state in the setting of illness, all reverse T3 determinations obtained over a 4-year period in a University teaching hospital were analyzed in the context of concurrent thyroid function tests, bilirubin, albumin, creatinine, subsequent treatment, and follow-up. Based on T4 (or free T4 index) and TSH, the thyroidal state of the patient and the appropriateness of the reverse T3 determination were assigned. A total of 262 reverse T3 determinations were made in 246 patients. There is an inverse linear relationship between the log TSH and the reverse T3. Patients with hypothyroidism plus illness may have a normal reverse T3 and patients with euthyroidism may have a low reverse T3. Reverse T3 is linearly related to bilirubin up to a bilirubin of approximately 171 microM (10 mg/dL). Sixty percent of the reverse T3 determinations were obtained for seemingly inappropriate indications. In association with a low free T4 index/T4, an unmeasurable reverse T3 did not lead to institution of thyroid hormone treatment in over 52% of cases. Although reverse T3 may be elevated in the setting of nonthyroidal illness, it is not reliable in distinguishing between the hypothyroid sick patient and the euthyroid sick patient. This is probably because of drug and disease effects on thyroid hormone metabolism as well as the presence of sufficient T4 substrate for conversion to reverse T3 in many hypothyroid sick patients.
—————————-
eating disorder
Eating Disorders – Treatment with Thyroid Hormone?
Anorexia is an eating disorder and is considered
a psychiatric disease. Patients with
anorexia may appear cachectic from starvation,
and as such, can represent a life-threatening
medical emergency requiring hospitalization
and hyper-alimentation. (70-72)
In 2011, Dr. Michelle Warren reviewed the
endocrine manifestations of eating disorders,
finding a similarity with the euthyroid sick syndrome,
with low Free T3 and high reverse T3,
a pattern suggesting central hypothyroidism,
writing:
Also typical in anorexia are changes seen
with the euthyroid sick syndrome. T3 levels
are low, whereas rT3 [reverse T3] is elevated.
In some patients, T4 is also decreased. TSH
levels are normal or occasionally slightly
reduced, suggesting a hypothalamic origin of
the suppressed thyroid function…Treatment
with thyroid hormone is inappropriate and
leads to undesirable weight loss and loss of
muscle mass. (73)
On the other hand, Dr. Richard Shames disagrees
with Dr. Warren regarding thyroid hormone
treatment for eating disorders. In 2022,
Dr. Richard Shames found eating disorder
patients have the low-T3 syndrome and should
be treated with T3-containing thyroid medication
with good results. He says T3 “acts directly
on the hypothalamus to stimulate feeding.” Dr.
Richard Shames writes:
Calorie restriction reduces circulating
triiodothyronine (T3) – the most active
thyroid hormone – inducing hypothyroidism,
constipation, and reduced appetite that
inhibit eating, acting to sustain and
sometimes precipitate eating disorders.
Thyroid-hormone treatment can be
effective but is rarely employed… Circulating
T3 levels decrease in eating disorders (EDs)
and in most severe and chronic illnesses
in the eponymous medical condition low-
T3 syndrome (LT3S)… LT3S occurs broadly
in severe and chronic illnesses including
trauma, sepsis, heart failure, COVID-19, and
during calorie restriction aside from EDs…
LT3S has major role in sustaining eating
disorders by causing chronic constipation
that inhibits eating and weight gain. T3
also acts directly on the hypothalamus to
stimulate feeding (independent of energy
expenditure). Reduced circulating T3 in LT3S
is likely a factor in appetite suppression…
Though thyroid-hormone dysfunction is
central to EDs, thyroid-hormone treatment
is rarely considered by doctors or presented
as an option to patients. Emphasis Mine
(Reference Shames 2022) Note: Euthyroid
sick syndrome is synonymous with low-T3
syndrome. (74)
Warren, Michelle P. “Endocrine Manifestations
of Eating Disorders.” The Journal of Clinical
Endocrinology & Metabolism 96.2 (2011): 333-343.
Shames, Richard, and Stuart Wenzel. “On the
Fundamental Efficacy of Thyroid Hormone Therapy
in Eating Disorders: Review of Mechanisms and Case
Study.” Journal of Restorative Medicine 12.1 (2022).
————————————————
https://europepmc.org/article/med/669...
Desai, M., et al. “The importance of reverse triiodothyronine in hypothyroid children on replacement treatment.” Archives of disease in childhood 59.1 (1984): 30-35.
Reverse triiodothyronine (rT3), triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) values were measured by radioimmunoassay in 40 children with congenital hypothyroidism who were being given levothyroxine (0.05-0.35 mg/day) and in 14 normal controls. In 15 of the children with hypothyroidism the treatment, judged by serum T4 and TSH values and thyrotrophin releasing hormone (TRH) test, seemed to be adequate and their mean rT3 value and rT3:T4 ratio were comparable with the controls. The remaining 25 children had a raised serum T4 and a low TSH value. Only 4 (16%) of these children had an abnormally high T3 concentration but the rT3 value was raised in 23 (92%) and their mean rT3 value and rT3:T4 ratio were significantly higher than in the control children. Less than 20% of this ‘overtreated’ group, however, had clinical hyperthyroidism. We suggest that in patients on T4 replacement treatment the peripheral thyroid homeostatic mechanisms produce larger amounts of rT3, thereby preventing high T3 values where serum T4 values are raised. This may explain why the ‘overtreated’ children showed no clinical evidence of hyperthyroidism. These findings emphasise the protective and selective role of peripheral monodeiodination.
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
http://sci.med.narkive.com/covV2Qo2/jeffrey-dach-book-announcment-natural-medicine-101
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2023 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given. See Repost Guidelines.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
Published on June 21st, 2023 by Jeffrey Dach MD
The post Reverse T3 Helpful or Waste of Time? appeared first on Jeffrey Dach MD.
April 27, 2023
New Weight Loss Anti-Diabetic Drugs Ozempic and GLP-1 Receptor Agonists
 New Weight Loss Anti-Diabetic Drugs Ozempic and GLP-1 Receptor Agonists by Jeffrey Dach MD
New Weight Loss Anti-Diabetic Drugs Ozempic and GLP-1 Receptor Agonists by Jeffrey Dach MD
What is the big deal about the new FDA approved weight loss anti-diabetic drugs such as Ozempic and other GLP-1 Receptor Agonists, liraglutide and semaglutide ? Maybe it is the celebrities going nuts on social media for these new weight loss drugs, thus creating massive demand and manufacturing shortages. April 25, 2023, Peter Loftus of The Wall Street Journal writes:
Demand for medications including Ozempic, for weight loss use, led to shortages that sometimes deprived people with diabetes of their prescription refills…A drug approved by the Food and Drug Administration to treat people with Type 2 diabetes has ignited a craze among social-media influencers, the rich and famous and everyday people alike. Ozempic, made by Novo Nordisk A/S, has gained popularity for its off-label use, helping users drop excess pounds within a matter of months. (7)
Header image, young woman wearing loose jeans courtesy of wikimedia commons.
The New Miracle Treatment for Weight Loss
In April 2023, Natan Ponieman, Editor for Benzinga writes demand for GLP-1 drugs for obesity may propel sales to $100 billion annually by 2031:
Ozempic, Wegovy and Mounjaro could very well have become the most sought-after weight-loss drugs of the past two years, as press reports and social media amplification help push these new medications as a new “miracle treatment” for weight management...A February analysis by Jefferies puts sales of GLP-1 drugs for obesity above $100 billion by 2031. But for the time being, most of these drugs continue to officially address the diabetes market, which could cause problems for the patients who need them the most.(9)
Weight Loss for Non-Diabetics
Although the GLP-1 agonist class of drugs were originally intended as anti-diabetic drugs to control blood sugar, these drugs were found to have significant weight loss effects as well. These weight loss effects in diabetes were found to extend to non-diabetics, making these drugs a viable competitor to bariatric surgery for the obese. (2)
Broad Health Benefits
GLP-1 agonists are incretin analogs and have additional broad health benefits, such as reduction in all-cause mortality, reduced rates for myocardial infarction, stroke. One must also include protective effects on kidney and brain function with reduction in dementia and cognitive impairment.Roughly half the patients treated with these drugs reach Hemoglobin A1C values below 5.7% (normal). Obese patients experience 20% or more weight loss. These new GLP-1 receptor agonists are on track to radically change modern medicine’s approach to treating Type Two Diabetes and Obesity. In 2022, Dr. Darlene M. Sanders, writes:
Recent studies show that incretin hormone analogues effectively control blood glucose while producing major weight losses and reducing the risk of all-cause mortality, myocardial infarction, stroke and kidney function impairment. Furthermore, the risk of dementia and cognitive impairment is reduced. A monomolecular coagonist (tirzepatide) of receptors for both incretin hormones (glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide) produced HbA1c values below 5.7% in 50% of the treated patients and weight losses exceeding 20% in obese individuals. These new agents will radically change our approach to the treatment of T2DM and obesity alike….Glucagon-like peptide-1 receptor agonists access specific brain areas important for appetite regulation, resulting in weight loss. These mechanisms may help explain how treatment with GLP-1 agonists led to reduced appetite and food cravings and better control of eating. Evidence supports both GLP-1 agonists liraglutide and semaglutide as effective agents for weight loss in patients with obesity without diabetes, with semaglutide data providing a more significant weight loss in clinical trials. Although GLP-1 agonists have side effects, the weight loss benefits may outweigh their risks. (2)
Not for Type One Diabetes, Only for Type Two
The benefits for Type Two Diabetics are not extended to Type One Diabetes, involving a different mechanism, namely, autoimmune destruction of the Beta Cells of the pancreas. These are the cells that produce insulin. In 2020, Dr. Maria Redondo found no apparent benefit for beta-cell function or glycemia using GLP-1 receptor agonists as adjuvant therapy in type one diabetes. As a result, semaglutide type drugs are not routinely recommended for Type One Diabetics. (13)
Another matter for concern, in 2023, Dr. Khary Edwards found more semaglutide users experienced diabetic ketoacidosis (DKA) at a rate of 12.8% of the study population. This is not a good thing. (14-16)
Not a Miracle Drug at All
In March 2023, Zee Krstic writes in Good Housekeeping magazine about his personal experience with Semaglutide. Zee was obese his entire life and was concerned when his doctor found his diabetic marker was very high. Zee’s Hemoglobin A1C was 9.8% (very high). After 5 months on treatment with Ozempic, his HgbA1C declined to normal, 5.4%, and he had lost 64 pounds. Yes, Zee did lose 64 pounds, yet he found Semaglutide not a “miracle drug” because of the significant diet and lifestyle modifications needed. For example, Zee wore a continuous glucose monitor, and found the drug associated with a long list of adverse side effects, most notably constipation, nausea, diarrhea, etc. Zee writes:
I also must wear a continuous glucose monitor to ensure anything I eat doesn’t impact my blood sugar control…Even those with diabetes, who found that Ozempic successfully tamed blood sugar levels, put up with nausea, vomiting and abdominal pain, and commonly, either diarrhea or a total blockage in firm constipation. Semaglutide may also lead to pancreatitis, some forms of kidney failure or thyroid tumors in some. Stories of people dealing with dire hospitalizations stemming from incessant vomiting or even heart failure became commonplace in most reports. Most patients experience the worst symptoms as dosages are initially ramped up, with your gastrointestinal tract beginning to slow down…The symptom that caused me the most pain was constipation. Within five days of my first dose, my digestive tract slowed to a crawl, and I simply couldn’t go. Almost four days went by before I woke up one morning with crippling pain in my lower gut â it took an hour on the toilet to relieve myself. I suffered a tear in my rectum that had me lying on my stomach for the next 18 hours, crying in pain…For the first few weeks, I was so nauseous that I wouldn’t eat normally for up to 2 or 3 days afterward, skipping meals altogether. Even now, after being settled on a 1mg dose for over four months, I still feel sore after injections for at least 12 hours. My constipation eventually swung in the opposite direction and stayed there â I had severe diarrhea that lasted on and off for two weeks each time I changed my dose, per my doctor’s directions. Dr. Mathur says my experience is typical. (10)
High Cost and Limited Availability
A major barrier to use of the drugs is high cost and limited availability. For example one carton of pre-filled 4 mg Ozempic pens (Novo-Nordisc)Â costs approximately $1,000 according to GoodRx.
Conclusion:
The GLP-1 receptor agonist class of drugs is a major advance and will no doubt change the medical landscape for management of Diabetes and Obesity.
Articles with Related Interest:
Diabetes, Arterial Calcification and Statin Drugs
Thiamine Deficiency and Diabetes
Improve Insulin Resistant Type 2 Diabetes
Metformin The Good Anti-Diabetes Drug
Testosterone Found Beneficial For Diabetes
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Key Words: GLP-1 receptor agonist incretin pancreas insulin Ozempic Wegovy Semaglutide glucagon-like peptide-1
Books:
OZEMPIC (SEMAGLUTIDE) SIMPLIFIED: ALSO WITH 50 QUESTIONS YOU ALWAYS ASK AND DOCTORS ANSWERS ABOUT HOW OZEMPIC (SEMAGLUTIDE) HELPS CURE DIABETES AND OBESITY. Paperback â Large Print, August 12, 2022 by DR JOHN JAX (Author)
Life On Semaglutide Kody and Krystal Dunn (Author)
During this Podcast Kody and Krystal will take you on there adventure of obtaining, using and everything in between of this new weight loss drug called Semaglutide.
Weight Loss Unlocked: The GLP-1 Breakthrough: Introduction to medicines like Wegovy and Mounjaro by Lisa Pieranunzi MD (Author) Format: Kindle Edition
The Semaglutide Way: A Scientific Approach to Losing Weight Kindle Edition by Scarlett Byrd (Author) Format: Kindle Edition
Achieve Weight Loss Journal: Designed For Those Taking Weekly Weight Loss Injections Paperback â January 3, 2023
by Julie N Hagaman (Author)
Ozempic (semaglutide): A Comprehensive Guide book that teach about Antidiabetes medication used for the treatment of type 2 diabetes and Anti-Obesity medication used for Long-term weight management Paperback â Large Print, April 2, 2023
by Dr. Olivia H. Philip (Author)
OZEMPIC (SEMIGLUTIDE)
by DR. CARL T. KEY | Aug 9, 2018
Links and References:
1) Mahapatra, Manoj K., Muthukumar Karuppasamy, and Biswa M. Sahoo. “Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: a narrative review.” Pharmaceutical Research 39.6 (2022): 1233-1248.
Many GLP-1 receptor agonists have shown hepatoprotective and neuroprotective activity in animal and human trials. As semaglutide is an already clinically approved drug, successful human trials would hasten its inclusion into therapeutic treatment of NASH and neurodegenerative diseases. Semaglutide improves insulin resistance, insulin signalling pathway, and reduce body weight which are responsible for prevention or progression of NASH and neurodegenerative diseases.
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
2) June 16, 2022 Efficacy of GLP-1 Agonists for Weight Loss in Adults Without Diabetes , Darlene M. Sanders, DMSc, MPAS, PA-C
Recent studies show that incretin hormone analogues effectively control blood glucose while producing major weight losses and reducing the risk of all-cause mortality, myocardial infarction, stroke and kidney function impairment. Furthermore, the risk of dementia and cognitive impairment is reduced. A monomolecular coagonist (tirzepatide) of receptors for both incretin hormones (glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide) produced HbA1c values below 5.7% in
50% of the treated patients and weight losses exceeding 20% in obese individuals. These new agents will radically change our approach to the treatment of T2DM and obesity alike.
Glucagon-like peptide-1 receptor agonists access specific brain areas important for appetite regulation, resulting in weight loss.17
These mechanisms may help explain how treatment with GLP-1 agonists led to reduced appetite and food cravings and better control of eating.3,17
Evidence supports both GLP-1 agonists liraglutide and semaglutide as effective agents for weight loss in patients with obesity without diabetes,
with semaglutide data providing a more significant weight loss in clinical trials.
Although GLP-1 agonists have side effects, the weight loss benefits may outweigh their risks.
3) pdf
Holst, Jens Juul. “Incretin-based therapy of metabolic disease.” Danish Medical Journal 70.1 (2022): A10220597-A10220597.
Incretin analogues (semaglutide, tirzepatide) provide effective glucose control in type 2 diabetes mellitus (T2DM) and major weight losses in people without diabetes. HbA1c may reach normal levels in 50% of patients and weight losses by > 20% may be achieved. The agents reduce the risk of mortality, myocardial infarction, stroke, reduced kidney function, dementia and cognitive impairment. These new agents will change our therapy of T2DM and obesity
4) Holst, Jens Juul. “The physiology of glucagon-like peptide 1.” Physiological reviews 87.4 (2007): 1409-1439.
Glucagon-like peptide 1 (GLP-1) is a 30-amino acid peptide hormone produced in the intestinal epithelial endocrine L-cells by differential processing of proglucagon, the gene which is expressed in these cells. The current knowledge regarding regulation of proglucagon gene expression in the gut and in the brain and mechanisms responsible for the posttranslational processing are reviewed. GLP-1 is released in response to meal intake, and the stimuli and molecular mechanisms involved are discussed. GLP-1 is extremely rapidly metabolized and inactivated by the enzyme dipeptidyl peptidase IV even before the hormone has left the gut, raising the possibility that the actions of GLP-1 are transmitted via sensory neurons in the intestine and the liver expressing the GLP-1 receptor. Because of this, it is important to distinguish between measurements of the intact hormone (responsible for endocrine actions) or the sum of the intact hormone and its metabolites, reflecting the total L-cell secretion and therefore also the possible neural actions. The main actions of GLP-1 are to stimulate insulin secretion (i.e., to act as an incretin hormone) and to inhibit glucagon secretion, thereby contributing to limit postprandial glucose excursions. It also inhibits gastrointestinal motility and secretion and thus acts as an enterogastrone and part of the “ileal brake” mechanism. GLP-1 also appears to be a physiological regulator of appetite and food intake. Because of these actions, GLP-1 or GLP-1 receptor agonists are currently being evaluated for the therapy of type 2 diabetes. Decreased secretion of GLP-1 may contribute to the development of obesity, and exaggerated secretion may be responsible for postprandial reactive hypoglycemia.
5) pdf
Bergmann, Natasha Chidekel, et al. “Semaglutide for the treatment of overweight and obesity: A review.” Diabetes, Obesity and Metabolism 25.1 (2023): 18-35.
6) https://www.mdpi.com/2077-0383/12/5/1909
Genser, Laurent, Dominique Thabut, and Judith Aron-Wisnewsky. “Precision Bariatric/Metabolic Medicine and Surgery.” Journal of Clinical Medicine 12.5 (2023): 1909.
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
7) What Is Ozempic and why is it such a big deal right now? FOX Business April 25, 2023 By Peter Loftus The Wall Street Journal
Demand for medications including Ozempic, for weight loss use, led to shortages that sometimes deprived people with diabetes of their prescription refills.
A drug approved by the Food and Drug Administration to treat people with Type 2 diabetes has ignited a craze among social-media influencers, the rich and famous and everyday people alike. Ozempic, made by Novo Nordisk A/S, has gained popularity for its off-label use, helping users drop excess pounds within a matter of months.
8) FDA News Release September 20, 2019
FDA approves first oral GLP-1 treatment for type 2 diabetes
The U.S. Food and Drug Administration today approved Rybelsus (semaglutide) oral tablets to improve control of blood sugar in adult patients with type 2 diabetes, along with diet and exercise. Rybelsus is the first glucagon-like peptide (GLP-1) receptor protein treatment approved for use in the United States that does not need to be injected. GLP-1 drugs are non-insulin treatments for people with type 2 diabetes.
9)Â Want To Lose 10 Pounds? ‘Miracle’ Weight Loss Drugs Create Winners And Losers
by Natan Ponieman, Benzinga Editor April 18, 2023
Ozempic, Wegovy and Mounjaro could very well have become the most sought-after weight-loss drugs of the past two years, as press reports and social media amplification help push these new medications as a new “miracle treatment” for weight management.
A February analysis by Jefferies puts sales of GLP-1 drugs for obesity above $100 billion by 2031. But for the time being, most of these drugs continue to officially address the diabetes market, which could cause problems for the patients who need them the most.
===========================================
10) I Lost 64 Pounds on Ozempic, But I’m Here to Tell You It’s Not a Miracle Weight Loss Drug   If you’re thinking a once-weekly injection will magically help you lose weight, think again. By Zee Krstic Mar 11, 2023 Medically reviewed by Rekha B. Kumar, M.D. and Joshua J. Joseph, M.D., M.P.H, FAHA Good Housekeeping
GLP-1 receptor agonist incretin pancreas insulin Ozempic Wegovy Semaglutide glucagon-like peptide-1,
The medication’s manufacturer, Novo Nordisk,
Semaglutide is an injectable medication, known to doctors as a GLP-1 receptor agonist. It mimics a gut hormone called incretin, prompting the pancreas to naturally produce more insulin when blood sugar levels are high, something that the pancreas usually does on its own. Long before it was regarded as a so-called “miracle” drug, Ozempic attracted the attention of obesity medicine specialists after extensive clinical trials in 2017 revealed weight loss was a significant side effect of the medication. Since over 80% of those with type 2 diabetes are clinically obese,
“When you look at studies that were done for [semaglutide use] in both obesity and diabetes, they were all in conjunction with lifestyle changes,” says Ruchi Mathur, M.D., an endocrinologist specializing in thyroid disorders at Cedars-Sinai Medical Center in Los Angeles. “Those clinical studies were all done with a calorie deficit of at least 500 calories a day, along with an exercise goal of about 150 minutes a week, plus monthly healthcare check-ins to ensure lifestyle was being optimized⦠Benefits are always achieved in conjunction with lifestyle changes, it can never occur without them.”
I also must wear a continuous glucose monitor to ensure anything I eat doesn’t impact my blood sugar control.
Even those with diabetes, who found that Ozempic successfully tamed blood sugar levels, put up with nausea, vomiting and abdominal pain, and commonly, either diarrhea or a total blockage in firm constipation. Semaglutide may also lead to pancreatitis, some forms of kidney failure or thyroid tumors in some. Stories of people dealing with dire hospitalizations stemming from incessant vomiting or even heart failure became commonplace in most reports. Most patients experience the worst symptoms as dosages are initially ramped up, with your gastrointestinal tract beginning to slow down, Dr. Mahali explains.
The symptom that caused me the most pain was constipation. Within five days of my first dose, my digestive tract slowed to a crawl, and I simply couldn’t go. Almost four days went by before I woke up one morning with crippling pain in my lower gut â it took an hour on the toilet to relieve myself. I suffered a tear in my rectum that had me lying on my stomach for the next 18 hours, crying in pain.
For the first few weeks, I was so nauseous that I wouldn’t eat normally for up to 2 or 3 days afterward, skipping meals altogether. Even now, after being settled on a 1mg dose for over four months, I still feel sore after injections for at least 12 hours. My constipation eventually swung in the opposite direction and stayed there â I had severe diarrhea that lasted on and off for two weeks each time I changed my dose, per my doctor’s directions. Dr. Mathur says my experience is typical.
Phase 1: 0.25mg
All patients start at a dosage of 0.25mg once weekly; they’ll be closely monitored by doctors while on this dose for at least two weeks, if not a full month. Early on-set symptoms may feel severe but may progress as doses increase.
Phase 2: 0.5mg
Patients graduate to a weekly 0.5mg dose if they can tolerate Ozempic well, and stay on this dose for at least a month, per manufacturer instructions. This is when many experience the bulk of their symptoms, doctors tell us.
Phase 3: 1mg
Those who need further blood sugar control may continue onto 1mg doses once each week, which is when the author’s own healthcare provider completed HbA1C tests to determine Ozempic’s efficacy in his case. He currently remains on 1mg weekly injections, more than 7 months into his treatment.
Phase 4: 2mg
The maximum dosage for Ozempic is 2mg, and is only used after patients remain at 1mg for at least one month, if not longer. Missing more than two doses at this strength means patients will likely start the schedule from 0.25mg once they resume injections.
=======================================================
11)Â Wegovy vs Ozempic for Weight Loss
Mochi Health, where board-certified obesity medicine physicians
Dr. Constantine Joseph Pella, MD
Boston University Medical Center
Wegovy (semaglutide) and Ozempic (semaglutide) are two prescription weight-loss medications that are cut from the same cloth. Both medications belong to the same class of drugs called glucagon-like peptide-1 (GLP-1) receptor agonists, and they work by mimicking the action of the hormone GLP-1, which regulates glucose metabolism and appetite. Despite their similarities, they are not interchangeable. There are differences between Wegovy and Ozempic that patients and healthcare providers should be aware of. In this blog post, we will compare and contrast the two medications to help you make an informed decision about which one may be right for you. April 21, 2023
=======================================================
12) A new treatment for obesity September 10, 2021
By Chika Anekwe, MD, MPH, Contributor; Editorial Advisory Board Member, Harvard Health Publishing
On June 4, 2021, the FDA announced the long-anticipated approval of Wegovy, an injectable medication taken once per week for weight management.
Wegovy received significant media attention in the months preceding approval, with a New York Times article declaring it a “game changer,” MedPage Today reporting its “unprecedented results,” and the BBC announcing it could mark a “new era” in treating obesity.
Wegovy, like all other prescription medications designated for the treatment of obesity, is approved for use in those with a body mass index (BMI) of 30 kg/m2 or greater, or those with a BMI of 27 kg/m2 with a weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol.
The widely reported STEP 1 trial, the results of which were published in the New England Journal of Medicine, demonstrated an average of 14.9% body weight reduction after 68 weeks of therapy in those assigned to the medication group, versus only 2.4% weight loss in those assigned to the placebo group. The average weight loss seen with existing anti-obesity medications is typically about 5% to 9%, while those engaged in lifestyle and behavioral therapy alone are expected to lose only 3% to 5% of their body weight.
The most common side effects of Wegovy are nausea, diarrhea, vomiting, and constipation. The medication also comes with a warning for risk of a specific tumor of the thyroid, and thus it is not recommended for those with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 (a genetic condition associated with endocrine tumors). It should be noted that tumors were only observed in animal studies, and not seen in the human trials.
Wegovy is the latest in a line of medications, starting with phentermine in 1959, that have achieved FDA approval for the treatment of obesity. Currently there are 10 FDA-approved anti-obesity medications in the US: phentermine, diethylpropion, benzphetamine, phendimetrazine, orlistat, phentermine/topiramate ER (Qsymia), bupropion/naltrexone (Contrave), liraglutide (Saxenda), setmelanotide (Imcivree), and now semaglutide (Wegovy). Of note, setmelanotide is only approved for the treatment of obesity caused by specific, rare genetic conditions. Other medications such as metformin, zonisamide, and other GLP-1 RAs normally used for treating diabetes are often prescribed “off label” and at the discretion of the prescriber for the treatment of obesity.
=======================================================Type One Diabetes
13) Redondo, Maria J., and Fida Bacha. “GLP-1 receptor agonist as adjuvant therapy in type 1 diabetes: No apparent benefit for beta-cell function or glycemia.” The Journal of Clinical Endocrinology & Metabolism 105.8 (2020): e3000-e3002.
14) Edwards, Khary, Xilong Li, and Ildiko Lingvay. “Clinical and Safety Outcomes with GLP-1 Receptor Agonists and SGLT2 Inhibitors in Type 1 Diabetes: A Real-World Study.” The Journal of Clinical Endocrinology & Metabolism 108.4 (2023): 920-930.
Context: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) are used off-label in the management of type 1 diabetes mellitus (T1DM) in real-world practice as adjuvant therapies to insulin. There are few real-world data regarding efficacy and safety of this practice.
Objective: This work aimed to determine the efficacy and safety of GLP-1RAs and sodium-glucose SGLT2is in the management of T1DM in real-world practice.
Methods: A retrospective chart review was performed of all instances of GLP-1RA and/or SGLT2i use greater than 90 days in adult patients with T1DM at a single academic center. We report the clinical and safety outcomes over the duration of use.
Results: We identified 104 patients with T1DM who ever used a GLP-1RA (76 patients) or SGLT2i (39 patients) for more than 90 days. After 1 year of therapy, GLP-1RA users had statistically significant reductions in weight (90.5 kg to 85.4 kg; P < .001), glycated hemoglobin A1c (HbA1c) (7.7% to 7.3%; P = .007), and total daily dose of insulin (61.8 units to 41.9 units; P < .001). SGLT2i users had statistically significant reductions in HbA1c (7.9% to 7.3%; P < .001) and basal insulin (31.3 units to 25.6 units; P = .003). GLP-1RA users compared to SGLT2i users had greater reduction in weight (P = .027) while HbA1c reduction was comparable between the groups. Over a mean total duration of use of 29.5 months/patient for both groups, more SGLT2i users experienced diabetic ketoacidosis (DKA) (12.8% vs 3.9%). Therapy was discontinued because of adverse events 26.9% of the time for GLP-1RA users vs 27.7% for SGLT2i users.
Conclusion: GLP-1RA and SGLT2i use in T1DM is associated with clinically relevant benefits. DKA remains a clinical concern with SGLT2i use, requiring careful patient selection and monitoring, with the risk to benefit ratio of treatment evaluated at an individual level.
MICE
15) Gu, Liangbiao, et al. “Combination of GLP-1 receptor activation and glucagon blockage promotes pancreatic β-cell regeneration in situ in type 1 diabetic mice.” Journal of Diabetes Research 2021 (2021): 1-7.
16) Wei, Tianjiao, et al. “Glucagon Acting at the GLP-1 Receptor Contributes to β-Cell Regeneration Induced by Glucagon Receptor Antagonism in Diabetic Mice.” Diabetes (2023): db220784.
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dachâs Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dachâs Online Store for Natureâs Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
http://sci.med.narkive.com/covV2Qo2/jeffrey-dach-book-announcment-natural-medicine-101
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician â patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this Article
Copyright (c) 2023 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given. See Repost Guidelines.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a âfair useâ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
Published on April 27th, 2023 by Jeffrey Dach MD
The post New Weight Loss Anti-Diabetic Drugs Ozempic and GLP-1 Receptor Agonists appeared first on Jeffrey Dach MD.
February 15, 2023
Pycnogenol and Gotu Kola for Calcium Score
 Gotu Kola (Centella Asiatica) and Pycnogenol for Calcium Score
Gotu Kola (Centella Asiatica) and Pycnogenol for Calcium Score
Thanks and credit goes to Joel Kahn MD and Kirk Hamilton for alerting me to the combination of two plant extracts, French maritime pine bark (Pycnogenol) and Centella asiatica (Goto Cola) for Reversing Calcium Score, Atherosclerosis and preventing in-stent stenosis. Most of the recent studies have been done by Dr. Gianni Belcaro’s group in Italy showing impressive efficacy and safety of this combination in preventing progression of cardiovascular disease and in-stent stenosis. Dr. Joel Kahn uses this combination in his cardiology practice and has written an excellent summary of the research studies reprinted in Life Extension Magazine. (1)
Reversing CalcificationsÂ
The 2019 study by Dr Hu showed reversal of arterial calcifications. Control subjects had a 34.88% increase while treated subjects has a decrease of -9.95%. However, this was not a calcium score study which would be the preferred technique. I would like to see this repeated using actual calcium scores. I would assume this efficacy would be translated to calcium scores. It would be nice to actually see this. (2)
There may also other uses for skin, obesity etc. Combinations with Aloe Vera and Nattokinase are aslo9 useful as antiseptic and anti-thrombotic. (1-11)
Pycnogenol from Pure Encapsulations
Life Extension has the combination product available on Amazon: Combination Gotu Kola and Pycnogenol:Â Click Link Here to Buy on Amazon
Articles with Related Content
Above Left header image: Low magnification micrograph of the distal right coronary artery with complex atherosclerosis and luminal narrowing. Masson’s trichrome. Courtesy of wikimedia commons
Jeffrey Dach MD
7450 Griffin Road Suite 180/190
Dave, Florida 33314
954 792 4663
Links and References
1) Reversal of Calcification and Atherosclerosis. two plant extracts.
French maritime pine bark and Centella asiatica Goto Cola. November 2022. Written by: Joel Kahn, MD.
arterial/cardiac calcifications in subjects with asymptomatic atherosclerosis.
2) Hu, Shu, et al. “Central cardiovascular calcifications: supplementation with Pycnogenol® and Centellicum®: variations over 12 months.” Minerva Cardioangiologica 68.1 (2019): 22-26.
BACKGROUND: This âconceptâ registry study evaluated the efficacy of Pycnogenol® and the combination Pycnogenol® and Centella Asiatica (Centellicum®) in controlling over 12 months the increasing number of arterial/cardiac calcifications in subjects with asymptomatic atherosclerosis.
METHODS: The study included 3 groups of 30 males with asymptomatic coronary calcifications. Group one was followed with standard management (SM); group 2 used SM and Pycnogenol® (150 mg/day); group 3 used the combination Pycnogenol® (150 mg/day) + Centellicum® (450 mg/day). All subjects took cardioaspirin (Bayer, 100 mg/day).
RESULTS: No dropouts, no clinical events were observed in 12 months. The 3 groups had comparable demographic and medical characteristics at baseline. No tolerability problems and no side effects from supplementation were reported. After 12 months, oxidative stress was significantly decreased (P<0.05) in both groups taking Pycnogenol®. The evaluation of the number of calcifications >1 mm indicated a trend in controls using SM towards a progressive increase in calcifications. At 12 months the decrease in the number of calcifications with the combined supplements (Pycnogenol® and Centellicum®) (group 3) was -9.952% and thus significantly better that in the other two groups (P<0.05). Pycnogenol® alone was more effective than SM alone in controlling the variation in calcifications (P<0.05). Considering a 34.88% increase in SM subjects, the total absolute difference between SM (34.8%) and the decrease observed in group 3 (-9.95%) was 44.75% (P<0.02). This indicates that supplementation with the combined supplements blocks the increase in calcified areas and, possibly, in time may decrease the number of calcified spots.
CONCLUSIONS: This study shows that there is a significant activity of the complex Pycnogenol®+ Centellicum® in reducing the progressive diffusion of central cardiovascular calcifications-associated with advanced plaques – in a relatively short period of time. Longer studies – focusing also on events – may better evaluate the efficacy of these standardized supplements combination on the evolution of atherosclerosis.
3) Belcaro, Gianni, et al. “Delayed progression of atherosclerosis and cardiovascular events in asymptomatic patients with atherosclerotic plaques: 3-year prevention with the supplementation with Pycnogenol®+ Centellicum®.” Minerva cardioangiologica 68.1 (2020): 15-21.
Background: The aim of this study was the evaluation of the progression of atherosclerosis and the occurrence of cardiovascular events in asymptomatic patients with atherosclerotic plaques (Class IV and V) and arterial wall atherosclerotic lesions and intima-media thickening (IMT).
Methods: Progression of atherosclerotic lesions, oxidative stress and IMT were measured in a 3-year concept, pilot registry study. All subjects were followed with standard management (SM) – including diet and exercise – to control cardiovascular risk factors.The target measurements were: the rate of progression of the atherosclerotic lesions (the passage of subjects from one atherosclerotic class to the next class); the occurrence of “hard” cardiovascular events (i.e. myocardial infarction or strokes; angina was not considered a “hard” event). The study included 3 groups: 1) SM): 2) subjects using cardioaspirin (100 mg/day) and SM; 3) subjects following SM, taking cardioaspirin and supplemented with Pycnogenol® (150 mg/day)+Centellicum® (450 mg/day).
Results: The groups were comparable for age and baseline evaluations. 54 subjects completed the 3 year study with standard management only, 74 with aspirin and 56 with aspirin and Pycnogenol®+Centellicum®. The BMI of all subjects was <26. No side effects and no tolerability problems were observed with the supplements. Progression was defined by the passage of the atherosclerotic lesions from one class to the next more advanced class. Progression in the supplement group was observed in 5.3% of the subjects in comparison with a progression >20% in the other groups (P<0.05).
In comparison with the SM group and the cardioaspirin group the rate of ‘hard’ cardiovascular events, requiring hospital admissions were <4% with the combined supplement in comparison with a value >12% in the other two groups (22.22% event rate in the SM group).
The reduction produced by the aspirin only was significantly lower (P<0.05) in comparison with supplemented patients. Antiplatelet management appears to reduce a significant number of events (P<0.05) without a real effect on progression of atherosclerotic lesions. The additional parameters of carotid IMT and oxidative stress were also lower (P<0.05) with the supplements.
Conclusions: In conclusion, this study indicates that the combined supplementation with Pycnogenol®+Centelicum® appears to control both the progression of atherosclerosis and the occurrence of cardiovascular events in this 3 year study. Larger studies, in a wider population with more complex and less standardized conditions may be needed.
prevent asymptomatic atherosclerosis progression
4) Belcaro, G., M. Dugall, E. Ippolito, M. Hosoi, U. Cornelli, A. Ledda, M. Scoccianti, M. R. Cesarone, L. Pellegrini, R. Luzzi, M. Corsi and B. Feragalli (2017). “Pycnogenol® and Centella asiatica to prevent asymptomatic atherosclerosis progression in clinical events.” Minerva Cardioangiol 65(1): 24-31.
BACKGROUND: The aim of this study was to evaluate the effect of the nutritional supplements Pycnogenol® and Centella asiatica (CA) on atherosclerosis progression in low-risk, asymptomatic subjects with carotid or femoral stenosing plaques. METHODS: The study included subjects aged 45-60 with stenosing atherosclerotic plaques (50-60%) in at least one carotid or common femoral bifurcation. Subjects were allocated into 3 groups. In Group 1 (controls), management was based on education, exercise, diet and lifestyle changes. This same management plan was used in the other two groups: Group 2 used Pycnogenol® (100 mg/day), while Group 3 used Pycnogenol® 100 mg/day plus CA(100 mg/day). The follow-up lasted 4 years. Plaque progression was assessed using the ultrasonic arterial score based on arterial wall morphology, considering plaque characteristics and the number of subjects that had cardiovascular events. Oxidative stress was also measured.
RESULTS: Of the 413 individuals that were admitted, 391 individuals completed 4 years. Group distribution was comparable. The rate of progression of ultrasound arterial score was significantly lower in the two supplement groups (P<0.05) in comparison with controls suggesting a beneficial effect of Pycnogenol® with a significant difference in favor of the combination (P<0.05). There was a reduction in plaques progression in the supplement groups with the best effects obtained by the combination, considering maximum plaque thickness and length and echogenicity (grey scale median) (P<0.05). Plaques became generally dense (more echogenic) achieving a mixed echogenicity. The occurrence of anginal events was less than 3% in the two supplement groups (in comparison with 6.25% in controls) (P<0.05) with the best results obtained by the combination (P<0.05). The occurrence in myocardial infarctions was significantly lower for the combination (P<0.05). Minor transient ischemic attacks were also less frequent with the supplements with the best results observed with the combination (P<0.05). Events in controls – requiring hospital admission – were globally seen in 16.4% of subjects (minor events) in comparison with 8.9% of subjects using Pycnogenol® and only 3.3% of patients using the combination. At 4 years, oxidative stress in the supplement groups was lower than in controls (P<0.05, with no significant difference between groups 2 and 3). CONCLUSIONS: Pycnogenol® and the combination of Pycnogenol® plus CAreduce the progression of arterial plaques and the progression to clinical stages. The reduction in plaques and clinical progression was associated with a reduction in oxidative stress. The results justify a larger study to define the efficacy of the combination of Pycnogenol® plus CAas a prophylaxis in preclinical atherosclerosis.
post-stent prevention of neointima and plaque re-growth.
5) Belcaro, Gianni, et al. “Pycnogenol®+ Centellicum®, post-stent evaluation: prevention of neointima and plaque re-growth.” Minerva cardioangiologica 67.6 (2019): 450-455.
Abstract
Background: The aim of this study was to evaluate the regrowth and progression of within-stent neointima after stenting as a model of accelerated atherosclerosis and the potential effects of the combination Pycnogenol® and Centellicum® in 12 months’ follow-up.
Methods: Progression was defined as the passage from one arterial risk class to next, more advanced risk class in 12 months of follow-up. Each class corresponds to a different risk of cardiovascular events and progression. Three management groups were formed, treated with either standard management (SM), Pycnogenol® 150 mg/day, or a combination of Pycnogenol® 150 mg/day and Centellicum® 450 mg/day.
Results: No side effects or tolerability problems were observed. 82 subjects with stented arteries in class 2 were evaluated for the passage into class 3 over 12 months. This group included 82 subjects; there were no dropouts. The management subgroups were comparable at baseline. At 12 months 66.7% of subjects in the SM subgroup progressed to class 3, versus 10.7% in the Pycnogenol® group; progression was seen in 6.7% (P<0.05) of subjects supplemented with the combination. In the second section of the registry study (78 subjects with stented arteries in class 3) we evaluated the percentage of patients passing into class 4. At 12 months 53.6% of subjects using the SM progressed versus 26.9% in the subgroup using Pycnogenol® (P<0.05) and 11.5% in the Pycnogenol®+Centellicum® group (P<0.05). Across all 160 subjects in the three management groups, progression of the stented artery at 12 months was seen in 59.6% of subjects in the SM group versus 18.5% (P<0.05) in the group managed with Pycnogenol® only. The Pycnogenol®+Centellicum® combination further decreased progression down to 8.9% (P<0.05). Oxidative stress was significantly reduced (P<0.05) in the two supplement groups.
Conclusions: In conclusion, the combination Pycnogenol®+Centellicum® appears to reduce the rate of progression of the neointima after stenting.
6) Pycnogenol-Nattokinase Combo Prevents In-Flight Venous Thrombosis
Thursday, 15 April 2004 0:38 By Erik Goldman | Editor in Chief – Vol. 5, No. 1. , 2004
Flite Tabs formula contains 150 mg of âPinokinase,â a proprietary combination of pycnogenol and nattokinase.
————————————————————-
7) Belcaro, G., et al. “Pycnogenol® and Centella asiatica in the management of asymptomatic atherosclerosis progression.” International Angiology: a Journal of the International Union of Angiology 34.2 (2014): 150-157.
8) Belcaro, Gianni, et al. “Pycnogenol® and Centella asiatica to prevent asymptomatic atherosclerosis progression in clinical events.” Minerva Cardioangiologica 65.1 (2015): 24-31.
Gotu Kola (Centella Asiatica (L.) Urban) and Aloe Vera Herb Extracts
9) Mayefis, Delladari, et al. “Effectiveness of Combination of Gotu Kola (Centella Asiatica (L.) Urban) and Aloe Vera Herb Extracts as a Natural Disinfectant.” Jurnal EduHealth 14.01 (2023): 182-193.
10) Sun, Boju, et al. “Therapeutic potential of Centella asiatica and its triterpenes: A review.” Frontiers in pharmacology 11 (2020): 568032.
Centella asiatica (also known as Centella asiatica (L.) Urb. or Gotu kola) is a traditional Chinese medicine with extensive medicinal value, which is commonly used in Southeast Asian countries. This study aimed to summarize the effects of C. asiatica and its main components on neurological diseases, endocrine diseases, skin diseases, cardiovascular diseases, gastrointestinal diseases, immune diseases, and gynecological diseases, as well as potential molecular mechanisms, to study the pathological mechanism of these diseases based on the changes at the molecular level. The results showed that C. asiatica and its triterpenoids had extensive beneficial effects on neurological and skin diseases, which were confirmed through clinical studies. They exhibited anti-inflammatory, anti-oxidative stress, anti-apoptotic effects, and improvement in mitochondrial function. However, further clinical studies are urgently required due to the low level of evidence and lack of patients.
Among the 109 studies included, 58 were in vivo experiments, 36 in vitro experiments, 11 in vivo and in vitro experiments, and 4 clinical trials (Figure 2).
Previous studies found that C. asiatica and its triterpenoids could effectively increase SOD and GPX activities, activate nuclear factor erythroid-2-related factor 2, improve the cognitive impairment of animals, and then alleviate the symptoms of related diseases (Gray et al., 2017a; Chintapanti et al., 2018; Welbat et al., 2018).
C. asiatica extract, asiatic acid, and asiaticoside could effectively increase the content of brain-derived neurotrophic factor (BDNF)
C. asiatica extracts are promising in treating endocrine diseases, especially type 2 diabetes and obesity (Table 3). As for specific compounds, asiatic acid was effective in obesity (Rameshreddy et al., 2018) and madecassoside might be a potential candidate for treating osteolytic bone diseases (Wang et al., 2019).
First, the C. asiatica extract seemed to improve the oxidative stress. Both the diabetic animal model and the obesity animal model demonstrated that the C. asiatica extract increased the GSH, CAT, and SOD activities, thereby improving the enzyme antioxidant system (Kumari et al., 2016; Masola et al., 2018; Rameshreddy et al., 2018). Second, the results of animal experiments showed that the C. asiatica extract could effectively decrease related inflammation factors (TNF-α, IL-1β, and IL-4). At the same time, it also reduced blood glucose and blood lipid levels (Oyenihi et al., 2017; Masola et al., 2018). Besides, the results showed that the extracts of C. asiatica lowered food and water intake and body weight, which suggested that C. asiatica extract may affect obesity by influencing the feeding center controlled by central nervous system (Halpern et al., 2008; Blanco et al., 2011).
anti-obesity agent
Moreover, the potential of asiatic acid as an anti-obesity agent can be proved from the facts that it suppresses weight gain, and enhance the sensitivity of leptin and insulin. At the molecular level, asiatic acid can increase the level of enzymatic antioxidants (CAT, GPx and SOD), reverse the expression of CPT-1 and UCP-2 that are suppressed by high-fat diet. Therefore, it can be deduced that asiatic acid can repair oxidative stress damage caused by obesity, and can also suppress weight gain by promoting fatty acid oxidation (Rameshreddy et al., 2018).
treatment of osteoporosis
The results of madecassoside intervention in a mouse model of osteoporosis, caused by estrogen deficiency and bone marrow monocytes showed that it can inhibit the expression of related genes by affecting the NF-κB and MAPK signaling pathways (NFATc1, c-Fos, Acp5, CTSK, VATPase-d2), inhibits the generation of osteoclasts, weaken the absorption activity of osteoclasts. It can be inferred that made cassoside can be a potential candidate for the treatment of osteoporosis (Wang et al., 2019).
EFFECTS ON SKIN DISEASES
The C. asiatica extract and its triterpenoids had certain therapeutic and relieving effects on acne, baldness, vitiligo, atopic dermatitis, and wounds
Effects on Cardiovascular Diseases
A clinical prospective, placebo-controlled, randomized, dose range trial found that after 4 weeks of treatment with C. asiatica total triterpenes (TTFCA), the capillary filtration rate, ankle circumference and ankle edema of patients with venous hypertension were improved, and the dose range showed that 180 mg/day was most effective in symptoms improvement (De Sanctis et al., 2001).
Clinical studies have shown that after 4 years of intervention in patients with Pycnogenol® 100 mg/day plus C. asiatica (100 mg/day), the combined treatment group has reduced plaque progression, reduced oxidative stress, and mild transient brain deficiency as compared to the control group. The incidence of angina events in combined treatment group was less than 3%, while the control group it was 6.25%. Therefore, it can be established that C. asiatica may have a role in preventing preclinical atherosclerosis (Belcaro et al., 2015; Belcaro et al., 2017).
11) Cesarone, Maria R., et al. “Pycnogenol®-Centellicum® supplementation improves lung fibrosis and post-COVID-19 lung healing.” Minerva Medica 113.1 (2021): 135-140.=================================
https://www.centellicum.com/Â Â Â References 2
S. Hu, G. Belcaro, M.R. Cesarone, B. Feragalli, R. Cotellese, M. Dugall, C. Scipione , V. Scipione , C. Maione (2020). Central cardiovascular calcifications: supplementation with Pycnogenol® and Centellicum®: variations over 12 months. Minerva Cardioangiologica 68(1):22-6
G. Belcaro, M.R. Cesarone, S. Hu, B. Feragalli, P. Peterzan, M. Corsi, R. Luzzi, M. Hosoi, R. Cotellese, M. Dugall, C. Scipione , V. Scipione , (2020). Delayed progression of atherosclerosis and cardiovascular events in asymptomatic patients with atherosclerotic plaques: 3-year prevention with the supplementation with Pycnogenol®+Centellicum®
Belcaro, G. and M. Cornelli (2017). “Variations in Echogenicity in Carotid and Femoral Atherosclerotic Plaques with Pycnogenol®+ Centella Asiatica Supplementation.” Int J Angiol 26: 95-101.
Cotellese, R., S. Hu, G. Belcaro, A. Ledda, B. Feragalli, M. Dugall, M. Hosoi and E. Ippolito (2018). “Centella asiatica (Centellicum®) facilitates the regular healing of surgical scars in subjects at high risk of keloids.” Minerva Chir 73(2): 151-156.
Hosoi, M., G. Belcaro, A. Ledda, U. Cornelli, M. Dugall, B. Feragalli, R. Luzzi, R. Cotellese, S. Hu and F. Fano (2018). “Effects of collagen remodulation with Centella asiatica (Centellicum®) in Dupuytren palmar fibromatosis: a pilot supplement study.” Minerva Ortopedica e Traumatologica 69(3): 78-83.
Hu, S., G. Belcaro, M. Hosoi, B. Feragalli, R. Luzzi and M. Dugall (2018). “Postpartum stretchmarks: repairing activity of an oral Centella asiatica supplementation (Centellicum®).” Minerva Ginecol 70(5): 629-634.
Luzzi, R., G. Belcaro and E. Ippolito (2016). “Carotid plaque stabilization induced by the supplement association Pycnogenol® and centella asiatica (Centellicum®).” Minerva Cardioangiol 64(6): 603-609.
Belcaro, G., M. Dugall, M. Hosoi, E. Ippolito, M. Cesarone, R. Luzzi, U. Cornelli and A. Ledda (2014). “Pycnogenol® and Centella Asiatica for asymptomatic atherosclerosis progression.” Int Angiol 33(1): 20-26.
Belcaro, G., M. Dugall, E. Ippolito, M. Hosoi, U. Cornelli, A. Ledda, M. Scoccianti, M. R. Cesarone, L. Pellegrini, R. Luzzi, M. Corsi and B. Feragalli (2017). “Pycnogenol® and Centella asiatica to prevent asymptomatic atherosclerosis progression in clinical events.” Minerva Cardioangiol 65(1): 24-31.
Belcaro, G., E. Ippolito, M. Dugall, M. Hosoi, U. Cornelli, A. Ledda, M. Scoccianti, R. D. Steigerwalt, M. R. Cesarone, L. Pellegrini, R. Luzzi and M. Corsi (2015). “Pycnogenol® and Centella asiatica in the management of asymptomatic atherosclerosis progression.” Int Angiol 34(2): 150-157.
Luzzi, R., G. Belcaro and E. Ippolito (2016). “Carotid plaque stabilization induced by the supplement association Pycnogenol® and centella asiatica (Centellicum®).” Minerva Cardioangiol 64(6): 603-609.
Published on February 15th, 2023 by Jeffrey Dach MD
The post Pycnogenol and Gotu Kola for Calcium Score appeared first on Jeffrey Dach MD.
February 12, 2023
The New Osteoporosis Drugs, the Good, Bad and Ugly
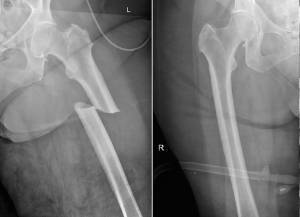 The New Osteoporosis Drugs, the Good, Bad and Ugly
The New Osteoporosis Drugs, the Good, Bad and Ugly
by Jeffrey Dach MD
Denosumab (Prolia, FDA approved 2010) and Romosozumab (Evenity, FDA approved 2019), are similar to the older bisphosphonate drugs (Fosamax, Alondronate) in two ways. Number one the mechanism of action is similar. They are all antiresorptive, osteoclast inhibitors. Number two, they all have same problems with Avascular Necrosis of the Jaw and Atypical Femur Fractures, similar to the bisphosphonates (Alondronate, Fosamax).
Header image: Atypical femur fracture after 5 years of denosomab coutesy of Kumar, Shejil, et al. “Atypical femoral fracture in a bisphosphonate-naïve patient on denosumab for osteoporosis.” Archives of Osteoporosis 17.1 (2022): 131.
The Good and Bad News
The good news is studies show these new humanized monoclonal antibody ostoporosis drugs increased bone density and reduced fragility fractures. The bad news is there is still a problem with atypical femur fractures and AVN of the jaw. The risk of AVN of the jaw with denosamab is an order of magnitude greater than the bisphosphonates (2.3% vs 0.3%). In 2022, Dr. Anthony Colella writes:
The risk of ONJ [Osteonecrosis of Jaw] in patients on denosumab for osteoporosis is a magnitude greater than for patients on the oral bisphosphonates 2.3% vs. zero to 0.3%, which is 7.7 times more likely. (7)
Rebound Phenomenon
Once Denosumab is started, it can not be stopped because fractures accelerate when stopped, a “rebound” phenomenon.
Only For Severe Cases
Romosozumab (Evenity) FDA approved in April 9, 2019 is a new drug, and recommended only for very severe cases which have already suffered osteoporotic fractures.
Anabolic Therapy – PTH Analog Drugs
Anabolic PTH analog drugs such as Forteo are available and have good efficacy. These are preferred by orthopedic surgeons to improve bone and fracture healing. For this reason, PTH analogs are my preference over the osteoclast inhibitor, antiresorptive drugs for severe cases of osteoporosis with ongoing fragility fractures. Discontinuing a PTH analog drug requires a replacement drug, Denosamab or a bisphosphonate. In 2022, Dr. Yevgeniya Kushchayeva writes:
Teriparatide (TPTD) [Forteo] and abaloparatide [TYMLOS] are anabolic medications that are recombinant fragments of human PTH…New bone formation with TPTD is characterized by an increased cancellous bone volume and connectivity, improved trabecular morphology, and a shift toward a more plate-like structure, with an increased cortical bone thickness…In general, anabolic therapy is preferred over antiresorptive medications for optimizing outcomes of orthopedic surgery… In general, treatment with Dmab or BPs is recommended after the discontinuation of any anabolic medication. Emphasis Mine (1)
Post Menopausal Hormone Replacement
The truth is that post menopausal osteoporosis is a hormone deficiency problem, and hormone replacement is the best way to prevent skeletal bone loss associated with menopause. In my opinion, human hormones, estradiol, progesterone, DHEA and testosterone (ie bioidential hormones) are preferred over the above mentioned osteoporosis drugs in the typical post-menopausal female. These women may be worried about their DEXA bone density report showing a low T score of less than -2.5, but have have not yet had fragility fractures, and are seeking prevention. For elderly women with far advanced osteoporosis, typically presenting with vertebral compression fractures, use of osteoporosis drugs could be justified, and a PTH analog would be my first choice over the osteoclast inhibitor, antiresorptive drugs. (13-21)
In 2021, Dr Anna Gosset reviewed menopausal hormone therapy for the management of osteoporosis, writing:
Postmenopausal osteoporosis is for a large part the consequence of both quantitative and qualitative bone alterations induced by estrogen deficiency which occurs within the first years of the menopause transition. Bone is a strong estrogen-dependent tissue and estrogens play a major role in the acquisition and maintenance of bone mineral content throughout life. (13)
For more information on Bioidentical Hormone Replacement, see my free e-book online at Bioidentical Homones 101.
Bone Building Supplements
In addition, a good prevention program includes a bone building supplement program such as:
1) Bone Guard by Perque. Ingredient List: Vitamins, C, D, K, Calcium, Magnesium, Strontium, Zinc, Manganese, Selenium, Chromium, Iodine, Copper, Boron, Vanadium, Silica
2) New Chapter Calcium Supplement â Bone Strength Ingredient List: Vitamin D, Vitamin K, Calcium, Magnesium , Strontium, Silica, Vanadium
There are many other widely available similar products.
Patient Selection – No Consensus on Initiating Drug Therapy
Which patients should be selected for treatment with the new osteoporosis drugs? In 2022, Dr. Hans Dimai says there is no consensus on whom and when exactly to treat with osteoporosis drugs, and suggest the criteria for initiating treatment includes a prior fragility fracture, writing:
Currently, there is no international consensus in whom and when exactly pharmacological osteoporosis treatment should be initiated. In short, many national guidelines recommend that women (and men) with a prior fragility fracture be considered for pharmacological intervention because such fractures are associated with a high risk of subsequent fractures.(12) Emphasis mine
Conclusion: The new Osteoporosis Drugs Denosumab (Prolia) and Romosozumab (Evenity) still have the problem with atypical femur fractures and osteonecrosis of the jaw. Granted, the reported incidence of these are rare, and are outweighed by reduction in fragility fractures. However, that is not the point. The fact that we are seeing atypical fracture indicates the drugs create pathological bone with disturbed bone architecture.
The other issue is the “Discontinuation Syndrome” for Denosumab with a rebound increase in fractures when discontinued. This is a factor which must be carefully considered. This is serious and requires the patient to start treatment with a bisphosphonate drug which also carries the risk of abnormal bone architecture with atypical femur fracture. This means either lifelong Denosamab or Denosamab followed by lifelong Bisphosphonate drug use. In order to obtain informed consent, the patient must be willing to commit to life long use of these drugs, and be willing to accept atypical femur fracture and osteonecrosis of the jaw as possible adverse side effects.
Articles with related interest
Fosamax Study Denies Link with Femur Fractures
Fosamax, Actonel, Osteoporosis and Toulouse Lautrec
FDA Says Osteoporosis Drugs Cause Femur Fractures
Jeffrey Dach MD
7450 Griffin Road Suite 180/190
Davie, Fl 33314
954-792 4663
=======================================================
References
Drug Prolia (denosumab) Injection
Company: Amgen, Inc.
Approval Date: 6/1/2010
1) Kushchayeva, Yevgeniya, et al. “Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing.” Journal of Clinical Medicine 11.24 (2022): 7477.
Dmab, which is not retained in the skeleton, should be followed by another medication, usually a BP, after discontinuation. Dmab is administered every 6 months; non-compliance with the dosing schedule can lead to a rebound increase in bone remodeling and bone loss, and an increased risk of multiple VFs ]vetebral faractures] [159]. The discontinuation of Dmab leads to an enhanced osteoclastogenesis and osteoblastogenesis, resulting in a loss in cortical thickness and trabecular bone volume along with a rapid acceleration of bone turnover and increased amount of unmineralized bone. Bone loss during the first year after Dmab discontinuation is approximately 5â11% at all skeletal sites [160]. BP-naïve patients may experience more bone loss after the discontinuation of Dmab in comparison with BP-treated patients [161]. BP-treated patients who have transitioned to Dmab have a greater BMD increase than those who continue BP therapy [162].
Teriparatide (TPTD) and abaloparatide are anabolic medications that are recombinant fragments of human PTH (1â34). TPTD is recombinant PTH (1â34); abaloparatide is a synthetic analog of PTH-related protein PTHrP (1â34). Abaloparatide has a 41% homology to PTH (1-34) and 76% homology to PTHrP (1â34).
Although a sustained PTH elevation in patients with hyperparathyroidism leads to an increased bone resorption and bone loss [167], the intermittent administration of TPTD stimulates bone remodeling and increases bone formation in excess of bone resorption [168]. Although TPTD also upregulates osteoclasts, the anabolic effect dominates [169]. New bone formation with TPTD is characterized by an increased cancellous bone volume and connectivity, improved trabecular morphology, and a shift toward a more plate-like structure, with an increased cortical bone thickness [170].
In general, anabolic therapy is preferred over antiresorptive medications for optimizing outcomes of orthopedic surgery.
Based on an analysis of four prospective observational studies, TPTD reduces rates of clinical VFs, non-VFs, clinical fractures, and hip fractures by 62%, 43%, 50%, and 56%, respectively, with >6 mo of therapy compared with 0 to 6 mo [182]. Abaloparatide has been shown to reduce rates of VFs, non-VFs, and major osteoporotic fractures by 86%, 43%, and 70%, respectively, compared with placebo [183].
TPTD was initially limited to 24-month lifetime use due to an increase in the risk of osteosarcoma in rats. However, this restriction has been recently removed based on reviews of long-term post-marketing data, showing no evidence of an increase in osteosarcoma risk in humans [184,185].
In general, treatment with Dmab or BPs is recommended after the discontinuation of any anabolic medication.
Romosozumab (Rmab) is a humanized monoclonal antibody against sclerostin with the dual effect of stimulating bone modeling and inhibiting resorption, in contrast to other anabolic agents that stimulate remodeling via an increased formation and resorption of bone. Sclerostin, the Rmab target, is a glycoprotein produced by osteocytes that inhibits bone formation due to the downregulation of the Wnt pathway. Rmab binds to sclerostin and inhibits it activity [188], resulting in an increase in osteoblastic differentiation, proliferation, and survival. In the presence of Rmab, the Wnt signaling pathway is activated, leading to bone formation and BMD gain.
In postmenopausal women, the dual effect of Rmab leads to a significant increase in BMD and a reduction in the fracture risk compared with alendronate and placebo [193,194]. Rmab 12 mo therapy led to a 73% lower relative risk of new VFs, 36% lower risk of clinical fractures, and no significant effect on non-VFs [194,195].
—————————-Â ——————————
2) Bone, Henry G., et al. “10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension.” The lancet Diabetes & endocrinology 5.7 (2017): 513-523.
Background: Long-term safety and efficacy of osteoporosis treatment are important because of the chronic nature of the disease. We aimed to assess the long-term safety and efficacy of denosumab, which is widely used for the treatment of postmenopausal women with osteoporosis.
Methods: In the multicentre, randomised, double-blind, placebo-controlled, phase 3 FREEDOM trial, postmenopausal women aged 60-90 years with osteoporosis were enrolled in 214 centres in North America, Europe, Latin America, and Australasia and were randomly assigned (1:1) to receive 60 mg subcutaneous denosumab or placebo every 6 months for 3 years. All participants who completed the FREEDOM trial without discontinuing treatment or missing more than one dose of investigational product were eligible to enrol in the open-label, 7-year extension, in which all participants received denosumab. The data represent up to 10 years of denosumab exposure for women who received 3 years of denosumab in FREEDOM and continued in the extension (long-term group), and up to 7 years for women who received 3 years of placebo and transitioned to denosumab in the extension (crossover group). The primary outcome was safety monitoring, comprising assessments of adverse event incidence and serious adverse event incidence, changes in safety laboratory analytes (ie, serum chemistry and haematology), and participant incidence of denosumab antibody formation. Secondary outcomes included new vertebral, hip, and non-vertebral fractures as well as bone mineral density (BMD) at the lumbar spine, total hip, femoral neck, and one-third radius. Analyses were done according to the randomised FREEDOM treatment assignments. All participants who received at least one dose of investigational product in FREEDOM or the extension were included in the combined safety analyses. All participants who enrolled in the extension with observed data were included in the efficacy analyses. The FREEDOM trial (NCT00089791) and its extension (NCT00523341) are both registered with ClinicalTrials.gov.
Findings: Between Aug 3, 2004, and June 1, 2005, 7808 women were enrolled in the FREEDOM study. 5928 (76%) women were eligible for enrolment in the extension, and of these, 4550 (77%) were enrolled (2343 long-term, 2207 crossover) between Aug 7, 2007, and June 20, 2008. 2626 women (1343 long-term; 1283 crossover) completed the extension. The yearly exposure-adjusted participant incidence of adverse events for all individuals receiving denosumab decreased from 165·3 to 95·9 per 100 participant-years over the course of 10 years. Serious adverse event rates were generally stable over time, varying between 11·5 and 14·4 per 100 participant-years. One atypical femoral fracture occurred in each group during the extension. Seven cases of osteonecrosis of the jaw were reported in the long-term group and six cases in the crossover group. The yearly incidence of new vertebral fractures (ranging from 0·90% to 1·86%) and non-vertebral fractures (ranging from 0·84% to 2·55%) remained low during the extension, similar to rates observed in the denosumab group during the first three years of the FREEDOM study, and lower than rates projected for a virtual long-term placebo cohort. In the long-term group, BMD increased from FREEDOM baseline by 21·7% at the lumbar spine, 9·2% at total hip, 9·0% at femoral neck, and 2·7% at the one-third radius. In the crossover group, BMD increased from extension baseline by 16·5% at the lumbar spine, 7·4% at total hip, 7·1% at femoral neck, and 2·3% at one-third radius.
Interpretation: Denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau.
3) Osteoporosis drugs: Which one is right for you? September 12, 2021 Harvard Health
Denosumab (Prolia) is a monoclonal antibody given as a twice-yearly injection. It prevents bone-dissolving osteoclast cells from forming. Denosumab may be an option if a woman cannot tolerate bisphosphonates. Once started, women usually stay on this therapy indefinitely because if stopped than bone resorption will accelerate.
Romosozumab (Evenity) is another monoclonal available for women with very severe osteoporosis, usually considered after a woman has had a fragility fracture. It acts by blocking sclerostin, a protein that inhibits bone formation. The medication is injected once a month using two separate prefilled syringes for a full dose.
——————————– —————-
4) Kobayakawa, T., Miyazaki, A., Saito, M. et al. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep 11, 11801 (2021).
Osteonecrosis of JAW
5) Tagliamento, Marco, et al. “Denosumab related osteonecrosis of the jaw: Unusual pattern with periosteal reaction.” European Journal of Cancer 166 (2022): 33-37.
6) Goss, Alastair N. “Osteonecrosis of the jaw and denosumab.” Australian Prescriber 45.6 (2022): 208.
Despite having no clear guidelines to favour denosumab, it has substantially replaced the oral bisphosphonates as the first-line treatment for osteoporosis in Australia.1 Denosumab is effective when given as a six-monthly 60 mg subcutaneous injection and has few adverse reactions. The main concern medically is that, if denosumab is discontinued or the injection is substantially delayed, there is a risk of vertebral fracture. This means effectively that, once started, the patient must remain on denosumab or another antiresorptive drug for the rest of their life.
Despite having no clear guidelines to favour denosumab, it has substantially replaced the oral bisphosphonates as the first-line treatment for osteoporosis in Australia.1 Denosumab is effective when given as a six-monthly 60 mg subcutaneous injection and has few adverse reactions. The main concern medically is that, if denosumab is discontinued or the injection is substantially delayed, there is a risk of vertebral fracture. This means effectively that, once started, the patient must remain on denosumab or another antiresorptive drug for the rest of their life.
Medication-related osteonecrosis of the jaw is a well-documented severe complication of dental extractions in patients on the oral bisphosphonates. It has been assumed that the risk with denosumab is similar to that with bisphosphonates.2 This is incorrect. In the 2022 update of its position paper, the American Association of Oral and Maxillofacial Surgeons has stated that the risk is a magnitude higher for denosumab than the oral bisphosphonates.3 The risk is 0.3%.
7) Colella, Anthony, et al. “What is the Risk of Developing Osteonecrosis Following Dental Extractions for Patients on Denosumab for Osteoporosis?.” Journal of Oral and Maxillofacial Surgery (2022).
Conclusions The risk of ONJ in patients on denosumab for osteoporosis is a magnitude greater than for patients on the oral bisphosphonates 2.3% v 0 – 0.3%, which is 7.7 times more likely. Number of extractions and early resumption of the next dose of denosumab increases the risk of ONJ.
Atypical Femur Fractures
8) Goh, Jeremy Keng Meng, et al. “Bilateral atypical femur fractures after denosumab in a bisphosphonate naïve patient: a case report.” Calcified Tissue International 111.1 (2022): 96-101.
A case report of bilateral atypical femur fractures (AFF) in a bisphosphonate naive patient. A 62-year-old female bisphosphonate naive patient was started on denosumab for osteoporosis. Approximately 3 years later she complained of right hip pain and was found to have a bilateral incomplete AFFs. She was asymptomatic on the left lower limb. Patient was managed conservatively and placed on protected weight bearing on both legs. Symptoms subsequently resolved over a period of 3 months, although radiographic findings remained at approximately 1 year. AFFs may be associated with patients on denosumab therapy even without a prior history of bisphosphonate use. Patients should be counselled appropriately and monitored for such complications.
9) Kumar, Shejil, et al. “Atypical femoral fracture in a bisphosphonate-naïve patient on denosumab for osteoporosis.” Archives of Osteoporosis 17.1 (2022): 131.
Summary A post-menopausal Caucasian woman sustained an atypical femoral fracture (AFF) after 5-years continuous denosumab for osteoporosis without prior bisphosphonate exposure. This is only the fifth case reported of AFF in a bisphosphonate-naïve patient receiving denosumab for osteoporosis. Although rare, physicians should consider AFF in patients taking denosumab even without prior bisphosphonate exposure.
Introduction Denosumab has demonstrated overwhelmingly favourable skeletal benefit/risk profile in managing post-menopausal osteoporosis with up to 10-year exposure in the extension of the pivotal FREEDOM randomised placebo-controlled trial. Four previous cases of atypical femoral fracture have been reported in bisphosphonate-naïve patients receiving deno-sumab for osteoporosis.
Methods We present an 85-year-old Caucasian post-menopausal woman without prior fragility fracture who sustained unilateral atypical femoral fracture after 5-years continuous subcutaneous denosumab for osteoporosis. She had no prior bisphosphonate or glucocorticoid exposure and had known chronic kidney disease.
Results X-ray scan demonstrated complete, non-comminuted left proximal femoral shaft fracture meeting radiographic criteria for an atypical femoral fracture. Computed tomography (CT) scan lower limbs revealed unusual side-by-side appearance of proximal and distal fragments of left proximal femur. DXA BMD was artefactually elevated at lumbar spine (1.504 g/cm 2 , T-score + 2.5) and low-normal at right femoral neck (0.856 g/cm2 , T-score -1.0). Serum biochemistry showed renal impairment at baseline (eGFR 30 mL/min/1.73m 2 ). Low-normal serum C telopeptide of type 1 collagen (CTX) (230 ng/L) indicated therapeutic suppression of bone resorption.
Conclusion The patient underwent intramedullary nailing of the femur and denosumab was ceased. This is only the fifth case reported of atypical femoral fracture in a bisphosphonate-naïve patient receiving denosumab for osteoporosis. Although rare, physicians should maintain a high index of suspicion for atypical femoral fracture in a patient taking denosumab even without prior bisphosphonate exposure
Denosumab
10) Kendler, David L., et al. “Denosumab in the treatment of osteoporosis: 10 years later: a narrative review.” Advances in therapy 39.1 (2022): 58-74.
11) Sølling, Anne Sophie, et al. “Denosumab Discontinuation.” Current Osteoporosis Reports (2022): 1-9.
12) Dimai, Hans P., and Astrid Fahrleitner-Pammer. “Osteoporosis and Fragility Fractures: Currently available pharmacological options and future directions.” Best Practice & Research Clinical Rheumatology (2022): 101780.
Currently, there is no international consensus in whom and when exactly pharmacological osteoporosis treatment should be initiated. In short, many national guidelines recommend that women (and men) with a prior fragility fracture be considered for pharmacological intervention because such fractures are associated with a high risk of subsequent fractures [5]. The latter has been shown to be even excessively high within the first and second year after the index fracture and any other subsequent fracture [6]. Consequently, the category of a âvery highâ or âimmanentâ fracture risk has been introduced recently and integrated into the FRAX® fracture risk assessment tool [9]. Furthermore, it has led to the recommendation that drugs with proven faster and more pronounced onset of antifracture efficacy should be considered in these patients [9]. In men and women without a prevalent fracture, the intervention threshold can be set at the FRAX®-based age-specific fracture probability equivalent to a person (of the same age) with a prior fragility fracture [10].
Interventions solely based on BMD T-scores have been shown to be less optimal because at a given T-score the fracture risk of different populations and ethnicities may vary considerably. Furthermore, the relevance of the T-score in terms of its fracture risk relevance is largely dependent on age [2].
Osteonecrosis of the jaw (ONJ), medication-related ONJ (MRONJ)
Initially, the occurrence of ONJ was believed to be associated exclusively to BP use, which led to the term âBP-related ONJ (BRONJ)â [51]. Later, when it was found to be associated to Dmab as well, the term âmedication-related ONJâ (MRONJ) was introduced. A multidisciplinary expert group developed a case definition with the consequence that all subsequent studies could report on the same condition [52]. ONJ was defined as the presence of exposed bone in the maxillofacial region that would not heal within 8 weeks after identification by a healthcare provider. The risk of BRONJ associated with oral BPs was estimated between 1 in 10,000 and < 1 in 100,000 patient-treatment years. According to a more recent consensus finding, BRONJ incidence in osteoporosis patients may range from 0.01% to 0.06%, with a somewhat higher incidence in the Asian population [53]. A critical review which was recently conducted by an expert working group of the European Calcified Tissue Society found that, as expected, the risk for MRONJ is much higher in patients with advanced malignancies compared to those with benign bone diseases [54]. It was concluded that the benefits of antiresorptive treatment far outweigh the potential risk of an MRONJ.
Atypical femoral fractures (AFF)
The occurrence of unusual subtrochanteric and shaft fractures of the femur, also referred to as atypical femoral fractures (AFF), in association with BP treatment has first been published in some case reports in the mid 2000s [55,56]. Later, similar events have been reported in patients treated with Dmab [57]. Consensus criteria for AFF have been published by an expert group with an update a few years later [58,59]. Typically, unilateral or bilateral cortical thickening (hypertrophy) is present in the region where the fracture occurs, but cortical thickening may also be more generalized. Another radiographic feature is a transverse fracture line at the point of origination in the lateral cortex, and a more oblique fracture line to the medial cortex with the latter exhibiting a typical spike in the case of complete fracture. Risk-benefit analyses have shown that benefits of treatment in terms of preventing osteoporotic fractures by far outweigh the risks of AFF [60]. For example, it has been shown that for 1 AFF associated with 3 years of BP treatment, roughly 1200 fractures would be prevented. Nevertheless, irrespective of this impressively positive benefit-risk ratio, BP use decreased dramatically not only in the United States in the following years [61].
DenosumabDenosumab (Dmab) is a fully human monoclonal antibody against the receptor activator of nuclear factor-κB ligand (RANKL) that plays a crucial role in formation, survival, and lifespan of osteoclasts and osteoclast precursors []. By binding to RANKL, Dmab blocks the interaction between RANKL and its receptor RANK and therefore reversibly inhibits bone resorption [].
Dmab significantly reduced the risk of new radiographic vertebral fractures (RRR â68%), hip fractures (RRR â40%), and nonvertebral fractures (RRR â20%). Interestingly, no safety issues were reported in the study.
A phenomenon commonly described as a ârebound phenomenonâ has been observed after cessation or discontinuation of Dmab treatment [107]. Histomorphometric analyses of patients who discontinued Dmab without subsequent medication demonstrated increased osteoclast number, osteoclast surface, and eroded bone surface, together with increased osteoblast numbers and osteoblast-covered bone surface [108]. The dramatic increase in bone turnover has been shown to revert many of the favorable skeletal effects that were gained during Dmab treatment. Accordingly, most patients experience a rapid and marked bone loss that leads to increased fragility and the occurrence of low trauma fractures specifically of the spine including multiple vertebral fractures
To avoid above-mentioned consequences, subsequent osteoprotective treatment with other antiresorptive drugs is necessary, whereby the time of consolidating treatment initiation has been shown to be crucial. Based on currently available evidence, initiation of either a potent oral (ALN) or intravenous (ZOL) BP is recommended. The question, whether treatment with a bone anabolic drug such as teriparatide could be beneficial to counteract the detrimental effects of the rebound phenomenon, cannot be answered at this time []. Bone turnover markers should be measured 3 months after initiation of oral BP to monitor adherence and effectiveness and 6 months after ZOL infusion as a second infusion might be necessary [,
One AFF occurred in each group during the extension phase. Seven cases of ONJ were reported in the long-term group and six cases in the crossover group. Hypocalcemia was reported at low rates (0.05â1.7%), but in a real-life study in community-dwelling osteoporotic men and women, a 7.4% rate of denosumab-induced hypocalcemia was found []. Furthermore, in a 3 year observational study, hypocalcemia developed in almost 4% of the patients treated with Dmab []. Consequently, it has been recommended to start Dmab treatment only in calcium replete patients [,].
Practice points
â¢There is increasing evidence that bisphosphonate drug holidays may be associated with an increased fracture risk, particularly if one or more additional strong fracture risk factors are present.
â¢Anti fracture efficacy of menopausal hormone treatment (MHT) has been demonstrated by numerous studies of different study designs. However, it should be kept in mind that so far no data are available from prospective randomized controlled trials with antifracture efficacy as the primary endpoint.
â¢After discontinuation of denosumab treatment, most patients experience a rapid and marked bone loss, which leads to increased fragility and the occurrence of low trauma fractures, specifically of the spine, including multiple vertebral fractures. To avoid such undesirable consequences, subsequent osteoprotective treatment with antiresorptive drugs, e.g., ALN or ZOL, is necessary. The optimal time point at which to begin this treatment is crucial.
â¢One of the very few potential and promising future osteoporosis treatment options is mesenchymal stem cell infusion, but first results from an ongoing clinical trial investigating safety and efficacy in patients with osteoporosis will not be available before 2026.
HRT
13) Gosset, Anna, Jean-Michel Pouillès, and Florence Trémollieres. “Menopausal hormone therapy for the management of osteoporosis.” Best Practice & Research Clinical Endocrinology & Metabolism 35.6 (2021): 101551.
Postmenopausal osteoporosis is for a large part the consequence of both quantitative and qualitative bone alterations induced by estrogen deficiency which occurs within the first years of the menopause transition. Bone is a strong estrogen-dependent tissue and estrogens play a major role in the acquisition and maintenance of bone mineral content throughout life.
14) Yong, Eu-Leong, and Susan Logan. “Menopausal osteoporosis: screening, prevention and treatment.” Singapore Medical Journal 62.4 (2021): 159.
Oestrogen is one of the very few drugs with both anabolic and anti-resorptive effects on bone cells.(2) Randomised, controlled trials and observational studies show that standard-dose MHT reduces hip fractures by 28% (relative risk [RR] 0.72 [0.53â0.98]), vertebrae fractures by 35% (RR 0.65 [0.46â0.92]) and other non-vertebral fractures by 27% (RR 0.73 [0.58â0.94]).(44) MHT utilises lower levels of hormones compared to oral contraceptive formulations that require supraphysiological doses to suppress ovulation, increasing its level of safety. The benefit-risk ratio is most favourable for women with oestrogen deficiency due to POI and early menopause. These women, especially those who experience surgical menopause, frequently suffer from distressing vasomotor symptoms, which MHT largely resolves. MHT also protects against genitourinary syndrome of menopause (GSM), which affects the lower genitourinary tract and is characterised by vulval itch, vaginal dryness, dyspareunia, urinary frequency, urgency, nocturia, urge incontinence and urinary tract infection. Therapy should continue until at least the age of menopause (49 years in Singapore). Observational studies suggest that benefits outweigh risks for effects on bone, heart, cognition, genitourinary symptoms, sexual function, mood and quality of life.(39)
A large trial involving women with a BMD T-score of less than â2.5 but not less than â4.0 at the lumbar spine or total hip showed that treatment with denosumab (60 mg administered twice yearly by subcutaneous injection) resulted in a significantly lower risk of vertebral fractures (by 68%), hip fractures (by 40%), and nonvertebral fractures (by 20%) compared to a placebo.(55) As with bisphosphonates, rare cases of atypical femur fractures and osteonecrosis of the jaw have been observed. Recent concerns about rapid rebound bone loss following cessation of denosumab therapy, exceeding those on placebo,(54) necessitate re-examination of its costs and benefits.(56)
15) Gamsjaeger, Sonja, Erik F. Eriksen, and Eleftherios P. Paschalis. “Effect of hormone replacement therapy on bone formation quality and mineralization regulation mechanisms in early postmenopausal women.” Bone reports 14 (2021): 101055.
16) Lin, Shih-Yin, et al. “Efficacy and safety of postmenopausal osteoporosis treatments: a systematic review and network meta-analysis of randomized controlled trials.” Journal of Clinical Medicine 10.14 (2021): 3043.
17) Vigneswaran, Kugajeevan, and Haitham Hamoda. “Hormone replacement therapyâCurrent recommendations.” Best Practice & Research Clinical Obstetrics & Gynaecology 81 (2022): 8-21.
18) Brommage, Robert. “New targets and emergent therapies for osteoporosis.” Bone Regulators and Osteoporosis Therapy (2020): 451-473.
19) Podfigurna, Agnieszka, et al. “Impact of hormonal replacement therapy on bone mineral density in premature ovarian insufficiency patients.” Journal of Clinical Medicine 9.12 (2020): 3961.
20) Gambacciani, M., A. Cagnacci, and S. Lello. “Hormone replacement therapy and prevention of chronic conditions.” Climacteric 22.3 (2019): 303-306\
21) Langer, R. D., et al. “Hormone replacement therapyâwhere are we now?.” Climacteric 24.1 (2021): 3-10.
22) Gupta, Anmol, et al. “DEXA sensitivity analysis in patients with adult spinal deformity.” The Spine Journal 20.2 (2020): 174-180.
=======================================================
Berberine for bone density
Qi, Guobin, et al. “Berbamine inhibits RANKL-and M-CSF-mediated osteoclastogenesis and alleviates ovariectomy-induced bone loss.” Frontiers in Pharmacology 13 (2022).
Osteoporosis is a common public health problem characterized by decreased bone mass, increased bone brittleness and damage to the bone microstructure. Excessive bone resorption by osteoclasts is the main target of the currently used drugs or treatment for osteoporosis. Effective antiresorptive drugs without side effects following long-term administration have become a major focus of anti-osteoporotic drugs. In the present study, we investigated the effect of berbamine, a small molecule natural product from Berberis amurensis Rupr, a traditional Chinese medicine, on RANKL-induced osteoclast differentiation in vitro and ovariectomy-induced bone loss in vivo. The results demonstrated that berbamine at a safe and effective dose inhibited osteoclastogenesis and bone resorption function in vitro by suppressing the nuclear factor-κB signaling pathway. In addition, berbamine protected against osteoporosis by inhibiting osteoclastogenesis and bone resorption function without affecting osteogenesis in the ovariectomy mouse model. These findings revealed that berbamine has a protective role against osteoporosis and may represent a novel promising treatment strategy for osteoporosis.
Published on February 12th, 2023 by Jeffrey Dach MD
The post The New Osteoporosis Drugs, the Good, Bad and Ugly appeared first on Jeffrey Dach MD.
February 7, 2023
The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two
The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two
Jeffrey Dach MD
Brenda is a young woman who came to my office for evaluation and upon testing, was found to have essentially no urinary iodine excretion, indicating iodine deficiency.
Above header image: Autonomous Nodule in right thyroid lobe (red arrow) showing “hot” , avid update of radio-tracer, with suppression of remaining thyroid gland (blue dotted line) Outline of neck (blue dotted line). Courtesy of wikimedia commons.
Although Brenda had been in the US for twenty years, she was born and grew up in a foreign country noted for low iodine levels. Because of her iodine deficiency, Brenda began a program of iodine supplementation with iodized salt. About a week later, Brenda experienced an episode of tachycardia, severe rapid heart rate, with a sustained pulse rate of 220 beats per minute. Shortly after arriving at the local hospital Emergency Room, the tachycardia resolved spontaneously. The doctors had no explanation for the tachycardia, and Branda was sent home with a Beta-Blocker Drug, called Atenolol to slow the heart rate should the tachycardia return.
What Caused Brenda’s Tachycardia? The Autonomous Nodule.
Brenda’s tachycardia was a manifestation of hyperthyroidism from an autonomous thyroid nodule. When Brenda started taking iodized salt, the increased iodine was converted rapidly into thyroid hormone by the autonomous nodule, a well described phenomenon of hyperthyroidism associated with dietary Iodine intake. The autonomous thyroid nodule manufactures thyroid hormone uncontrollably depending on iodine availability, outside of the normal control mechanism of TSH (thyroid stimulating hormone). When these patients ingest dietary iodine, they become thyrotoxic with very low TSH, high Free T3 and FreeT4, yet the autonomous nodule continues to take up iodine and produce large amounts of thyroid hormone despite the very low TSH. Yet, the low TSH will suppress the iodine uptake in the surrounding normal thyroid tissue, rendering the nodule “hot” on a suppressed background of normal thyroid on the radionuclide scan.
The Sonogram and Radio-Nuclide Thyroid Scan
Brenda was advised to stop the dietary Iodine and sent for diagnostic evaluation for an autonomous thyroid nodule, also called a “hot nodule”. Sure enough, Brenda’s thyroid sonogram showed a thyroid nodule in the right upper lobe, and her Technetium 99M radionuclide scan showed the nodule avidly took up radio-tracer, a “Hot Nodule”, indicating an autonomous nodule. (25-29)
Thyroxine Suppression Test
In some cases, the nodule may not be visible on the radionuclide scan. The radioactive tracer uptake may not be increased enough or “Hot” enough to be visible. To make the nodule more visible, standing out from the surrounding normal thyroid gland, and an additional step may be required. This is called a Thyroxine suppression test. The scan is repeated after giving the patient Levothyoxine which suppresses uptake in the remaining normal thyroid tissue, yet does not affect the autonomous nodule, making the hot nodule stand out from the suppressed background thyroid tissue. (25-29)
In 1998, Dr John Stanbury concluded the most common cause of iodine induced hyperthyroidism is the autonomous nodule, writing:
The biological basis for IIH (Iodine induced hyperthyroidism) appears most often to be mutational events in thyroid cells that lead to autonomy of function. When the mass of cells with such an event becomes sufficient and iodine supply is increased, the subject may become thyrotoxic. These changes may occur in localized foci within the gland or in the process of nodule formation. IIH may also occur with an increase in iodine intake in those whose hyperthyroidism (Graves’ disease) is not expressed because of iodine deficiency. The risks of IIH are principally to the elderly who may have heart disease, and to those who live in regions without medical care.(21)
What is an Autonomous Nodule?
Since 1998, more recent medical research reveals that autonomous thyroid nodules are clones of cells that have a mutation in the TSH Receptor (Thyroid Stimulating Hormone Receptor). These mutations usually arise as a result of iodine deficiency in patients who spend their childhood in iodine deficient countries or geographic regions. Mutations in the gene for the TSH receptor make these nodules independent of TSH control usually found in normal thyroid tissue. Thus, based on the availability of Iodine, the autonomous nodule makes thyroid hormone uncontrollably. (9-13)(35)
Etiology of Autonomous Nodules
What causes the autonomous nodule? Remember, iodine is needed for the production of thyroid hormone, and Iodine deficiency impairs this process causing hypothyroidism. The pituitary responds to low thyroid hormone levels by secreting more TSH, causing elevation of the TSH, which then travels to the thyroid gland and instructs increased generation of hydrogen peroxide, as well as all other steps in thyroid hormone production. If the patient is also selenium deficient, the the selenium-based antioxidants system will be deficient, with lack of degradation of hydrogen peroxide. The excess hydrogen peroxide is mutagenic, leading to mutations in the TSH receptor. A clone of thyroid cells with such a mutation is an autonomous nodule. In 2007, Dr. Knut Krohn writes:
We reconstruct a line of events that could explain the predominant neoplastic character (i.e. originating from a single mutated cell) of thyroid nodular lesions. This process might be triggered by the oxidative nature of thyroid hormone synthesis or additional oxidative stress caused by iodine deficiency or smoking. If the antioxidant defense is not effective, this oxidative stress can cause DNA damage followed by an increase in the spontaneous mutation rate, which is a platform for tumor genesis. The hallmark of thyroid physiology—H2O2 production during hormone synthesis—is therefore very likely to be the ultimate cause of frequent mutagenesis in the thyroid gland. DNA damage and mutagenesis could provide the basis for the frequent nodular transformation of endemic goiters. (51-53) Emphasis Mine.
The Autonomous Nodule Originates in an Iodine Deficient Region
Chronic iodine deficiency, usually from living in an iodine deficient region, is considered the main cause of autonomous nodules. Iodine deficiency is a risk factor for mutations in the TSH receptors and development of the autonomous thyroid nodule. Here in the US, we started a program of iodine fortification of table salt back in 1924, and since iodine deficiency has been considerably reduced, autonomous nodules are now quite rare in the US. Most of the cases we see here in the US are patients who migrate here from iodine deficient regions outside the US. These people may harbor autonomous nodules, and may present with features of transient hyperthyroidism when supplemented with iodine in the diet. (1-13)
Hyperthyroidism After Iodized Salt Fortification
Even the small amounts of dietary iodine found in Iodized Salt can cause transient hyperthyroidism in patients with autonomous nodules. Public records show a transient increase in mortality from thyrotoxicosis in 1926-1928 after the introduction of Iodized Salt. This was thought to be due to presence of pre-existing autonomous nodules in the population. Likewise, in various other countries, Iodized salt and Iodized Bread programs were introduced, and again a transient increase in thyrotoxicosis was reported shortly afterwards. Again, these cases were thought to be autonomous nodules responding to dietary iodine supplements. Note: some of these cases were toxic nodular goiter, with one or more autonomous nodules. In addition to iodized salt programs, thyrotoxicosis in the autonomous nodule patient may be caused by various iodine containing drugs such as SSKI, amiodorone, and iodinated radiographic contrast. In case reports of iodine causing hyperthyroidism in normal thyroid glands, one might suggest these normal glands harbor autonomous nodules that are simply missed. (1-13)(20-23) (33-34)
Marine-Lenhart Syndrome
In Japan, potassium iodine is commonly used as a thyroid blocking drug in Grave’s disease patients. However about 10 per cent of cases do not respond or are made worse by iodine. Could some of these cases be explained by autonomous functioning thyroid tissue? This is the Marine-Lenhart Syndrome defined as Graves’ disease with thyroid nodular lesions and clinical characteristics of both Graves’ disease and Plummer disease (toxic nodular goiter). In 2021, Dr. Hirosuke Danno found 0.26 per cent prevalence of MLS among Graves Disease patients in Japan. This is not high enough prevalence to explain the 9-10 per cent rate of iodine escape found in 2015 by Dr. Yoshihara when switching pregnant Graves’ disease patients from Methimazole to Iodine. However, the co-existence of Plummers’ with Graves’ is something to keep in mind when clinical features are atypical. (54-58)(64)
Over Expression of NIS in autonomous nodules
In 1999, Dr. Meller demonstrated over expression of the Na+/I- symporter (NIS) in autonomous nodules, providing an explanation for enhanced uptake and clearance of iodine on radionuclide scan. (27)
Treatment of Toxic Adenoma and Toxic Multinodular Goiter
Treatment of thyro-toxicosis caused by autonomous thyroid nodule or toxic nodular goiter consists of the following: (38-48)(59-63)
1) Thyroid Blocking Drugs, also called medical treatment. The most used drug is Methimazole, 15 -30 mg per day. Lithium Carbonate 300 mg TID has also been found useful as a thyroid blocking drug, and may be given prior to radioactive iodine (I-131) therapy to enhance retention of Iodine in the thyroid gland. To slow the heart rate, Beta Blockers (atenolol 25-40 mg/day) are commonly prescribed for relief of tachycardia. (44-48)
2) Thyroid Ablation with Radiation. Radioactive Iodine (I-131) therapy for autonomous nodule is a form of radiation therapy which ablates the nodule, and is quite successful. Autonomous nodules are highly active and soak up most of the radioactive iodine, sparing the remaining normal gland. (45-47)
3) Surgical removal of the nodule with thyroid lobectomy procedure. (63)
4) Percutaneous ethanol injection into the autonomous thyroid nodule under ultrasound control. (38-40)
Another way to check Iodine Sufficiency- Serum Thyroglobulin
Serum thyroglobulin is also useful in evaluating iodine status. (17-19)
Conclusion: Although quite rare in the US, the autonomous nodule is an important cause of iodine induced hyperthyroidism. Unlike Graves’ Disease in which iodine serves as a useful thyroid blocking agent, iodine is contraindicated for the autonomous nodule or toxic nodular goiter patient, as in these cases, iodine causes thyrotoxicosis, which may represent a life-threatening medical emergency. The autonomous nodule is frequently missed by mainstream medicine, representing another error.
References
1) Azizi, F. “Iodized oil: its role in the management of iodine deficiency disorders.” International Journal of Endocrinology and Metabolism 5.2 (2007): 91-98.
2) Kohn, LAWRENCE A. “The Midwestern American” epidemic” of iodine-induced hyperthyroidism in the 1920s.” Bulletin of the New York Academy of Medicine 52.7 (1976): 770.
3) Connolly, R. J., G. I. Vidor, and J. C. Stewart. “Increase in thyrotoxicosis in endemic goitre area after iodation of bread.” The Lancet 295.7645 (1970): 500-502.
4) Vidor, G. I., et al. “Pathogenesis of iodine-induced thyrotoxicosis: studies in northern Tasmania.” The Journal of Clinical Endocrinology & Metabolism 37.6 (1973): 901-909.
5) Todd, C. H., et al. “Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe.” The Lancet 346.8989 (1995): 1563-1564.
6) Bourdoux, Pierre, et al. “Iodine-induced thyrotoxicosis in Kivu, Zaire.” Lancet 347.9000 (1996): 552-553.
7) Delange, F., B. De Benoist, and D. Alnwick. “Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt.” Thyroid 9.6 (1999): 545-556.
8) Livadas, D. P., et al. “The toxic effects of small iodine supplements in patients with autonomous thyroid nodules.” Clinical endocrinology 7.2 (1977): 121-127.
9) Tonacchera, Massimo, et al. “Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter.” The Journal of Clinical Endocrinology & Metabolism 85.6 (2000): 2270-2274.
10) Bülow Pedersen, Inge, et al. “Large differences in incidences of overt hyper-and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey.” The Journal of Clinical Endocrinology & Metabolism 87.10 (2002): 4462-4469.
11) Bülow Pedersen, Inge, et al. “Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark.” The Journal of Clinical Endocrinology & Metabolism 91.10 (2006): 3830-3834.
12) Davies, Terry F., et al. “Thyrotropin receptor–associated diseases: from adenomata to Graves disease.” The Journal of clinical investigation 115.8 (2005): 1972-1983.
13) Gozu, Hulya Ilıksu, et al. “Similar prevalence of somatic TSH receptor and Gsα mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey.” European journal of endocrinology 155.4 (2006): 535-545.
14) Beck-Peccoz, Paolo. “Antithyroid drugs are 65 years old: time for retirement?.” Endocrinology 149.12 (2008): 5943-5944.
15-16) deleted
17) Brodowski, Jacek, et al. “Thyroglobulin concentration after introduction of population iodine prophylaxis in random selected group of children and adolescents in Szczecin region.” Polski Merkuriusz Lekarski: 20.116 (2006): 164-167.
18) van den Briel, Tina, et al. “Serum thyroglobulin and urinary iodine concentration are the most appropriate indicators of iodine status and thyroid function under conditions of increasing iodine supply in schoolchildren in Benin.” The Journal of nutrition 131.10 (2001): 2701-2706.
19) Vejbjerg, Pernille, et al. “Thyroglobulin as a marker of iodine nutrition status in the general population.” European Journal of Endocrinology 161.3 (2009): 475-481.
20) Gołkowski, Filip, et al. “Increased prevalence of hyperthyroidism as an early and transient side-effect of implementing iodine prophylaxis.” Public health nutrition 10.8 (2007): 799-802.
21) Stanbury, John Burton, et al. “Iodine-induced hyperthyroidism: occurrence and epidemiology.” Thyroid 8.1 (1998): 83-100.
22) Corvilain, Bernard, et al. “Autonomy in endemic goiter.” Thyroid 8.1 (1998): 107-113.
23) Schwarzfischer, P., et al. “Iodine-induced hyperthyroidism in the aged. 2. Pathomechanism, differential diagnosis and therapy problems.” Fortschritte der Medizin 100.5 (1982): 153-158.
24) Joseph, K., J. Mahlstedt, and U. Welcke. “Early recognition of autonomous thyroid tissue by a combination of quantitative thyroid pertechnetate scintigraphy with the free T4 equivalent (author’s transl).” Nuklearmedizin. Nuclear Medicine 19.2 (1980): 54-63.
25) Fricke, Eva, et al. “Scintigraphy for risk stratification of iodine-induced thyrotoxicosis in patients receiving contrast agent for coronary angiography: a prospective study of patients with low thyrotropin.” The Journal of Clinical Endocrinology & Metabolism 89.12 (2004): 6092-6096.
26) Ratnos, Celso D., et al. “Thyroid suppression test with L‐thyroxine and [99mTc] pertechnetate.” Clinical endocrinology 52.4 (2000): 471-477.
27) Meller, J., and W. Becker. “Scintigraphy with (99m) Tc-pertechnetate in the evaluation of functional thyroidal autonomy.” The Quarterly Journal of Nuclear Medicine and Molecular Imaging 43.3 (1999): 179.
28) Meller, J., and W. Becker. “The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound.” European journal of nuclear medicine and molecular imaging 29.2 (2002): S425-S438.
29) Meller, J., and W. Becker. “Scintigraphic evaluation of functional thyroidal autonomy.” Experimental and clinical endocrinology & diabetes 106.S 04 (1998): S45-S51.
30) Nolte, Wilhelm, et al. “Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study.” European journal of endocrinology 134.3 (1996): 337-341.
31) Hehrmann, R., et al. “Risk of hyperthyroidism in examinations with contrast media.” Aktuelle Radiologie 6.5 (1996): 243-248.
32) Rieu, Max, et al. “Prevalence of subclinical hyperthyroidism and relationship between thyroid hormonal status and thyroid ultrasonographic parameters in patients with non‐toxic nodular goitre.” Clinical endocrinology 39.1 (1993): 67-71.
33) Savoie, J. C., et al. “Iodine-induced thyrotoxicosis in apparently normal thyroid glands.” The Journal of Clinical Endocrinology & Metabolism 41.4 (1975): 685-691.
34) Shilo, Shmuel, and Harry J. Hirsch. “Iodine-induced hyperthyroidism in a patient with a normal thyroid gland.” Postgraduate Medical Journal 62.729 (1986): 661-662.
35) Krohn, Knut, and Ralf Paschke. “Progress in understanding the etiology of thyroid autonomy.” The Journal of Clinical Endocrinology & Metabolism 86.7 (2001): 3336-3345.
36) Lima, N., and G. Medeiros‐Neto. “Transient thyrotoxicosis in endemic goitre patients following exposure to a normal iodine intake.” Clinical endocrinology 21.6 (1984): 631-637.
37) Laurberg, Peter, et al. “The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives.” European journal of endocrinology 155.2 (2006): 219-228.
38) Monzani, Fabio, et al. “Five‐year follow‐up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients.” Clinical endocrinology 46.1 (1997): 9-15.
39) Tarantino, Luciano, G. Froncica, and Ignazio Sordelli. “Percutaneous ethanol injection of hyperfunctioning thyroid nodules: long-term follow-up in 125 patients.” American Journal of Roentgenology 190.3 (2008): 800.
40) Lippi, Francesco, et al. “Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group.” The Journal of Clinical Endocrinology & Metabolism 81.9 (1996): 3261-3264.
41) Iagaru, Andrei, and I. Ross McDougall. “Treatment of thyrotoxicosis.” Journal of nuclear medicine 48.3 (2007): 379-389.
42) Rose, Noel R., et al. “Linking iodine with autoimmune thyroiditis.” Environmental Health Perspectives 107.suppl 5 (1999): 749-752.
43) Xue, Haibo, et al. “Selenium upregulates CD4+ CD25+ regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD. H-2h4 mice.” Endocrine Journal 57.7 (2010): 595-601.
44) Azizi, Fereidoun. “Long-term treatment of hyperthyroidism with antithyroid drugs: 35 years of personal clinical experience.” Thyroid 30.10 (2020): 1451-1457.
45) Płazińska, Maria Teresa, Leszek Królicki, and Marianna Bąk. “Lithium carbonate pre-treatment in 131-I therapy of hyperthyroidism.” Nuclear Medicine Review 14.1 (2011): 3-8.
46) Ross, Douglas S., et al.. “Successful treatment of solitary toxic thyroid nodules with relatively low-dose iodine-131, with low prevalence of hypothyroidism.” Annals of internal medicine 101.4 (1984): 488-490.
47) Huysmans, Dyde A., Frans H. Corstens, and Peter W. Kloppenborg. “Long-term follow-up in toxic solitary autonomous thyroid nodules treated with radioactive iodine.” Journal of Nuclear Medicine 32.1 (1991): 27-30.
48) Ross, Douglas S. “Treatment of toxic adenoma and toxic multinodular goiter.” UpToDate. 12th ed. Waltham, MA: Wolters Kluwer (2019).UpToDate_
49) Ratcliffe, Guy E., et al. “Radioiodine treatment of solitary functioning thyroid nodules.” The British Journal of Radiology 59.700 (1986): 385-387.
50) Nygaard, Birte, et al. “Long‐term effect of radioactive iodine on thyroid function and size in patients with solitary autonomously functioning toxic thyroid nodules.” Clinical endocrinology 50.2 (1999): 197-202.
51) Song, Yue, et al. “Roles of hydrogen peroxide in thyroid physiology and disease.” The Journal of Clinical Endocrinology & Metabolism 92.10 (2007): 3764-3773.
52) Krohn, Knut, Jacqueline Maier, and Ralf Paschke. “Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors.” Nature clinical practice Endocrinology & metabolism 3.10 (2007): 713-720.
53) Maier, J., et al. “Iodine deficiency activates antioxidant genes and causes DNA damage in the thyroid gland of rats and mice.” Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1773.6 (2007): 990-999.
54) Nishikawa, Mitsushige, et al. “Coexistence of an Autonomously Functioning Thyroid Nodule in a Patient with Graves’ Disease An Unusual Presentation of Marine-Lenhart Syndrome.” Endocrine journal 44.4 (1997): 571-574.
55) Charkes, N. David. “Graves’ disease with functioning nodules (Marine-Lenhart syndrome).” Journal of Nuclear Medicine 13.12 (1972): 885-892.
56) Danno, Hirosuke, et al. “Prevalence and Treatment Outcomes of Marine-Lenhart Syndrome in Japan.” European Thyroid Journal 10.6 (2021): 461-467. 0.26% in Japan
57) The Marine-Lenhart syndrome (MLS), first described by Charkes in 1972 [1], is now commonly defined as “a combination of Graves’ disease and autonomous functioning thyroid nodule(s) (AFTN)”
58) Miyazaki, Megumi, et al. “A case of Marine-Lenhart syndrome with predominance of Plummer disease.” Journal of UOEH 41.2 (2019): 165-170.
59) Azizi, Fereidoun, et al. “Treatment of toxic multinodular goiter: comparison of radioiodine and long-term methimazole treatment.” Thyroid 29.5 (2019): 625-630.
60) Bawand, Rashed, et al. “Comparison of clinical efficacy of antithyroid drugs, radioactive iodine, and thyroidectomy for treatment of patients with graves’ disease, toxic thyroid adenoma, and toxic multinodular goiter.” Biomedical and Biotechnology Research Journal (BBRJ) 6.4 (2022): 569.
61) Roque, Catarina, et al. “Long-term effects of radioiodine in toxic multinodular goiter: thyroid volume, function, and autoimmunity.” The Journal of Clinical Endocrinology & Metabolism 105.7 (2020): e2464-e2470.
62) Racaru, L. Vija, et al. “Management of adenomas and toxic multinodular goiters with Iodine 131.” Médecine Nucléaire 44.4 (2020): 272-276.
63) Kırdak, Türkay. “Surgery in Hyperthyroidism: Toxic Adenoma and/or Multinodular Goiter.” Thyroid and Parathyroid Diseases. Springer, Cham, 2019. 45-49.
64) Yoshihara, Ai, et al. “Substituting potassium iodide for methimazole as the treatment for Graves’ disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan.” Thyroid 25.10 (2015): 1155-1161.
Published on February 7th, 2023 by Jeffrey Dach MD
The post The Autonomous Thyroid Nodule and Iodine Induced Hyperthyroidism Part Two appeared first on Jeffrey Dach MD.
February 2, 2023
Dr Peter McCullough on Ivermectin and Covid Vaccines
 Dr Peter Mcullough on Ivermectin and Covid Vaccines
Dr Peter Mcullough on Ivermectin and Covid Vaccines
Over the past three years of the Covid Pandemic and Rollout of the Covid Vaccines, our office policy has been to treat early on, all Covid infections as outpatients using repurposed antiviral drugs and supplements such as Ivermectin, Hydroxychloriquine, Zinc, Quercitin, Doxycycline, Zithromycin, Steroid inhalers, Vitamin D3, Vitamin C, etc. All such patients treated in my office have recovered uneventfully without hospitalization. That is 100 per cent recovery without hospitalization with Early Treatment !
Above header image: The War on Ivermectin, Courtesy of Dr Pierre Kory.
Regarding the Covid 19 vaccine first released December 14, 2020:
Five months after release of the vaccine on May 23 , 2021, we alerted people thorough our newsletter that the VAERS system (Vaccine Adverse Event reporting System) was showing adverse side effects and deaths far beyond any previous vaccine, and to me was similar to a previous drug, Vioxx, which was eventually pulled from the marketplace. The Covid Vaccine has only short lived efficacy, and is not a safe product, and the entire program should be halted. In the video clip below, Dr McCullough discusses the experience in Australia, where 99 per cent of all hospitalized patients with COVID have been vaccinated.
Dr. McCullough Discusses Efficacy and Safety of Ivermectin for Covid
Ivermectin gives Immediate Relief for Pulmonary Symptoms of Covid Infection. Horse Paste? Ivermectin Is the ‘Most Dynamic’ and ‘Most Rapidly-Acting’ Medication for COVID …
====================================
=======================================
Covid Vaccines? Dr. Peter McCullough: “The Vaccines Have Backfired”
==================================================
Articles with Related Interest
Explaining Damar Hamlin Cardiac Arrest on Field
Covid Vaccines, a time for Re-Assessment
Director of CDC, Rochelle Walensky Warns of ADE, Antibody Dependent Enhancement From Israel Data.
Israel Should Stop Ṗẝiẕḗr and Start l\/ḗrmḗctin Distribution
The Covid Vaccine is Safe and Effective ?
Could the Covid Vaccine be the Next Vioxx ?
Ivermectin for Covid, The Failure of American Medicine
Ivermectin Antiparasitic Anticancer Antiviral Wonder Drug
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
http://sci.med.narkive.com/covV2Qo2/jeffrey-dach-book-announcment-natural-medicine-101
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2023 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given. See Repost Guidelines.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
Published on February 2nd, 2023 by Jeffrey Dach MD
The post Dr Peter McCullough on Ivermectin and Covid Vaccines appeared first on Jeffrey Dach MD.
January 31, 2023
Medical Aspects of Global Nuclear War
 Medical Aspects of Global Nuclear War
Medical Aspects of Global Nuclear War
by Jeffrey Dach MD
Steven Starr is a professor at the University of Missouri who teaches a college level course on the consequences of Nuclear War. In this 16 minute video, Dr. Starr explains what happens after an “accident” triggers a nuclear first strike with subsequent exchange of the remaining arsenal of nuclear weapons.
Thousands of nuclear detonations obliterate all major cities in massive fireballs. These massive fireballs create many tons of black smoke that rise to the stratosphere blocking sunlight for the next 3-10 years. This creates a nuclear winter with freezing temperatures killing all crops. With complete cessation of food production, all humans and animals die of starvation. We haven’t even touched on health effects of radiation contamination.
Nuclear winter is a mass extinction phenomenon. What are the medical aspects of nuclear winter? This is a terminal medical catastrophe with no known treatment other than prevention.
===============================================
Jeffrey Dach MD
7450 Griffin Road Suite 1890/190
Davie, Fl 33314
954-792-4663
References
1) Nuclear War, Nuclear Winter, and Human Extinction By Federation of American Scientists • October 14, 2015 by Steven Starr
That is, a nuclear winter would cause most humans and large animals to die from nuclear famine in a mass extinction event similar to the one that wiped out the dinosaurs.\
These mass fires, many of which would rage over large cities and industrial areas, would release many tens of millions of tons of black carbon soot and smoke (up to 180 million tons, according to peer-reviewed studies), which would rise rapidly above cloud level and into the stratosphere
The smoke from a war fought with strategic nuclear weapons would quickly prevent up to 70% of sunlight from reaching the surface of the Northern Hemisphere and 35% of sunlight from reaching the surface of the Southern Hemisphere. Such an enormous loss of warming sunlight would produce Ice Age weather conditions on Earth in a matter of weeks. For a period of 1-3 years following the war, temperatures would fall below freezing every day in the central agricultural zones of North America and Eurasia.
Nuclear winter would cause average global surface temperatures to become colder than they were at the height of the last Ice Age. Such extreme cold would eliminate growing seasons for many years, probably for a decade or longer. Can you imagine a winter that lasts for ten years?
The results of such a scenario are obvious. Temperatures would be much too cold to grow food, and they would remain this way long enough to cause most humans and animals to starve to death.
the catastrophic environmental consequences of nuclear war – has been effectively dropped from the global discussion of nuclear weaponry.
2) Nuclear Famine Steven Starr …The long-term environmental consequences of a nuclear war between the US and Russia could kill most humans and land animals. An India-Pakistan nuclear war could cause 2 billion people to starve to death. Nuclear war threatens all nations and peoples.
3) Nuclear Famine . org About Steven Starr
4) Steven Starr is the director of the University of Missouri’s Clinical Laboratory Science Program, as well as a senior scientist at the Physicians for Social Responsibility. He has been published in the Bulletin of the Atomic Scientists and the Strategic Arms Reduction (STAR) website of the Moscow Institute of Physics and Technology; he also maintains the website Nuclear Darkness. Starr also teaches a class on the Environmental, Health and Social Effects of nuclear weapons at the University of Missouri.
5) Atomic Bombing of Japan images Courtesy of Wikimedia
Left picture : At the time this photo was made, smoke billowed 20,000 feet above Hiroshima while smoke from the burst of the first atomic bomb had spread over 10,000 feet on the target at the base of the rising column. Six planes of the 509th Composite Group participated in this mission: one to carry the bomb (Enola Gay), one to take scientific measurements of the blast (The Great Artiste), the third to take photographs (Necessary Evil), while the others flew approximately an hour ahead to act as weather scouts (08/06/1945). Bad weather would disqualify a target as the scientists insisted on a visual delivery. The primary target was Hiroshima, the secondary was Kokura, and the tertiary was Nagasaki.
Right picture : Atomic bombing of Nagasaki on August 9, 1945, taken by Charles Levy.
Published on January 31st, 2023 by Jeffrey Dach MD
The post Medical Aspects of Global Nuclear War appeared first on Jeffrey Dach MD.
January 29, 2023
Fenbendazole Effective for Renal Cell Carcinoma Case Report

Fenbendazole Effective for Renal Cell Carcinoma Case Report
by Jeffrey Dach MD
Fenbendazole and Mebendazole are discussed in Chapter 24 of my book, Cracking Cancer Toolkit, use of repurposed drugs for cancer treatment. Fenbendazole has become a popular anti-cancer drug on social media where stories of success are posted. Fenbendazole is inexpensive and widely available without a prescription. Fenbendazole is an anti-parasitic veterinary drug similar to the human drug, mebendazole.
Renal Cell carcinoma is notoriously difficult to treat, not responsive to conventional chemotherapy or radiation therapy. This is a case report of complete resolution of metastatic renal carcinoma using the veterinary drug, Fenbendazole in a 63 year old human male.
Renal Cell Carcinoma Case Report
A 63 yr old male presented with metastatic kidney cancer, with spread to pancreas, lungs, inferior vena cava, and bone. Treatment with immunotherapy was unsuccessful and poorly tolerated. His doctors advised him he had six months to live. The patient learned about Fenbendazole, and started 222 mg orally for 3 days then 4 days off each week. Within 2 months of taking fenbendazole, the largest mass (red arrows above image) in the kidney was gone (green arrow) and the other tumors shrank considerably. By 5 months, all the tumors were gone. This Case Report was also written up in a medical journal : Chiang, R. S., et al. “Fenbendazole enhancing anti-tumor effect: a case series.” Clin. Oncol. Case Rep 4 (2021): 2-5
As mentioned in my book, Cracking Cancer Toolkit, anticancer effects of fenbendazole have considerable synergy with DCA (Dichloracetate), and probably also with autophagy inhibitors such as omeprazole, A common PPI antacid drug. Dogra, et al. “Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways.” Scientific reports 8.1 (2018): 1-15.
Above header image: Left Image: Before fenbendazole treatment showing large mass left kidney with extension into renal vein (red arrows). Right image after 2 months of fenbendazole, mass has resolved (green arrow) . C ourtesy of: Chiang, R. S., et al. “Fenbendazole enhancing anti-tumor effect: a case series.” Clin. Oncol. Case Rep 4 (2021): 2-5.
Articles with related Interest
Jeffrey Dach MD
7450 Griffin road
Suite 180/190
Davie, Fl 33314
954-792-4663
References and Links
Credit and Thanks goes to substack article by Ben Fen Dec 10, 2022, Case Report: Renal Cell Carcinoma, age 63, Male Traditional Chemo Failed, Dosage, No Other Co-Factors Used, Maintenance by Ben Fen Dec 10, 2022
Kang, Kyung-Sun, and Da-Hyun Kim. “Fenbendazole induces cell cycle arrest in colorectal cancer cells and patient-derived colon cancer organoids.” Cancer Research 82.12_Supplement (2022): 2313-2313.
Colorectal cancer is one of leading cause of cancer-related deaths. Therefore, there have been various attempts to cure the cancer by developing new efficient anti-cancer therapy in addition to surgical resection and chemotherapy. In this study, we investigated the effects of fenbendazole, an anti-helminthic drug, both colon cancer cells and patient-derived colon tumor organoids. Notably, we employed 3D tumor organoid models because 2D-cultured cell lines were not able to recapitulate the physiology of solid tumors. We first observed that treatment of fenbendazole to colon cancer cells induced apoptosis within 24 hours, which was extended for a long-term. We revealed that fenbendazole markedly suppressed proliferation rate via cell cycle arrest. Cell cycle progression is elaborately regulated by multiple genes, such as cyclins and cyclin-dependent kinases (CDKs). From a screening of cell cycle-related factors, we found that the protein levels of CDK1 phosphorylated at Tyr15 and cyclin B1 which was known to regulate M phase transition, were drastically downregulated when the tumor cells were exposed to fenbendazole. Next, colorectal tumor-bearing mouse model was established using AOM/DSS. Oral administration of fenbendazole into the mouse not only reduced the number of tumor cells but also lowered tumor grades. Overall, our study suggested a possibility that fenbendazole could be applied for anti-cancer therapy by targeting cell cycle arrest…
Case Report: Colon cancer, age 40, F
I’m a forty-year old woman in good health who was diagnosed with colon cancer in November 2021.
PET scans had shown that the liver was affected as well, which was confirmed by biopsy.
Chemo was ineffective as I had to have another surgery and more chemo was necessary. After two more rounds of chemo but not surgery, the tumors returned and another new growth was seen on the left kidney. That was in April.I read about fenbendazole and felt that I had nothing to lose. So I started in on May 5, 2022 with 2000 mg a day. About 8 scoops of the powder. I did split it into two 1000 mg doses. Within two weeks I did feel better but I wasn’t sure if it was wishful thinking. I was done with chemo, so now fenben was the only hope for me.I read about fenbendazole and felt that I had nothing to lose. So I started in on May 5, 2022 with 2000 mg a day. About 8 scoops of the powder. I did split it into two 1000 mg doses. Within two weeks I did feel better but I wasn’t sure if it was wishful thinking. I was done with chemo, so now fenben was the only hope for me.On Sept 9 I had a followup with my oncologist as well as a scan. The cancer was gone!
83 yr old female with metastatic breast cancer in lungs, liver, kidneys, spleen, pancreas, gall bladder and bones (spine, ribs, pelvis). Refused traditional chemotherapy and radiation. Took fenbendazole 222 mg once per day for 8 months. Added targeted spot radiation of two tumors on spine causing pain. Cancer-free by two corroborating measures: serum CA 27.29 tumor marker and PET scan. Fenbendazole was without adverse side effects. Monthly monitoring of CA 27.29 to guide future use of fenbendazole.
The following is a self-report from a 27 yr old man with a glioma who treated himself with fenbendazole.
Within a span of 3-4 months the tumor was markedly reduced using 888 mg fenbendazole per day.
Fenbendazole Index for the Book:
p. 13
Joe Tippens’s Fenbendazole Story: Joe
Tippens was diagnosed with non-smallcell
lung cancer with extensive metastatic
disease and sent home to hospice with
a projected three months to live. An old
friend, a veterinarian, suggested he take a
dog de-wormer drug called Fenbendazole.
Three months after starting the drug, his
PET scan, done at MD Anderson Cancer
Center in Houston was completely clear
of cancer. I had the pleasure of meeting
and briefly chatting with Joe at the Annie
Appleseed meeting February 2020. Joe
Tippens has a large Facebook group where
thousands of cancer patients share stories.
p.58
Synergy Combination of DCA and Fenbendazole
The anti-cancer activity of the antiparasitic
drug fenbendazole (FZ) was found to be
“strongly synergistic” with DCA. FZ increased
P53 protein, which translocated to the mitochondria,
resulting in a mitochondrial celldeath
pathway. FZ inhibited glucose uptake,
reduced lactate levels, and reduced HK2 activity.
Dr. Nilambra Dogera et al. write (2018) :
We evaluated the effect of FZ in combination
with the microtubule targeting drug
taxol, glycolytic inhibitor 2 deoxyglucose
[2DG] and dichloroacetate [DCA] – a pyruvate
dehydrogenase kinase inhibitor which
acts by shifting the metabolism towards
glucose oxidation over glycolysis…. there
was a strong synergism by FZ and DCA.
(96)
96) Dogra, Nilambra, Ashok Kumar, and Tapas
Mukhopadhyay. “Fenbendazole acts as a moderate
microtubule destabilizing agent and causes cancer
cell death by modulating multiple cellular pathways.”
Scientific reports 8.1 (2018): 1-15.
p 252
Synergy of Chloroquine with Mebendazole
In 2019, Dr. So Jung Sung et al. reported that
the autophagy inhibitor chloroquine synergizes
with mebendazole, dramatically augmenting
anti-cancer efficacy. (93)
One might expect similar synergy with
fenbendazole, another microtubule inhibitor
structurally similar to mebendazole in the
“azole” drug family. Fenbendazole is a veterinary
antiparasitic drug that has been repurposed
as an anti-cancer drug. (94)
p. 252
The Joe Tippens Story
The Joe Tippens story has created considerable
interest in fenbendazole as an anti-cancer
agent. I had the pleasure of meeting Joe
Tippens at the 2020 Annie Appleseed meeting
in West Palm Beach, Florida. Joe Tippens
grew up on a ranch in western Oklahoma,
and was a healthy, successful venture capitalist
until 2016, when he was diagnosed with a
left-lower lobe asymptomatic lung mass that
proved to be small-cell lung cancer with spread
to mediastinal nodes. In September 2016, Joe
underwent chemotherapy and left lung radiotherapy
at MD Anderson Cancer Center. By
December 2016, the left lung mass was gone,
yet the cancer was now disseminated widely
with metastatic disease. His doctors offered
him a clinical trial of Keytruda® (an immune
checkpoint inhibitor) and sent him home, with
the understanding of a poor prognosis and a
three-month life expectancy. Upon returning
home, Joe received a telephone call from an old
friend, a large-animal veterinarian in western
Oklahoma, who told Joe about fenbendazole, a
canine dewormer, and suggested he had nothing
to lose and should try it. In January of 2017,
Joe started the fenbendazole (222 grams per
day 3 days a week), which was available online
without a prescription at a number of pet medicine
online shops. Joe added curcumin (600
mg/day), tocotrienol and tocopheral vitamin
E (800mg/day) and “broad spectrum” CBD oil
(1–2 droppers/day). In May of 2017, a repeat
PET scan was completely clean, showing “no
evidence of disease.”
Joe writes that when his MD Anderson doctor
saw the PET scan, the doctor scratched his
head and replied:
We don’t quite know what to make of this
as you are the only patient in the clinical
trial with this kind of response. [Read the
Joe Tippens story at www.mycancerstoryrocks.
com.]
p 298
Some anti-cancer drugs (such as fenbendazole) depend on
a functioning P53.
p 314
Chapter 24 Mebendazole and Fenbendazole
p. 317
Fenbendazole Microtubule Agent and Glycolysis Inhibitor
The benzimidazole drug family includes
mebendazole, albendazole, flubendazole and
fenbendazole, all antiparasitic drugs of similar
structure. All use microtubule inhibition as the
main mechanism of action. A number of very
effective chemotherapy drugs (taxanes and
vinca alkaloids) also target the microtubule
system of the cancer cell. Disrupting microtubule
activity blocks the spindle formation
needed for cell division and blocks cell division
in metaphase (a phase in mitosis), causing cell
death (apoptosis).
p 318
Fenbendazole is effective at low micromolar
concentrations and is remarkably safe for animals
and humans, resulting in relatively mild
changes in the microtubule structure of cancer
cells.
In 2018, Dr. Nilambra Dogra et al. studied
the effects of fenbendazole (FZ) on a NSCLC
(non-small-cell lung cancer) cell line in vitro
and in vivo mouse xenografts and make this
favorable conclusion:
FZ [Fenbendazole] … is a safe and inexpensive
anthelmintic drug possessing an
efficient antiproliferative activity…. potent
growth-inhibitory activity…. moderate
affinity for mammalian tubulin and exerts
cytotoxicity to human cancer cells at micromolar
concentrations. Simultaneously, it
caused mitochondrial translocation of p53
and effectively inhibited glucose uptake,
mRNA expression of GLUT transporters
as well as HK2 [hexokinase 2]—a key
glycolytic enzyme that most cancer cells
thrive on. It blocked the growth of human
xenografts in nu/nu mice model when mice
were fed with the drug orally…. potential
therapeutic agent because of its effect
on multiple cellular pathways leading to
effective elimination of cancer cells. (48)
Treatment of the NSCLC lung cancer cells
with 1.0 micromolar FZ for 24 hours resulted in:
partial alteration of the microtubule network
… the microtubule cage around the
nucleus appeared to have lost its intactness
when compared with control cells
… relatively mild tubulin depolymerizing
activity of FZ as compared to other known
microtubule-disrupting agents like nocodazole
and colchicine… (48)
p 318
Fenbendazole GLYCOLYSIS Inhibitor
FZ mimics the structure of glucose and
decreases glucose consumption by binding to
the enzymatic pocket of HK2, thus impairing
the function of hexokinase 2 (HK2). FZ shows
augmented anti-cancer effects in combination
with GLYCOLYSIS inhibitors DCA (dichloroacetate),
and 2DG (2-deoxyglucose). There was
“strong synergism by FZ and DCA.” See the
chapter 5 on DCA for more on this. Dr. Dogra
et al. studied oral dosing of fenbendazole to
mice bearing lung cancer xenografts over 12
days finding “marked reduction in tumor size
and weight” as well as reduced tumor vascularity.
(48)
p 318
Increased P53 in Nucleus and
Mitochondrial Fraction
In addition to its microtubule binding and
disruption activities, FZ has a unique ability to
induce p53 to a considerably high level, with
increased nuclear accumulation and increased
P53 protein in the mitochondrial fraction, targeting
the VDAC, resulting in activation of
apoptosis, mitochondrial cell death pathways.
(48–54)
p 218
Screening for Compounds that
Promote P53 Activity
In cancer cell types with a functioning (wildtype)
P53 gene, promoting that function is a
worthy anti-cancer activity. In 2019, Dr. Zuzana
Mrkvová et al. performed a high-throughput
screen of 2448 compounds on melanoma cells
with functional (wild-type) P53 gene looking
for compounds that promote P53 activity.
Fenbendazole and albendazole were the best
candidates, not only increasing P53-P21 pathway,
but also decreasing the MDM2 and MDMx
suppressor pathway. (60)
Note: Mdm2 and MdmX are negative regulators
of P53, so suppressing them with Fenbendazole
restores P53 function.
p 319
Niclosamide Effective
Regardless of P53 Status
P53 protein translocation to the mitochondria
is part of the mechanism of cell death for
fenbendazole (FZ). One should ask the following
question: Does the cancer cell type in question
have a functioning P53 gene (wild-type) or
a mutant P53? If we are dealing with a mutant
P53, since FZ relies on P53 accumulation in the
mitochondria to induce apoptosis, this mode
of action may not be effective. If the cancer is
not responding to the fenbendazole, then one
might consider alternate drugs that maintain
effect regardless of P53 status. For example,
Niclosamide induces apoptosis via mitochondrial
ETC (electron transport chain) uncoupling
and is effective in P53 mutated cancer
cell types, regardless of P53 status.
p 320
Fenbendazole for Human
Lymphoma Xenografts
In 2008, Dr. Ping Gao et al. studied the effect
of fenbendazole in human lymphoma mouse
xenografts, finding an “unexpected anti-tumorigenic
effect.” (61)
p. 322
29) Lai, Serene Ruth, et al. “In vitro anti‐tubulin effects
of mebendazole and fenbendazole on canine glioma
cells.” Veterinary and comparative oncology 15.4
(2017): 1445-1454.
48) Dogra, Nilambra, Ashok Kumar, and Tapas
Mukhopadhyay. “Fenbendazole acts as a moderate
microtubule destabilizing agent and causes cancer
cell death by modulating multiple cellular pathways.”
Scientific reports 8.1 (2018): 11926
61) Gao, Ping, Chi V. Dang, and Julie Watson.
“Unexpected antitumorigenic effect of fenbendazole
when combined with supplementary vitamins.”
Journal of the American Association for Laboratory
Animal Science 47.6 (2008): 37-40.
63) Duan, Qiwen, Yanfeng Liu, and Sara Rockwell.
“Fenbendazole as a potential anticancer drug.”
Anticancer research 33.2 (2013): 355-362.
64) Aycock-Williams, Ari N., et al. “Effects of fenbendazole
and vitamin E succinate on the growth and survival
of prostate cancer cells.” J Cancer Res Exp Oncol
3.9 (2011): 115-121.
65) Lai, Serene Ruth, et al. “In vitro anti‐tubulin effects
of mebendazole and fenbendazole on canine glioma
cells.” Veterinary and comparative oncology 15.4
(2017): 1445-1454
p 336
Blocking PIBF with Microtubule
Inhibitor Mebendazole
As luck would have it, elimination of PIBF
may be accomplished with another drug that
is widely available, a microtubule-disrupting
agent such as nocodazole, an imidazole
similar to mebendazole and fenbendazole
p 478
Once might speculate on taxane synergy
with other FASN inhibitors, such as orlistat,
fenofibrate and quercetin. (99)
One might also speculate on synergy of fenofibrate
with microtubule-disrupting agents,
mebendazole, and fenbendazole
Published on January 29th, 2023 by Jeffrey Dach MD
The post Fenbendazole Effective for Renal Cell Carcinoma Case Report appeared first on Jeffrey Dach MD.
January 6, 2023
Explaining Damar Hamlin Cardiac Arrest on Field

Explaining Damar Hamlin Cardiac Arrest on Field by Jeffrey Dach MD
Over 38 years (1966-2004), 1101 athletes under age 35 died due to heart related conditions. This is 29 deaths per year. Half ( 50%) had congenital anatomic heart disease or cardiomyopathies, and 10 per cent atherosclerotic vascular disease early onset.
Since introduction of COvid-19 vaccination in January 2021, athletes have suffered 1598 cardiac arrests and 1101 deaths over the past 2 years (550 deaths per year). This is 550 per year. Before Covid Vaccine, this was 29 per year.
Ninety Five per cent of NFL athletes are fully vaccinated. The NFL dropped the vaccine mandates in March of 2022. The NFL received massive government funding to impose the vaccine mandates. NFL athletes are screened for heart conditions before allowed on the field.
Regarding any athlete suffering a cardiac arrest on the field, the top diagnostic consideration is Covid 19 subclinical myocarditis and a primary cardiac arrest . Other things have been relatively ruled out now, including commotio cordis. High levels of adrenalin surge (Catecholamines) triggers an arrythmia, and subsequent cardiac arrest as seen in Hamlin. (1)
Dr Peter McCullough Interview (below) with Del Bigtree on The HighWire explains adrenalin surge on field plus underlying myocarditis caused by spike protein equals cardiac arrest.
The Covid Vaccine contains mRNA which programs our cells to manufacture the spike protein associated with the Covid-19 viral genome. The spike protein is a foreign protein which induces an inflammatory reaction in the heart muscle.(7-9)
mRNA has been found to circulate and may be found in other organ systems, such as brain, ovaries etc. which will then create similar inflammatory reactions.(7-9)
https://jeffreydachmd.com/wp-content/uploads/2023/01/Damar-Hamlin-Peter-McCullugh-Del-Bigtree-primary-cardiac-arrest.mp4Conclusion: The FDA has openly admitted that Myocarditis is an adverse effect of Covid Vaccine which programs our cells to make spike protein. (5)
In Dr McCullough;s opinion, Adrenalin surge with subclinical myocarditis caused by spike protein is the most likely cause of cardiac arrest among NFL athletes on the playing field.(1-6)
Articles with Related Content
Covid Vaccines, a time for Re-Assessment
Director of CDC, Rochelle Walensky Warns of ADE, Antibody Dependent Enhancement From Israel Data.
The Covid Vaccine is Safe and Effective ?
Could the Covid Vaccine be the Next Vioxx ?
Ivermectin for Covid, the Failure of American Medicine
Povidone Iodine Nouth Wash Gargle, Nasal Spray for Prevention and Treatment of Covid
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
References
1) Cadegiani, Flavio A. “Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings.” Cureus 14.8 (2022).
2) Polykretis, Panagis, and Peter A. McCullough. “Rational harm‐benefit assessments by age group are required for continued COVID‐19 vaccination.” Scandinavian Journal of Immunology: e13242.
3) Peter McCullough Tweet
Dr. Panagis Polykretis, Florence, Italy, presents a clear understanding that the foreign genetic code and the presentation of the Spike protein and its fragments ignites autoimmune attack and cellular destruction of normal cells within the human body. He is dead certain! pic.twitter.com/doJGmgiKT2
— Peter A. McCullough, MD, MPH
(@P_McCulloughMD) March 22, 2022
Dr. Panagis Polykretis, Florence, Italy, presents a clear understanding that the foreign genetic code and the presentation of the Spike protein and its fragments ignites autoimmune attack and cellular destruction of normal cells within the human body. He is dead certain!
5) FDA adds a warning to Covid-19 vaccines about risk of heart inflammation By Lauren Mascarenhas Updated Sat June 26, 2021
=========================================
6) Attack from within After COVID-19 Vaccination
With the genetic vaccines, particularly mRNA, for the first-time human cells are forced to produce a highly abnormal, pathogenic Spike protein. The body reacts to this almost immediately with an attack on any cell that has taken up mRNA and expressing the Spike protein. Dr. Panagis Polykretis explains that every human cell, tissue, organ, and body that has taken a COVID-19 vaccine has been damaged…
7) Baumeier, Christian, et al. “Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series.” International Journal of Molecular Sciences 23.13 (2022): 6940.
8) Mörz, Michael. “A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19.” Vaccines 10.10 (2022): 1651.
9) Khan, Shahanshah, et al. “SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway.” Elife 10 (2021): e68563.
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
www.drdach.com
Heart Book by Jeffrey Dach
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
http://sci.med.narkive.com/covV2Qo2/jeffrey-dach-book-announcment-natural-medicine-101
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2023 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given. See Repost Guidelines.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
Published on January 6th, 2023 by Jeffrey Dach MD
The post Explaining Damar Hamlin Cardiac Arrest on Field appeared first on Jeffrey Dach MD.
Jeffrey Dach's Blog
- Jeffrey Dach's profile
- 3 followers



